Chemistry Notes for Chapter 9 Amines Class 12 - FREE PDF Download
FAQs on CBSE Notes Class 12 Chemistry Chapter 9 - Amines - 2025-26
1. What are the core topics I should focus on when using these revision notes for the Amines chapter?
For a quick and effective revision of Amines, you should focus on the following key areas as per the CBSE 2025-26 syllabus:
- Classification and Nomenclature: Quickly recap how to classify amines as primary, secondary, and tertiary.
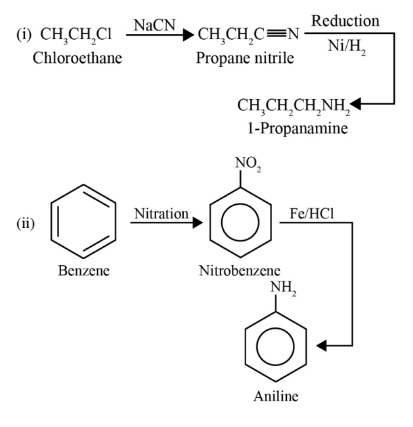
- Preparation Methods: Summarise key methods like reduction of nitro compounds, ammonolysis, reduction of nitriles, and Gabriel phthalimide synthesis.
- Physical Properties: Note the trends in boiling point and solubility, and the role of hydrogen bonding.
- Basicity of Amines: This is a crucial concept. Revise the comparison of basic strength between aliphatic amines, aromatic amines, and ammonia.
- Key Chemical Reactions: Focus on distinctive tests like the Carbylamine test and Hinsberg test.
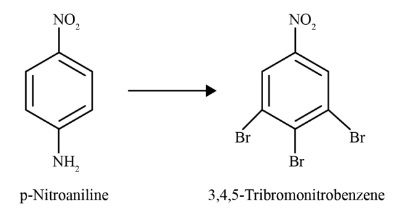
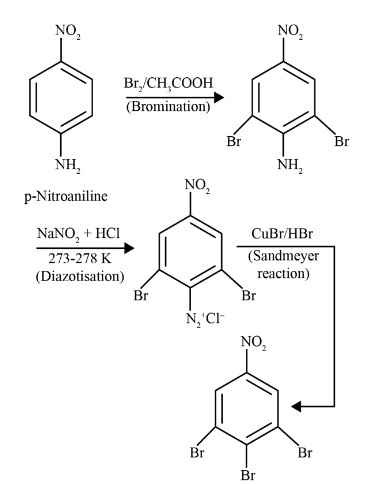
- Diazonium Salts: Understand their preparation (diazotization) and importance in synthetic organic chemistry, especially coupling reactions.
2. How can I quickly recall the difference between primary, secondary, and tertiary amines?
A simple way to remember the classification is to link it to the ammonia molecule (NH₃). The classification depends on how many hydrogen atoms are replaced by alkyl or aryl groups:
- Primary (1°) Amine: One hydrogen atom is replaced (e.g., R-NH₂). It has a -NH₂ group.
- Secondary (2°) Amine: Two hydrogen atoms are replaced (e.g., R₂-NH). It has a -NH group.
- Tertiary (3°) Amine: All three hydrogen atoms are replaced (e.g., R₃-N). It has only a Nitrogen atom bonded to three organic groups.
3. What is the key concept to remember about the boiling points of amines?
The key concept is hydrogen bonding. Primary (1°) and secondary (2°) amines can form intermolecular hydrogen bonds because they have N-H bonds. Tertiary (3°) amines cannot form hydrogen bonds with each other as they lack an N-H bond. Therefore, for isomeric amines, the boiling point order is: Primary > Secondary > Tertiary. However, all amines have lower boiling points than alcohols of similar mass because the N-H bond is less polar than the O-H bond.
4. Why are aliphatic amines generally more basic than ammonia?
Aliphatic amines are stronger bases than ammonia due to the positive inductive effect (+I effect) of the alkyl groups (like -CH₃, -C₂H₅). These alkyl groups are electron-donating, which increases the electron density on the nitrogen atom. This makes the lone pair of electrons on the nitrogen more readily available for donation to a proton, thus increasing the amine's basicity.
5. In contrast, why are aromatic amines like aniline much weaker bases than ammonia?
Aromatic amines, such as aniline, are significantly weaker bases than ammonia because the lone pair of electrons on the nitrogen atom is involved in resonance with the benzene ring. This delocalisation of electrons into the ring (a -R effect) makes the lone pair less available for donation to a proton. The positive charge developed on the nitrogen in the resonance structures further decreases its basic character.
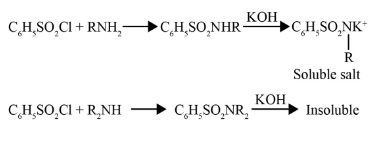
6. What is the main purpose of the Hinsberg test in the context of revising amines?
The main purpose of the Hinsberg test is to distinguish between primary, secondary, and tertiary amines. The reagent used is benzenesulphonyl chloride (C₆H₅SO₂Cl). The key outcomes to remember are:
- Primary amines form a precipitate which is soluble in alkali (like KOH).
- Secondary amines form a precipitate which is insoluble in alkali.
- Tertiary amines do not react with the Hinsberg reagent at all.
7. For quick revision, what is the key takeaway from the Carbylamine test (Isocyanide test)?
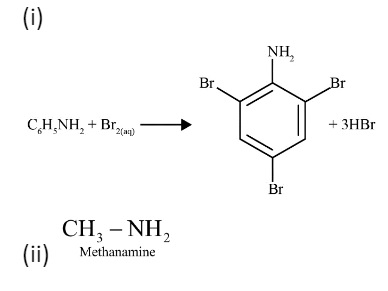
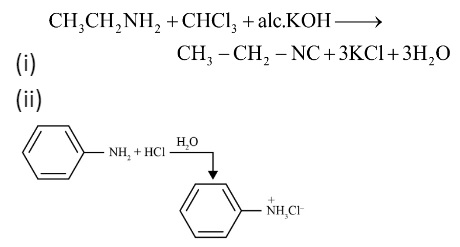
The key takeaway is that the Carbylamine test is a specific test for primary amines only (both aliphatic and aromatic). When a primary amine is heated with chloroform (CHCl₃) and alcoholic potassium hydroxide (KOH), it forms an isocyanide (or carbylamine), which has a very foul and easily identifiable smell. Secondary and tertiary amines do not give this test, making it a reliable method for identifying a primary amino group.
8. What is the critical limitation of Gabriel Phthalimide Synthesis that I should remember?
The critical limitation to remember is that Gabriel Phthalimide Synthesis can only be used to prepare primary aliphatic amines. It cannot be used to prepare aromatic primary amines (like aniline). This is because the reaction involves nucleophilic substitution on an alkyl halide, but aryl halides (like chlorobenzene) do not undergo nucleophilic substitution easily due to the partial double bond character between the carbon and halogen atom caused by resonance.
9. How does the Hoffmann Bromamide Degradation reaction help in preparing amines?
The Hoffmann Bromamide Degradation is a key method for preparing primary amines. The most important feature to remember for revision is that it produces a primary amine with one carbon atom less than the starting amide. The reaction involves treating an amide with bromine (Br₂) in an aqueous or ethanolic solution of sodium hydroxide (NaOH). This 'step-down' reaction is very useful for decreasing the length of a carbon chain.
10. What is the crucial difference in the reaction of primary aliphatic and aromatic amines with nitrous acid (HNO₂)?
This is a fundamental difference to note for your revision.
- Primary Aliphatic Amines react with nitrous acid at low temperatures (273-278 K) to form a highly unstable aliphatic diazonium salt, which quickly decomposes to produce alcohol and evolve nitrogen gas.
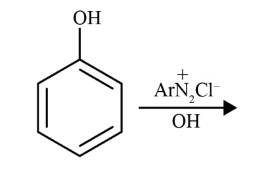
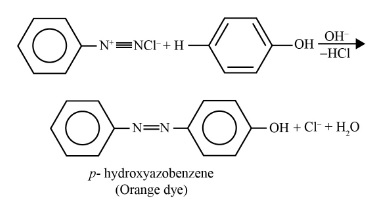
- Primary Aromatic Amines (like aniline) react with nitrous acid at the same low temperature to form a relatively stable benzenediazonium salt. This stability allows the diazonium salt to be used in further synthetic reactions, such as Sandmeyer and coupling reactions to form azo dyes.
11. How can I structure my revision for the various preparation methods of amines to avoid confusion?
A good strategy is to group the preparation methods by their starting material or reaction type. For your notes, consider this structure:
- Reduction Reactions: Group together the reduction of nitro compounds, nitriles, and amides. Note the common reducing agents used (e.g., H₂/Ni, Sn/HCl, LiAlH₄).
- Reactions of Halides: Focus on Ammonolysis and the Gabriel Phthalimide synthesis, both of which start with an alkyl halide.
- Degradation Reaction: Isolate the Hoffmann Bromamide Degradation as a unique method that shortens the carbon chain (a step-down reaction).