Chlorophyll a vs b: Structure, Formula, and Biological Significance
The concept of chlorophyll structure is essential in biology and helps explain real-world biological processes and exam-level questions effectively. Understanding the molecular structure of chlorophyll is crucial for students studying plant biology, photosynthesis, and plant physiology. It also helps in differentiating between plant pigments and similar molecules like hemoglobin.
Understanding Chlorophyll Structure
Chlorophyll structure refers to the molecular makeup of chlorophyll, the green pigment found in plants, algae, and some bacteria. This structure is important in areas like photosynthesis, plant nutrition, and coordination compounds in chemistry. Chlorophyll’s ability to absorb light is directly related to the arrangement of its atoms and functional groups. There are different types of chlorophyll, mainly chlorophyll a and b, with slight differences in their chemical formulas.
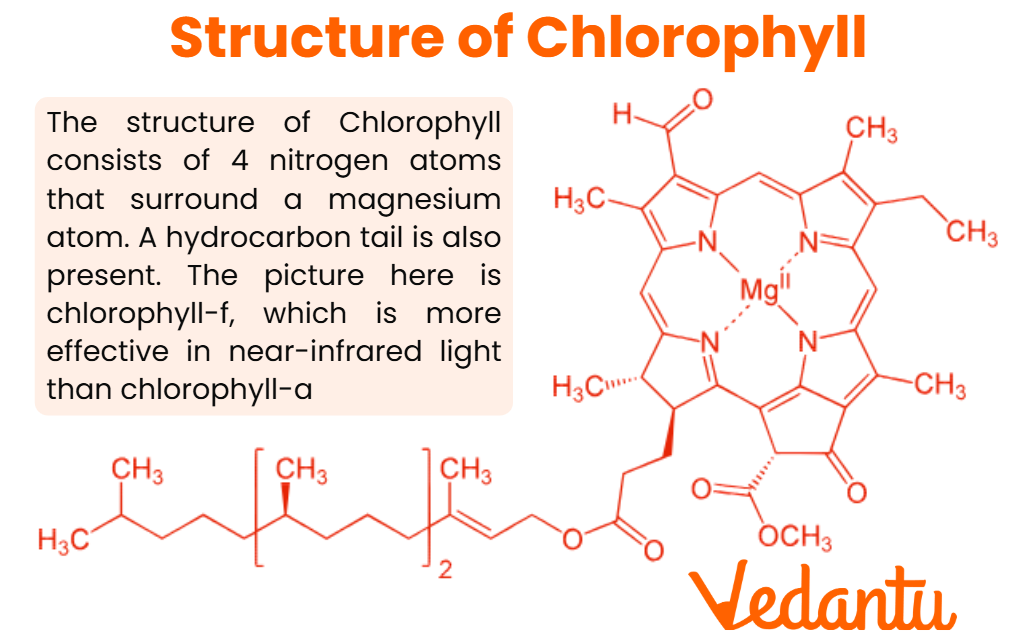
Chlorophyll Structure: Key Components
The chlorophyll structure consists of the following main components:
- Porphyrin Ring (Chlorin Ring): A large, flat, cyclic molecule made up of four pyrrole rings (each contains nitrogen and carbon).
- Magnesium Ion (Mg2+): Located at the center of the porphyrin ring and coordinated by nitrogen atoms from each ring. This central atom is critical for absorbing light energy.
- Phytol Tail: A long hydrocarbon chain that anchors the chlorophyll molecule to the thylakoid membrane in the chloroplast.
- Side Chains: Different functional groups (such as methyl, formyl, or carbonyl) attached to the ring, varying between different types of chlorophyll.
Chlorophyll Structure Formula
The general chemical formula for chlorophyll a is C55H72O5N4Mg. For chlorophyll b, the formula is C55H70O6N4Mg. Here’s a breakdown of each molecular part:
- C (Carbon): Forms the skeleton of the rings and tail.
- H (Hydrogen): Attached to carbons and in the tail groups.
- O (Oxygen): Found in side groups (especially in carbonyl or formyl side chains).
- N (Nitrogen): Central in the pyrrole rings.
- Mg (Magnesium): The metal ion at center of the ring.
Types of Chlorophyll: Chlorophyll a vs b
There are two main types of chlorophyll in higher plants. Here’s a simple table for comparison:
| Aspect | Chlorophyll a | Chlorophyll b |
|---|---|---|
| Formula | C55H72O5N4Mg | C55H70O6N4Mg |
| Main Side Group | Methyl (-CH3) | Formyl (-CHO) |
| Color | Blue-green | Yellow-green |
| Role | Primary pigment (main light absorber) | Accessory pigment |
Chlorophyll Structure vs Hemoglobin
An interesting comparison in biology is between chlorophyll and hemoglobin structures. Both have similar porphyrin ring backbones, but the central ion differs. See the table below:
| Aspect | Chlorophyll | Hemoglobin |
|---|---|---|
| Central Ion | Magnesium (Mg) | Iron (Fe) |
| Ring Type | Porphyrin (chlorin ring) | Porphyrin |
| Function | Light absorption for photosynthesis | Oxygen transport in blood |
| Color | Green | Red |
Chlorophyll Structure in Chemistry
In chemistry, chlorophyll structure is studied as a coordination compound. The magnesium ion at the center is coordinated by four nitrogen atoms, making it stable and perfect for its light-absorbing role. This structure is a textbook example of metal coordination in organic molecules.
Chlorophyll Structure and Function
Chlorophyll’s structure directly relates to its biological function. The porphyrin ring absorbs red and blue wavelengths of sunlight, while the phytol tail keeps it anchored in the thylakoid membranes. This structural design enables plants to efficiently capture solar energy and begin the process of photosynthesis.
Common Mistakes to Avoid
- Confusing chlorophyll structure with other plant pigments or hemoglobin.
- Forgetting the importance of the magnesium ion at the chlorophyll’s core.
- Not learning the different side groups that differentiate chlorophyll a and b.
Real-World Applications
The concept of chlorophyll structure is used in fields like agriculture, biotechnology, and plant science. For example, understanding the differences between chlorophyll a and b is important for crop improvement and environmental monitoring. Vedantu helps students relate such topics to practical examples in daily life and exams.
Practice Questions
- Draw a simple labeled diagram of chlorophyll structure.
- What is the role of the magnesium ion in chlorophyll?
- State two differences between chlorophyll a and chlorophyll b.
- Compare the structure of chlorophyll and hemoglobin.
- Write the chemical formula of chlorophyll a.
In this article, we explored chlorophyll structure, its key processes, real-life significance, and how to solve questions based on it. To learn more and build confidence, keep practicing with Vedantu. For detailed notes and revision, refer to related topics below.
Related Topics and Internal Links
- Photosynthesis Process – Understand how chlorophyll structure enables photosynthesis.
- Plant Cell – Learn where chlorophyll is found inside plants.
- Nutrition in Plants – See how chlorophyll supports plant food production.
- Difference Between Mitochondria and Plastids – Chlorophyll is present in plastids called chloroplasts.
- Cell Structure and Function – Broader context about where chlorophyll is contained in cells.
- Biotic and Abiotic – The role of chlorophyll in the living components of the ecosystem.
- Structure of Nucleus – Compare with other important plant cell organelles.
- Difference Between Cell Membrane and Plasma Membrane – Other fundamental cell biology concepts.
- Tissues – How chlorophyll contributes to plant tissue functions.
- Differences Between Photosynthesis and Cellular Respiration – Connect chlorophyll’s role to plant metabolism.
- What is Photosynthesis? – A concise definition and overview of the entire process.


FAQs on Chlorophyll Structure Made Simple for Students
1. What is chlorophyll and its structure?
Chlorophyll is a green pigment found in plants, algae, and cyanobacteria that plays a vital role in photosynthesis. Its structure consists of a porphyrin ring—a flat, nitrogen-containing ring with a central magnesium (Mg) ion—and a long hydrophobic phytol tail which anchors it into the thylakoid membranes of chloroplasts. This unique structure allows chlorophyll to absorb light energy efficiently, primarily in the blue and red wavelengths, helping plants convert sunlight into chemical energy.
2. What is the formula for chlorophyll?
Chlorophyll’s chemical formula is generally represented as C55H72O5N4Mg. This formula highlights the key elements including carbon, hydrogen, oxygen, nitrogen, and a central magnesium ion (Mg). Different types of chlorophyll (a, b, etc.) may have slight variations in side groups but maintain this core molecular framework essential for its light-absorbing function.
3. What are the differences in structure between chlorophyll and hemoglobin?
Chlorophyll and hemoglobin share a similar porphyrin ring structure, but differ mainly in the central metal ion: chlorophyll contains magnesium (Mg) at its center, whereas hemoglobin contains iron (Fe). This difference reflects their biological roles—chlorophyll captures light energy for photosynthesis, while hemoglobin transports oxygen in blood. Additionally, chlorophyll has a hydrophobic phytol tail which anchors it in membranes, a feature absent in hemoglobin.
4. What is the structure of chlorophyll a versus chlorophyll b?
Chlorophyll a and chlorophyll b differ slightly in their side groups attached to the porphyrin ring. Chlorophyll a has a methyl group (-CH3) while chlorophyll b has a formyl group (-CHO) at the same position. This structural variation causes chlorophyll b to absorb light at slightly different wavelengths, expanding the range of light energy captured during photosynthesis. Both contain the central magnesium ion and phytol tail.
5. Why is the magnesium ion important in chlorophyll?
The magnesium ion (Mg) at the center of the porphyrin ring is essential for chlorophyll’s function. It stabilizes the ring structure and plays a key role in absorbing light energy during photosynthesis. The Mg ion enables electron excitation upon light absorption, which triggers the conversion of solar energy into chemical energy that plants use to synthesize food.
6. Why do students confuse chlorophyll’s ring structure with that of hemoglobin?
Students often confuse the structures because both chlorophyll and hemoglobin contain a similar porphyrin ring system composed of four nitrogen-containing pyrrole rings. However, the central metal ion differs (magnesium in chlorophyll and iron in hemoglobin), as does their biological function. Understanding this key difference helps clarify their distinct roles in plants and animals respectively.
7. Why is a phytol tail present in chlorophyll?
The phytol tail is a long hydrophobic hydrocarbon chain attached to chlorophyll that anchors the molecule within the lipid membranes of the chloroplast’s thylakoid. This anchoring is crucial because it positions chlorophyll optimally to capture light energy during the photosynthetic process, maintaining the structure and organization of photosystems.
8. How does the structural difference between chlorophyll a and b affect their function?
The slight structural difference between chlorophyll a (methyl group) and chlorophyll b (formyl group) shifts their absorption spectra. Chlorophyll b absorbs light at wavelengths that chlorophyll a absorbs less efficiently. This complementary absorption broadens the range of light that plants can use for photosynthesis, improving overall energy capture and efficiency.
9. Is chlorophyll found in all photosynthetic organisms?
Chlorophyll is found in most photosynthetic organisms such as higher plants, algae, and cyanobacteria. However, some photosynthetic bacteria use related pigments like bacteriochlorophylls with similar structures but adapted properties. Thus, while chlorophyll is widespread, not all photosynthetic organisms use the exact same chlorophyll molecules.
10. Can the structure of chlorophyll be easily memorized using mnemonics?
Yes, students can use mnemonics to memorize chlorophyll’s structure by focusing on its main parts: Porphyrin ring (four nitrogen-containing pyrrole rings), Magnesium ion at the center, and the Phytol tail. For example, remember "P-M-P" for Porphyrin-Magnesium-Phytol. Visual diagrams combined with these keywords improve recall for exams.










