Class 12 Chemistry Chapter 16 Summary Notes PDF Download
FAQs on CBSE Notes Class 12 Chemistry Chapter 16 - Chemistry in Everyday Life - 2025-26
1. What are the key topics covered in the Class 12 Chemistry chapter on 'Chemistry in Everyday Life'?
This chapter provides a summary of how chemical compounds are used in our daily lives. The main topics for revision include:
- Drugs and their Classification: Understanding different types like analgesics, tranquillizers, antiseptics, antibiotics, etc.
- Chemicals in Food: Exploring food additives such as preservatives, artificial sweeteners, and antioxidants.
- Cleansing Agents: The chemistry of soaps and detergents, their cleansing mechanism, and differences.
- Dyes: Basic concepts of colour, chromophores, and auxochromes.
- Rocket Propellants: A brief introduction to different types of propellants and their properties.
2. How are drugs classified for systematic study?
Drugs can be classified based on four main criteria to facilitate their study and application:
- Pharmacological Effect: Based on the effect they have on the body. For example, analgesics are painkillers.
- Drug Action: Based on the specific biochemical process they influence. For example, antihistamines inhibit the action of histamine.
- Chemical Structure: Grouping drugs with similar structural features. For example, sulphonamides all share a common chemical skeleton.
- Molecular Targets: Based on the biomolecules they interact with, such as proteins, nucleic acids, carbohydrates, or lipids.
3. What is the fundamental difference between an antiseptic and a disinfectant?
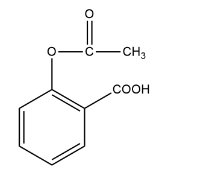
The primary difference lies in their application and safety for living tissues. Antiseptics are applied to living tissues, like wounds and skin, to prevent or stop the growth of microbes (e.g., Dettol). In contrast, disinfectants are applied to inanimate objects, such as floors and instruments, to kill microorganisms, but they are generally toxic to living tissues. For instance, a 1% solution of phenol acts as a disinfectant, while a 0.2% solution is an antiseptic.
4. Why do soaps fail to work in hard water, while detergents are effective?
Soaps are sodium or potassium salts of long-chain fatty acids. In hard water, which contains calcium (Ca²⁺) and magnesium (Mg²⁺) ions, soap molecules react to form insoluble precipitates called scum. This scum prevents proper lathering and cleansing. Detergents, being salts of long-chain alkyl sulphonates or sulphates, also react with these ions but form soluble salts, allowing them to lather and cleanse effectively even in hard water.
5. What are 'drug targets' in medicinal chemistry?
Drug targets are key biomolecules in the body with which a drug interacts to produce a therapeutic effect. These are typically macromolecules such as proteins (like enzymes and receptors), nucleic acids (DNA and RNA), carbohydrates, and lipids. The drug binds to the active site of these targets, altering their function and thereby correcting a metabolic imbalance or disease.
6. Why is the use of the artificial sweetener aspartame restricted to cold foods and drinks?
Aspartame is a popular artificial sweetener, but its use is limited to cold products because it is unstable at cooking temperatures. When heated, it breaks down and decomposes, causing it to lose its sweetening property. This makes it unsuitable for use in baked goods or hot beverages that require heating during preparation.
7. How do tranquillizers and analgesics differ in their neurological action?
Both are neurologically active drugs but serve different purposes. Tranquillizers act on the central nervous system to treat stress, anxiety, and mental diseases by affecting the message transfer mechanism from nerve to receptor. In contrast, analgesics are used to reduce or abolish pain without causing impairment of consciousness or paralysis. They are broadly divided into non-narcotics (like aspirin) and narcotics (like morphine).
8. What is the chemical principle behind the cleansing action of soap?
The cleansing action of soap relies on the structure of its molecules and the formation of micelles. Each soap molecule has a long hydrophobic (water-repelling) hydrocarbon tail and a short hydrophilic (water-attracting) ionic head. In water, these molecules form spherical clusters called micelles, trapping oily dirt in their hydrophobic core. This allows the dirt to be suspended in water and washed away.
9. Why are both antioxidants and preservatives added to food? Don't they serve the same purpose?
While both extend the shelf-life of food, they have distinct functions. Preservatives, like sodium benzoate, prevent food spoilage by inhibiting the growth of microorganisms such as bacteria and moulds. Antioxidants, such as BHT (Butylated hydroxytoluene), prevent the chemical oxidation of fats and oils in food. This oxidation can make food go rancid, so preservatives target microbial growth while antioxidants target chemical degradation.
10. Explain the difference between narcotic and non-narcotic analgesics with an example of each.
The key difference between these painkillers lies in their addictive properties and mechanism of action.
- Non-narcotic analgesics, like aspirin and paracetamol, are not habit-forming and work by inhibiting the synthesis of pain-causing chemicals called prostaglandins.
- Narcotic analgesics, like morphine, are highly potent but addictive. They bind to opioid receptors in the brain to relieve severe pain and are used only under strict medical supervision.
11. What distinguishes a dye from a regular coloured substance, and what is the role of an auxochrome?
A substance is a dye not just because it is coloured, but because it can bind to a substrate (like fabric) and impart a stable colour. The colour arises from molecular parts called chromophores. An auxochrome is a functional group (like –OH or –NH₂) that, while not coloured itself, deepens the colour of the chromophore and helps the dye bind firmly to the fabric.
12. How do liquid propellants offer a key advantage over solid propellants in rocketry?
The main advantage of liquid propellants (like liquid hydrogen) over solid propellants is their controllability. The thrust of a rocket using liquid propellants can be precisely controlled by regulating the fuel flow, allowing the engine to be throttled, shut down, and even restarted. Solid propellants, once ignited, burn uncontrollably at a predetermined rate until exhausted.
13. How do antihistamine drugs work to relieve allergy symptoms?
Histamine is a chemical released by the body during an allergic reaction, causing symptoms like sneezing and inflammation. Antihistamines work as competitive inhibitors. They have a structure similar enough to histamine to block its receptors. By occupying these receptors, they prevent histamine from binding and triggering the allergic response, thus providing relief from the symptoms.