Class 12 Chemistry Chapter 7 Summary Notes PDF Download
FAQs on CBSE Notes Class 12 Chemistry Chapter 7 - Alcohol Phenol and Ether - 2025-26
1. What are the key topics to summarise from the Alcohols, Phenols, and Ethers Class 12 notes?
For a quick revision of this chapter, you should summarise the following key topics as per the CBSE 2025-26 syllabus:
- Nomenclature and Classification: Rules for naming primary, secondary, and tertiary alcohols, phenols, and ethers.
- Preparation Methods: Key reactions for synthesising each class of compound, like hydration of alkenes, Williamson synthesis, and the Cumene process.
- Physical Properties: Trends in boiling points and solubility, focusing on the role of hydrogen bonding.
- Chemical Reactions: Important reactions like oxidation, dehydration, and electrophilic substitution in phenols.
- Acidity Comparison: The relative acidity of alcohols and phenols.
- Distinguishing Tests: Tests like the Lucas test and Ferric Chloride test to differentiate between the compounds.
2. What is the most effective order to revise the concepts in Alcohols, Phenols, and Ethers?
A logical revision flow helps in connecting concepts. Start with the basics of structure and nomenclature for all three compounds. Then, move to the methods of preparation for each. Follow this with a comparative study of their physical properties (boiling point, solubility). Next, revise the chemical reactions of alcohols, then phenols, paying special attention to the acidity difference. Finally, cover the reactions of ethers and the key distinguishing chemical tests.
3. Why are phenols significantly more acidic than alcohols?
Phenols are more acidic than alcohols because the conjugate base formed after losing a proton, the phenoxide ion, is highly stabilised by resonance. The negative charge is delocalised over the benzene ring. In contrast, the alkoxide ion formed from an alcohol has its negative charge localised on the oxygen atom, making it less stable and thus, the alcohol is less acidic.
4. How are the physical properties of alcohols, phenols, and ethers interconnected for revision?
The key connection is intermolecular hydrogen bonding. Alcohols and phenols can form strong hydrogen bonds due to their -OH group, resulting in high boiling points and good solubility in water. Ethers, lacking an -OH group, cannot form hydrogen bonds with themselves, leading to much lower boiling points, comparable to alkanes of similar mass. This distinction is crucial for revision.
5. What is the core principle of Williamson's synthesis for preparing ethers?
Williamson's synthesis is a key laboratory method for preparing both symmetrical and unsymmetrical ethers. The core principle is an SN2 reaction where a sodium or potassium alkoxide (R-O⁻Na⁺) acts as a nucleophile and attacks a primary alkyl halide (R'-X). This displaces the halide ion and forms an ether (R-O-R').
6. How can you quickly differentiate between primary, secondary, and tertiary alcohols during revision?
The most effective method for quick differentiation is the Lucas test. This test uses a reagent of concentrated HCl and anhydrous ZnCl₂.
- Tertiary alcohols react immediately to form a cloudy solution (turbidity).
- Secondary alcohols react within 5-10 minutes.
- Primary alcohols show no reaction at room temperature.
7. Why does the dehydration of ethanol yield different products at 413 K and 443 K?
Temperature controls the reaction pathway in the acid-catalysed dehydration of ethanol. At a lower temperature (413 K), the reaction follows an SN2 mechanism where one ethanol molecule attacks another protonated one, leading to the formation of ethoxyethane (ether). At a higher temperature (443 K), an elimination (E2) reaction is favoured, resulting in the formation of ethene.
8. How do electrophilic substitution reactions in phenol differ from those in benzene?
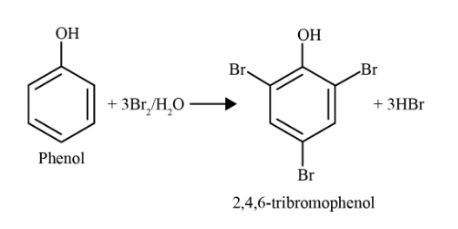
The -OH group in phenol is a strongly activating group that increases electron density on the benzene ring, particularly at the ortho and para positions. This makes phenol much more reactive towards electrophilic substitution than benzene. Reactions like bromination can occur even without a Lewis acid catalyst and often lead to polysubstitution, such as the formation of 2,4,6-tribromophenol.
9. What common misconceptions about this chapter should be clarified during revision?
During revision, it's crucial to clarify these common misconceptions:
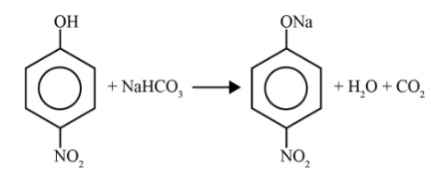
- Not all -OH compounds give a positive Ferric Chloride test: This is a characteristic test for phenols only, which give a violet colour; alcohols do not react.
- Ethers are not completely inert: While less reactive than alcohols, ethers can be cleaved by strong acids like HI or HBr.
- All alcohols do not oxidise to carboxylic acids: Only primary alcohols can be oxidised to carboxylic acids. Secondary alcohols oxidise to ketones, and tertiary alcohols resist oxidation under normal conditions.
10. Why is Williamson's synthesis not suitable for preparing ethers with tertiary alkyl groups?
Williamson's synthesis fails with tertiary alkyl halides because the alkoxide (e.g., CH₃O⁻) is a strong nucleophile and also a strong base. With a sterically hindered tertiary alkyl halide, the alkoxide favours an elimination (E2) reaction over substitution (SN2). Instead of an ether, the major product is an alkene.
11. What are the key points to summarise for the preparation of phenols?
For efficient revision, focus on these four main industrial and lab methods for preparing phenol:
- From Haloarenes (Dow's Process): Involves heating chlorobenzene with aqueous NaOH at high temperature and pressure.
- From Benzene Sulphonic Acid: Benzene is sulphonated and then fused with molten NaOH.
- From Diazonium Salts: Aniline is treated with NaNO₂/HCl to form a diazonium salt, which is then warmed with water.
- From Cumene: This is the most common commercial method, involving the oxidation of cumene (isopropylbenzene) followed by acid hydrolysis.
12. What is the summary of oxidation products for different types of alcohols?
A quick summary of alcohol oxidation for revision is:
- Primary (1°) Alcohols: Oxidise first to an aldehyde. With strong oxidising agents like KMnO₄, they further oxidise to a carboxylic acid.
- Secondary (2°) Alcohols: Oxidise to form a ketone. The reaction stops at this stage.
- Tertiary (3°) Alcohols: They are resistant to oxidation under normal conditions due to the absence of a hydrogen atom on the carbinol carbon. Strong conditions cause cleavage of C-C bonds.