How Does ELISA Work? Key Concepts and Real-Life Examples
ELISA full form is Enzyme-Linked Immunosorbent Assay. It is a widely used ELISA test that helps detect and quantify various substances like proteins, hormones, peptides, and antibodies in a sample, typically blood. This ELISA technique relies on the specific interaction between an antigen and an antibody, making it highly sensitive and specific for diagnosing infections, pregnancy, and other conditions.
When you want to check if your body has produced antibodies against a particular disease agent (antigen), the ELISA procedure becomes a crucial diagnostic tool. It is commonly used in medical labs, research facilities, and even for at-home testing kits in some cases.
Principle of ELISA
The principle of ELISA (also known as ELISA principle) is based on the specific binding of an antigen to its corresponding antibody. Here is a simple breakdown:
Antigen-Antibody Interaction: A specific antibody captures the target antigen or a specific antigen captures the target antibody.
Enzyme Conjugation: A secondary antibody is linked with an enzyme to detect the presence of the antigen-antibody complex.
Substrate Addition: A suitable substrate is introduced, which the enzyme converts into a coloured product.
Measurement: The colour change is measured using a spectrophotometer. The intensity of the colour indicates the amount of antigen or antibody present in the sample.
This principle of ELISA ensures high accuracy and sensitivity. The more antigen or antibody you have in your sample, the stronger the colour development.
Types of ELISA
There are three main types you need to understand to get a complete picture of the ELISA principle and procedure:
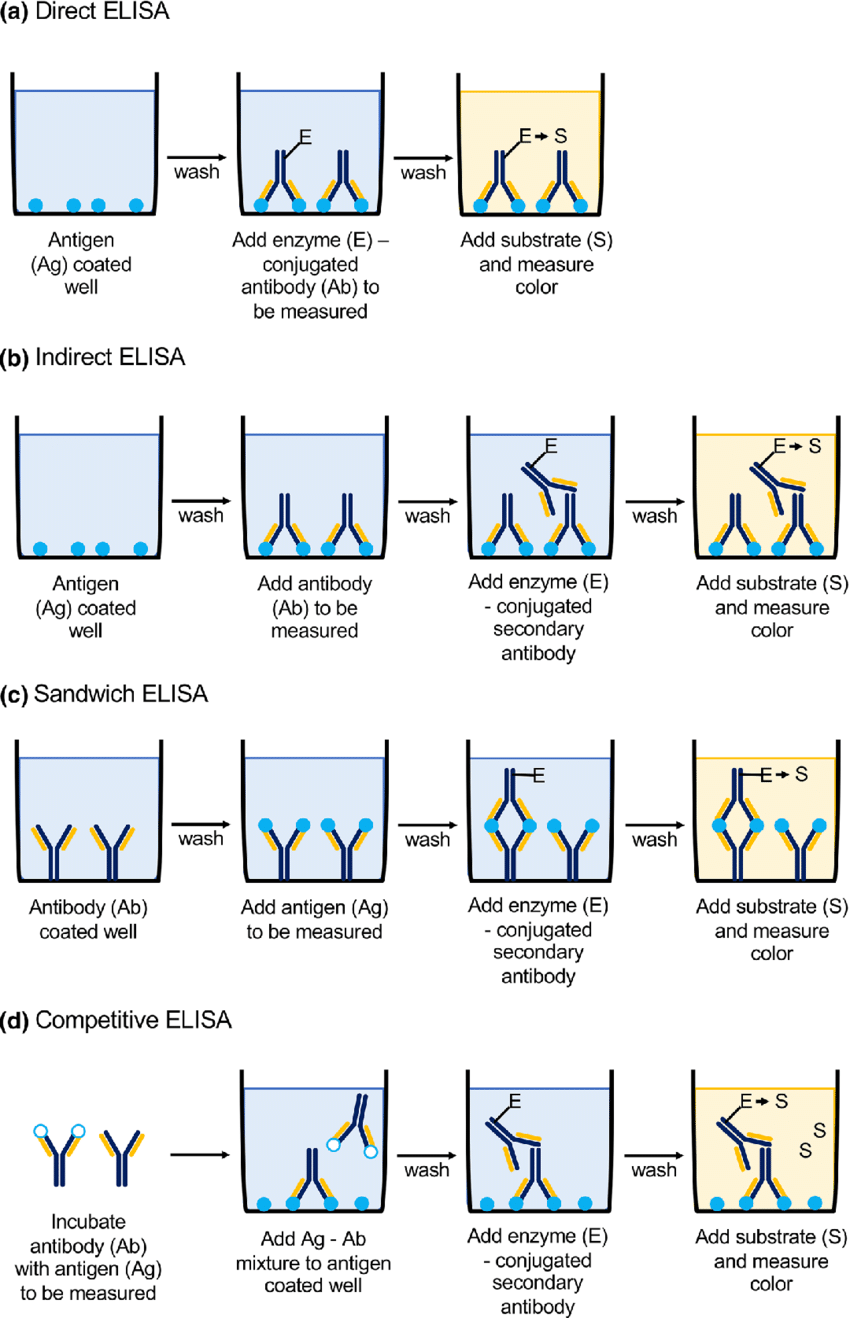
Indirect ELISA
Used to detect the presence (or concentration) of specific antibodies in a sample.
The antigen is first coated on the microtitre well.
The sample (containing the primary antibody) is added. If the antibody is present, it binds to the antigen.
A secondary, enzyme-linked antibody binds to the primary antibody.
Upon adding the substrate, the enzyme reacts to produce a visible signal (colour), which can be measured.
Sandwich ELISA
Commonly used to detect the presence (or concentration) of a specific antigen in a sample.
Antibody is coated onto the microtitre plate.
The sample (with the suspected antigen) is added. The antigen, if present, will bind to the immobilised antibody.
A second enzyme-conjugated antibody, which recognises a different epitope on the antigen, is added.
Substrate is added, leading to a colour change proportional to the amount of antigen present.
Competitive ELISA
Typically used to measure the concentration of an antigen when it’s present in low quantities or to study antigen-antibody affinity.
The microtitre well is coated with a known antigen.
The sample (unknown concentration of the same antigen) is mixed with a known amount of antibody, forming an antigen-antibody complex.
This mixture is transferred to the antigen-coated plate. If the sample contains a high concentration of the antigen, fewer free antibodies remain to bind the plate’s antigen.
After adding the enzyme-linked secondary antibody, the signal measured is inversely proportional to the antigen concentration in the sample.
ELISA Diagram
A typical ELISA diagram includes a 96-well microtitre plate. Each well can be coated with either antigen or antibody, depending on the type of ELISA you are performing. Then, the other components (primary or secondary antibodies and substrates) are sequentially added. The final colour change indicates the presence and quantity of your target molecule.
ELISA Procedure
While there can be slight variations depending on the type of ELISA, the general ELISA procedure usually involves the following steps:
Coating
A known antibody or antigen is adsorbed onto the solid surface (microtitre plate).
Excess unbound molecules are washed away using a suitable buffer.
Blocking
Any remaining protein-binding sites on the plate are blocked to prevent non-specific binding. Common blocking agents include BSA (bovine serum albumin) or skimmed milk.
Incubation with Sample
The sample containing the target antigen or antibody is added to the well.
If the target analyte is present, it will bind to the coated antibody or antigen.
Detection Antibody (if required)
A secondary enzyme-conjugated antibody is added. This secondary antibody will specifically bind the target if it is captured in the well.
Washing
Unbound materials are removed through several wash cycles.
Substrate Addition
A suitable substrate for the enzyme is added. The substrate reacts with the enzyme to produce a coloured or luminescent product.
Reading Results
The intensity of the developed colour or light is measured, often using a spectrophotometer or plate reader.
Higher absorbance or luminescence indicates a higher concentration of the target molecule in the sample.
Diagnosis with ELISA
The ELISA test plays a vital role in diagnosing various diseases and conditions. Some examples include:
HIV/AIDS: Detects antibodies against HIV.
Lyme Disease: Checks for antibodies against Borrelia burgdorferi.
Ebola: Identifies antibodies specific to the Ebola virus.
Zika Virus: Detects antibodies formed against Zika infection.
Rotavirus: Identifies proteins or antibodies related to rotavirus infection.
Toxoplasmosis: Recognises antibodies against Toxoplasma gondii.
Pernicious Anaemia: Helps detect intrinsic factor antibodies.
Carcinoma of Epithelial Cells: Sometimes used to measure tumour markers.
In pregnancy tests, ELISA is used to detect the HCG (Human Chorionic Gonadotropin) protein in blood or urine.
Advantages of ELISA
ELISA offers several benefits over other diagnostic tools:
High Specificity and Sensitivity: Uses antigen-antibody binding, so results are very accurate.
Quantitative, Semi-Quantitative, or Qualitative: Measurements can be taken in different formats.
Simple and Rapid: Procedures are straightforward and can be done relatively quickly.
No Need for Radioactive Substances: Unlike older assays, it relies on enzymes, making it safer to handle.
Adaptability: Can be customised for various applications, including medical diagnosis, food safety, and more.
Cost-Effective: Basic lab equipment (microtitre plate reader, pipettes) is generally sufficient.
Application of ELISA
The application of ELISA extends beyond just diagnosing infectious diseases. It includes:
Medical Diagnosis
Rapid screening and confirmation of several infections and autoimmune disorders.
Food Industry
Detects allergens like peanuts, soy, milk, and gluten in food products to ensure consumer safety.
Environmental Monitoring
Monitors the presence of contaminants (e.g., pesticides) in water, soil, or food.
Pharmaceutical and Drug Research
Measures the concentration of drugs, antibodies, or hormones in various samples to test efficacy or toxicity.
Epidemiological Studies
During disease outbreaks, large-scale ELISA test procedures help track infection rates within populations.
Quick Quiz (with Answers)
1. What does ELISA stand for?
Answer: Enzyme-Linked Immunosorbent Assay.
2. Which type of ELISA is commonly used to detect antibodies in a sample?
Answer: Indirect ELISA.
3. True or False: In Competitive ELISA, the colour intensity is directly proportional to the antigen concentration in the sample.
Answer: False (it is inversely proportional).
4. Name an enzyme commonly linked to the secondary antibody in ELISA.
Answer: Horseradish Peroxidase (HRP) or Alkaline Phosphatase (AP).
5. Which ELISA type is often used to measure cytokines or other proteins at very low concentrations?
Answer: Sandwich ELISA.
Related Topics


FAQs on ELISA Test: Principle, Steps, Types, and Applications
1. What is the basic principle of an ELISA test?
The ELISA test works on the principle of antigen-antibody interaction. It uses antibodies or antigens coupled to an enzyme to detect a specific substance in a liquid sample. If the target substance is present, it binds to the antibody, and the attached enzyme then reacts with a substrate to produce a measurable colour change.
2. What are the main types of ELISA used in laboratories?
There are four primary types of ELISA, each designed for a specific purpose:
- Direct ELISA: An enzyme-linked primary antibody reacts directly with an antigen.
- Indirect ELISA: Detects a primary antibody by using a secondary, enzyme-linked antibody.
- Sandwich ELISA: The antigen is 'sandwiched' between two antibodies (a capture antibody and a detection antibody). This is highly specific.
- Competitive ELISA: The target substance in the sample competes with a labelled reference for binding spots. A weaker signal means more of the substance is present.
3. What are the general steps involved in performing an ELISA procedure?
A typical ELISA procedure involves a few key steps:
- Coating: A microplate well is coated with either the antigen or a capture antibody.
- Blocking: A blocking buffer is added to cover any remaining empty surfaces on the plate to prevent non-specific binding.
- Detection: The sample (and detection antibodies, if needed) is added. If the target is present, binding occurs.
- Washing: The plate is washed to remove any unbound molecules.
- Signal Generation: A substrate is added, which reacts with the enzyme to produce a visible colour change.
- Reading: A spectrophotometer measures the intensity of the colour to determine the result.
4. Why is the ELISA test considered both highly specific and sensitive?
The power of ELISA comes from two factors:
- Specificity: This is due to the unique, precise binding of antibodies to their target antigens, much like a lock and key. This minimises the chance of binding to the wrong molecule.
- Sensitivity: This comes from enzyme amplification. A single enzyme attached to an antibody can trigger thousands of reactions, creating a strong colour signal that can be detected even if only a tiny amount of the substance is present.
5. How does the enzyme in an ELISA test actually create the result?
The enzyme itself doesn't detect anything. It is chemically linked to a detection antibody. Its job is to act as a signal amplifier. After all unbound materials are washed away, a colourless chemical called a substrate is added. The enzyme converts this substrate into a coloured product. The amount of colour produced is directly proportional to the amount of target substance captured in the well.
6. What is the main difference between how a Sandwich ELISA and an Indirect ELISA work?
The key difference is what they are designed to detect:
- A Sandwich ELISA directly detects the presence of an antigen (like a virus particle or a hormone) in the sample.
- An Indirect ELISA detects the presence of antibodies in the sample. This is useful for finding out if a patient's immune system has responded to an infection.
7. In what real-world situations is the ELISA test commonly used?
ELISA is a versatile tool used in many fields. Common examples include:
- Medical Diagnosis: Detecting infections like HIV, Hepatitis B, and COVID-19 antibodies.
- Pregnancy Tests: Identifying the pregnancy hormone (hCG) in urine.
- Food Safety: Screening for allergens like peanuts or gluten in food products.
- Toxicology: Detecting the presence of specific drugs in a sample.
8. What could cause a false positive or false negative result in an ELISA test?
While reliable, errors can occur. A false positive might happen if the antibody cross-reacts with a similar-looking but incorrect molecule. A false negative could occur if there is too little of the target substance to be detected or if there was an error in the procedure, such as improper washing or using expired reagents.










