What Are the Key Steps and Significance of the Urea Cycle?
The principal metabolic mechanism involved in the elimination of nitrogenous wastes generated by the breakdown of protein and other nitrogen-containing molecules is called the urea cycle. In the mitochondria of liver cells, the urea cycle converts excess ammonia to urea. Urea is formed, enters the bloodstream, is filtered by the kidneys, and is eventually excreted in the urine.
The biochemical part of excretion is urea synthesis using the urea cycle, also known as the Ornithine Cycle. It is also known as the Kreb-Henseleit cycle and occurs in the liver. In this article, we will see the regulation and significance of this cycle.
What is Urea Cycle?
The urea cycle is a series of biochemical reactions that results in the formation of urea CO(NH2)2 from ammonia (NH3).
Ureotelic animals are amphibians and mammals that use this cycle.
The urea cycle converts highly toxic ammonia to urea, which is then excreted. This cycle was discovered five years before the TCA cycle by Hans Krebs and Kurt Henseleit (Hans Krebs and Kurt Henseleit, 1932). Ratner and Cohen went into greater detail about this cycle later on.
The urea cycle is primarily carried out in the liver and, to a lesser extent, in the kidneys.
The urea cycle is irreversible and requires 4 ATP to complete.
Only the liver has all of the enzymes needed to generate urea from ammonia, and this route is only located in periportal hepatocytes.
The urea cycle is made up of four different enzyme processes, one mitochondrial and three cytosolic. Carbamoyl phosphate synthetase (CPS), ornithine carbamoyltransferase (OCT), argininosuccinate synthetase, argininosuccinate lyase, and arginase are all implicated.
The urea cycle enzymes ornithine carbamoyltransferase and arginase are also found in the mitochondria, whereas the cytoplasm contains argininosuccinate synthetase and argininosuccinate lyase.
Urea Cycle Biochemistry and Steps Involved
Carbamoyl Phosphate Synthesis
The mitochondrial carbamoyl phosphate synthetase I (CPS I) catalyses the condensation of NH4+ ions with CO2 to generate carbamoyl phosphate. This process is irreversible and rate-limiting, consuming 2 ATP. CPS I requires N-acetyl glutamate to function. Another enzyme involved in pyrimidine production, carbamoyl phosphate synthetase II (CPS-II), is found in the cytosol. It accepts the amino group from glutamine and does not need N-acetyl glutamate to function.
Citrulline Formation
Citrulline is produced by ornithine transcarbamoylase from carbamoyl phosphate and ornithine. Ornithine is recycled and utilised in the urea cycle.
Argininosuccinate Synthesis
Argininosuccinate synthase combines with citrulline and aspartate to generate argininosuccinate. This process incorporates the second amino group of urea. This process requires the breakdown of ATP into AMP and pyrophosphate (PPi). The PPi is instantly degraded to inorganic phosphate (Pi).
Argininosuccinate Cleavage
Arginosuccinase cleaves argininosuccinate to produce arginine and fumarate. Urea is a direct precursor of arginine.
Urea Formation
Arginase is the last enzyme that cleaves arginine to produce urea and ornithine. The regenerated ornithine enters the mitochondria for reuse in the urea cycle. Both CO2 and Mn2+ are activated by arginase.
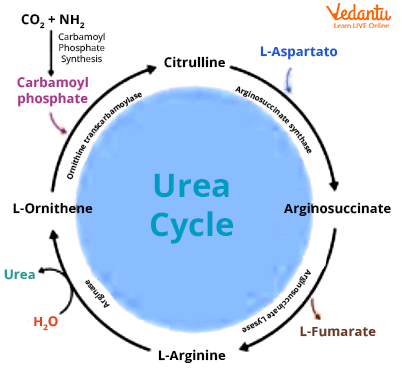
Urea Cycle
Regulation of Urea Cycle
The first reaction, catalysed by Carbamoyl Phosphate Synthetase I (CPS I), is a rate-limiting or essential step in the production of urea. N-acetyl glutamate (NAG) activates CPS I allosterically. The rate of urea production in the liver is linked to N-acetyl-glutamate concentration. NAG levels rise when arginine levels rise. A protein-rich meal raises the amount of NAG in the liver, resulting in increased urea production.
The mitochondria include carbamoyl phosphate synthetase I and glutamate dehydrogenase. They collaborate in the creation of NH3 and its usage in the synthesis of carbamoyl phosphate. The remaining four urea cycle enzymes are primarily regulated by the concentration of their respective substrates.
Overall Reaction and Energetics
The urea cycle is irreversible and requires 4 ATP to complete. The production of carbamoyl phosphate requires two ATPs. One ATP is converted to AMP and PPi to yield argininosuccinate, which is equivalent to two ATP. As a result, 4 ATP is actually consumed.
NH4+ + CO2 + Aspartate + 3ATP → Urea+ Fumarate + 2ADP + 2 Pi + AMP + PPi
Significance of Urea Cycle
The urea cycle, also known as the “Ammonia Detox Cycle”, is the process by which ammonia is removed from the body.
Toxic ammonia is transformed into harmless urea. It eliminates two waste products: ammonia and CO2.
It produces arginine, a semi-essential amino acid. It helps to regulate blood pH, which is determined by the dissolved CO2 ratio, e.g. H2CO3 to HCO3.
Ornithine is a precursor of polyamines such as spermidine and spermine.
Ammonia is a byproduct of protein metabolism that is hazardous to the human body, particularly the central nervous system. As a result, ammonia is transformed into urea, a harmless water-soluble molecule that is excreted through urine.
Importance of Urea Cycle
NH4+ is a byproduct of amino acid degradation.
Cells require NH4+ for the production of nitrogen-containing molecules.
Excess NH4+ is extremely harmful to our body. So, the excess NH4+ is transformed into urea and eliminated via the urea cycle. The urea cycle accounts for 80% of the nitrogen excreted.
TCA cycle intermediates are recycled.
Amino acids and keto acids are recycled.
Urea Cycle Disorder
The table describes metabolic disorders related to each of the five urea cycle enzymes. All of the disorders always result in an increase in blood ammonia (hyperammonemia), which leads to toxicity. Another urea cycle byproducts accumulate as well, depending on the individual enzyme deficiency. Clinical signs of urea cycle enzyme deficiencies include vomiting, drowsiness, irritability, stiffness, and mental retardation.
Conclusion
Urea cycle is a series of metabolic reactions that occur in the liver to convert ammonia to urea. The urea cycle is made up of six enzymes that rid the body of nitrogen produced during amino acid metabolism. They break it down into urea, which is excreted in the urine. This article talks about the importance and regulation of the Urea Cycle. There are different disorders also related to the improper functioning of the enzymes of the urea cycle.


FAQs on Urea Cycle: Steps, Importance & Exam Tips
1. What is the primary purpose of the urea cycle in the human body?
The primary purpose of the urea cycle is to convert highly toxic ammonia (NH₃), a byproduct of protein and amino acid metabolism, into urea, which is a far less toxic substance. This process mainly occurs in the liver. The urea is then transported through the bloodstream to the kidneys and safely excreted in the urine, preventing a toxic buildup of ammonia in the body.
2. What are the five main steps of the urea cycle?
The urea cycle is a sequence of five enzymatic reactions. The first two steps occur in the mitochondria, and the remaining three take place in the cytosol:
- Step 1 (Mitochondria): Formation of Carbamoyl Phosphate from ammonia and bicarbonate, catalysed by Carbamoyl Phosphate Synthetase I (CPS I). This is the rate-limiting step.
- Step 2 (Mitochondria): Formation of Citrulline from Carbamoyl Phosphate and Ornithine, catalysed by Ornithine Transcarbamylase (OTC).
- Step 3 (Cytosol): Formation of Argininosuccinate from Citrulline and Aspartate, catalysed by Argininosuccinate Synthetase.
- Step 4 (Cytosol): Cleavage of Argininosuccinate into Arginine and Fumarate, catalysed by Argininosuccinate Lyase.
- Step 5 (Cytosol): Hydrolysis of Arginine to yield Urea and regenerate Ornithine, catalysed by Arginase. The ornithine re-enters the mitochondria to continue the cycle.
3. Where in the liver cell do the different stages of the urea cycle occur?
The urea cycle is unique because it spans two different compartments within the liver cell. The initial two reactions occur inside the mitochondria, while the subsequent three reactions take place in the cytosol. This compartmentalisation allows for efficient regulation and separation of metabolic pathways. The intermediate citrulline is transported out of the mitochondria, and ornithine is transported back in to keep the cycle running.
4. Why is converting ammonia to urea so important for terrestrial animals like humans?
Converting ammonia to urea is a crucial adaptation for terrestrial life. Ammonia is extremely toxic, especially to the central nervous system, and requires a large amount of water to be diluted and excreted safely. By converting it to urea, which is about 100,000 times less toxic, the body can:
- Conserve Water: Urea can be concentrated in the urine, allowing for significant water conservation, which is vital for animals living on land.
- Prevent Toxicity: It allows for the safe transport of nitrogenous waste in the blood from the liver to the kidneys without causing cellular damage. This process is known as ureotelism.
5. What are the clinical consequences if the urea cycle is defective?
A defect in any of the urea cycle enzymes can lead to a group of genetic disorders known as Urea Cycle Disorders (UCDs). The primary clinical consequence is hyperammonemia, which is the accumulation of toxic levels of ammonia in the blood. Since ammonia can cross the blood-brain barrier, this condition can cause severe neurological damage, leading to symptoms like lethargy, vomiting, seizures, cognitive impairment, and can result in a coma or even be fatal if not treated promptly.
6. How is the urea cycle connected to the Krebs cycle (Citric Acid Cycle)?
The urea cycle and the Krebs cycle are interconnected through a series of reactions sometimes called the 'Krebs Bicycle'. The link is established by the molecule fumarate. In step 4 of the urea cycle, argininosuccinate is cleaved to produce arginine and fumarate. This fumarate can then enter the Krebs cycle to be converted into malate and then oxaloacetate, which can be used to generate energy (ATP) or be converted to aspartate. This aspartate then provides the second nitrogen atom for the urea cycle by combining with citrulline in step 3.
7. How is the urea cycle regulated in the body?
The regulation of the urea cycle is crucial to manage ammonia levels. The primary point of regulation is the first enzyme, Carbamoyl Phosphate Synthetase I (CPS I). Its activity is controlled in two main ways:
- Allosteric Activation: CPS I is allosterically activated by N-acetylglutamate (NAG). The synthesis of NAG itself is stimulated by high levels of arginine, signalling an excess of amino acids that need to be processed.
- Substrate Availability: The rate of the cycle also increases with higher concentrations of ammonia and other substrates. During periods of high protein intake or starvation (when muscle protein is broken down), the enzyme levels increase to handle the larger nitrogen load.
8. Is there a simple mnemonic to remember the intermediates of the urea cycle?
Yes, a popular and easy way for students to remember the main intermediates of the urea cycle in order is with the mnemonic: Ordinarily, Careless Crappers Are Also Frivolous About Urination. This stands for:
- Ornithine
- Carbamoyl Phosphate
- Citrulline
- Aspartate
- Argininosuccinate
- Fumarate
- Arginine
- Urea










