How IgG Antibodies Work in Immunity and Lab Tests
The concept of IgG antibody is essential in biology and helps explain real-world biological processes and exam-level questions effectively.
Understanding IgG Antibody
IgG antibody, also known as Immunoglobulin G, is the most abundant type of antibody found in human blood and extracellular fluids. IgG plays a vital role in the body's immune response by neutralizing bacteria, viruses, and toxins, and is involved in immunity, infection diagnosis, and medical testing. This concept is important in areas like immunity, humoral immunity, and medical diagnostics.
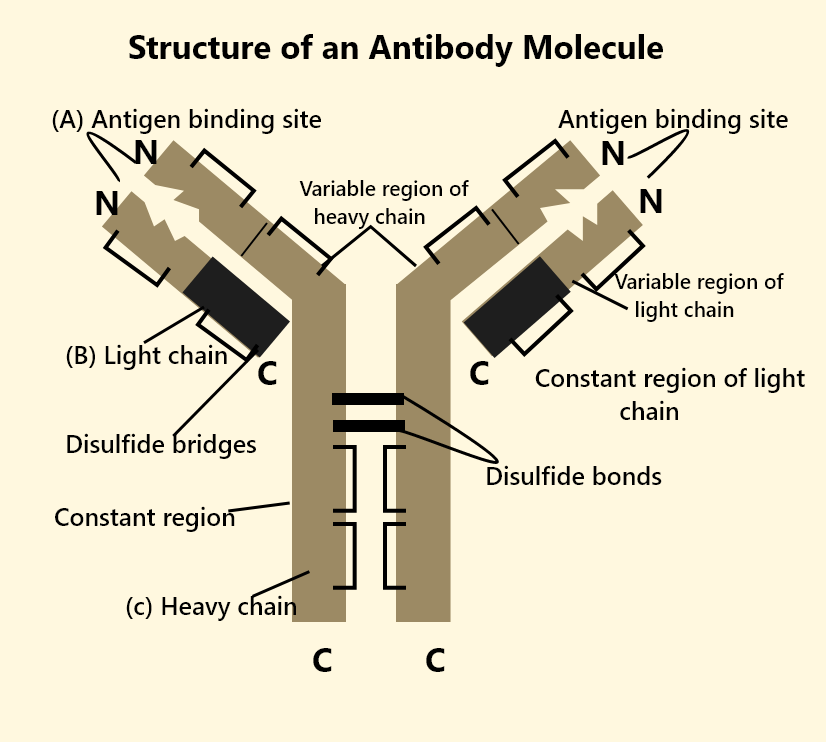
Structure of IgG Antibody
IgG antibody has a distinct Y-shaped structure. Each molecule consists of four peptide chains: two identical heavy (gamma) chains and two identical light chains, held together by disulfide bonds. The arms (Fab fragments) are responsible for binding antigens, while the stem (Fc region) determines the biological function of the antibody. The variable regions of the Fab arms contribute to the antibody's ability to recognize a wide array of pathogens. This structure makes IgG flexible and efficient in targeting invaders.
Functions of IgG Antibody
The IgG antibody offers protection through several key roles in the human immune system:
- Neutralization: Binds to toxins and pathogens, preventing them from harming cells.
- Opsonization: Tags microbes for easier recognition and destruction by phagocytes.
- Complement Activation: Triggers a cascade of immune proteins to remove pathogens.
- Antibody-dependent cell-mediated cytotoxicity (ADCC): Helps recruit natural killer cells to attack infected or abnormal cells.
- Crosses Placenta: Only antibody class providing passive immunity to newborns by transferring from mother to fetus.
Subclasses of IgG Antibodies
There are four subclasses of IgG in humans: IgG1, IgG2, IgG3, and IgG4. Each has slightly different roles and properties. For example, IgG1 and IgG3 are especially effective at activating the complement system and responding to protein antigens, while IgG2 is more involved with responses to polysaccharide antigens.
IgG Antibody in Testing and Diagnosis
The IgG antibody test measures the level of IgG in blood and is widely used to diagnose previous infection or exposure to pathogens such as measles, rubella, and herpes simplex virus. A result of "IgG antibody positive" means the person has encountered the pathogen before, either through infection or vaccination, and has developed a memory immune response.
Interpretation of IgG levels helps doctors assess immunity, determine vaccination needs, and detect certain autoimmune conditions or immunodeficiencies. Remember, high or low IgG levels should always be interpreted by a healthcare professional as results can vary due to different medical reasons.
Here’s a helpful table to understand IgG antibody subclasses better:
IgG Antibody Subclass Table
| Subclass | Main Role | Serum % | Placental Transfer |
|---|---|---|---|
| IgG1 | Response to proteins, strong complement activation | ~60% | Yes |
| IgG2 | Response to polysaccharides, weak complement activation | ~32% | Limited |
| IgG3 | Strong complement activation, antiviral/antibacterial | ~4% | Yes |
| IgG4 | Chronic antigen exposure, minimal complement activation | ~4% | Yes |
Difference Between IgG and IgM
IgG and IgM antibodies are often compared in medical diagnostics. Understanding their differences helps in interpreting results from infection tests:
| Feature | IgG | IgM |
|---|---|---|
| Appearance in Infection | Later, indicates past infection or immunity | First, indicates current or recent infection |
| Structure | Monomer (Y-shaped) | Pentamer (5 Y-shaped units) |
| Placental Transfer | Yes | No |
| Test Example | IgG positive = past exposure/immunity | IgM positive = current/recent infection |
Common Mistakes to Avoid
- Confusing IgG antibody with IgM or IgA antibodies in test results.
- Assuming a positive IgG means you are currently infected (it usually means past infection or immunity).
- Not considering all subclasses and their unique properties.
Real-World Applications
The concept of IgG antibody is used in medicine for diagnosing diseases, checking vaccine effectiveness, and monitoring immune status in conditions like autoimmune disorders. Vedantu helps students relate these concepts to practical scenarios, exam cases, and health awareness. IgG testing is also vital in blood banks, pregnancy health, and epidemiological studies.
In this article, we explored IgG antibody, its structure, subclasses, key functions, clinical uses, and how to avoid common mistakes. To learn more and build confidence, keep practicing with Vedantu.
Explore More Related Topics
- Immunity
- Humoral Immunity
- Immunoglobulin Structure
- Antibodies
- Antigen-Antibody Reaction Types
- Immunology
- Virus
- Measles
- Microbiology
- NEET Biology MCQs


FAQs on IgG Antibody: Structure, Key Functions & Test Significance
1. What is IgG antibody?
IgG antibody is the most abundant type of immunoglobulin found in human blood and extracellular fluid. It plays a crucial role in the immune system by recognizing and neutralizing pathogens such as viruses and bacteria, helping protect the body from infection. IgG antibodies also cross the placenta to provide passive immunity to the fetus and newborn.
2. What is the difference between IgG and IgM?
IgG and IgM are two different classes of antibodies with distinct functions and structures. IgM is the first antibody produced in response to an infection and exists as a pentamer, making it large and mostly limited to the bloodstream. IgG is produced later during the immune response, is smaller (monomeric), circulates widely, and can cross the placenta to provide neonatal immunity. IgG also has a longer half-life and higher affinity for antigens compared to IgM.
3. What are the main functions of IgG?
The key functions of IgG antibodies include:
- Neutralization: Binding and neutralizing viruses and toxins.
- Opsonization: Coating pathogens to enhance uptake by phagocytes.
- Complement activation: Triggering the classical complement pathway to eliminate pathogens.
- Antibody-dependent cell-mediated cytotoxicity (ADCC): Helping immune cells destroy infected or abnormal cells.
- Placental transfer: Crossing the placenta to provide passive immunity to the fetus.
4. What does it mean if IgG is high or positive?
A high or positive IgG antibody test usually indicates past exposure or immunity to a specific pathogen due to infection or vaccination. It means the immune system has produced IgG antibodies against that antigen. However, elevated IgG levels can also be seen in chronic infections, autoimmune diseases, or certain immune disorders. Always interpret in the clinical context.
5. What is the structure of IgG antibody?
IgG antibody is a Y-shaped molecule composed of four polypeptide chains: two identical heavy chains and two identical light chains held together by disulfide bonds. Each arm of the Y contains an antigen-binding fragment (Fab) with variable regions that recognize specific antigens, while the stem (Fc region) interacts with immune cells and activates complement proteins.
6. What is an IgG antibody test used for?
An IgG antibody test is used to detect the presence of specific IgG antibodies in blood, indicating immunity or prior exposure to infections such as measles, herpes simplex virus (HSV-1), rubella, or hepatitis. It helps diagnose infections, assess immune status post-vaccination, or monitor responses in autoimmune and allergic conditions.
7. Why is IgG antibody often confused with IgM in test reports?
IgG and IgM antibodies are both immunoglobulins but represent different phases of the immune response. Confusion arises because some tests detect both antibodies or report antibody classes without clarity. IgM indicates early or acute infection, while IgG indicates past exposure or immunity. Proper interpretation depends on timing, test type, and clinical symptoms.
8. How does IgG structure help it cross the placenta?
The Fc region of IgG antibodies specifically binds to the neonatal Fc receptor (FcRn) expressed in placental cells. This receptor-mediated transport allows IgG to cross the placenta, providing passive immunity to the fetus by transferring maternal antibodies before birth.
9. How to interpret “IgG to HSV-1 detected” in a report?
Detection of IgG antibodies to HSV-1 indicates past or latent infection with herpes simplex virus type 1. It means that the immune system has responded to the virus at some point, and the person has developed immunity. This does not necessarily confirm active infection but shows prior exposure.
10. In what other body fluids is IgG antibody found besides blood?
Besides blood serum, IgG antibodies are also present in extracellular fluids such as lymph, cerebrospinal fluid, synovial fluid, and breast milk. They provide immune protection in tissues and contribute to neonatal immunity through transfer in breast milk.
11. Why is knowing IgG vs IgA/IgM important for competitive exams?
Understanding the differences between IgG, IgA, and IgM is crucial for biology exams as these antibodies have different structures, functions, and roles in immunity. Knowing their unique features and responses helps answer questions on immunology, diagnostics, and pathogen defense effectively.
12. What are the subclasses of IgG?
IgG antibodies are divided into four subclasses: IgG1, IgG2, IgG3, and IgG4. Each subclass varies in abundance, ability to activate complement, bind Fc receptors, and cross the placenta. For example, IgG1 and IgG3 are strong complement activators, while IgG4 generally does not activate complement. These differences affect immune response types and clinical significance.










