How Do Aerobic and Anaerobic Respiration Impact Cellular Energy?
Have you ever wondered how cells have the energy in our food? Living organisms require energy to perform all kinds of tasks – from lifting a school bag to powering microscopic functions inside each cell. This energy is mainly produced through a biochemical process known as cellular respiration. There are two primary modes in which it can occur: aerobic and anaerobic respiration.
In this article, we will discuss what is the difference between aerobic and anaerobic respiration, explore their processes, and highlight their role in everyday life. We’ll also provide additional insights that go beyond the basics – all in clear, student-friendly language.
What is Cellular Respiration?
Cellular respiration is the step-by-step chemical process by which cells convert the energy stored in glucose (sugar) into a form that the cell can use (ATP – Adenosine Triphosphate). This process can happen in two distinct ways:
Aerobic respiration: This takes place in the presence of oxygen.
Anaerobic respiration: This takes place in the absence of oxygen.
Although many associate aerobic respiration mainly with advanced organisms like humans and animals, both processes (aerobic and anaerobic) are crucial for different living organisms at different times.
Overview: Aerobic vs Anaerobic Respiration
When we talk about aerobic vs anaerobic respiration, the most critical factor is the availability of oxygen. Aerobic respiration requires oxygen, producing carbon dioxide, water, and a substantial amount of energy. Anaerobic respiration, on the other hand, occurs when oxygen is not present (or is scarce), generating energy along with by-products like lactic acid or alcohol (ethanol) and carbon dioxide.
If you’re studying this topic for an exam, especially if you need to understand the difference between aerobic and anaerobic respiration class 10, keep in mind that these differences form a core concept in Biology and are tested frequently.
What is Aerobic Respiration?
“Aerobic” literally means “with air” or “with oxygen.” During aerobic respiration, glucose molecules are fully broken down using oxygen, releasing carbon dioxide (CO₂), water (H₂O), and energy (ATP). Here’s a simplified equation:
Glucose + Oxygen → Carbon dioxide + Water + Energy (ATP)
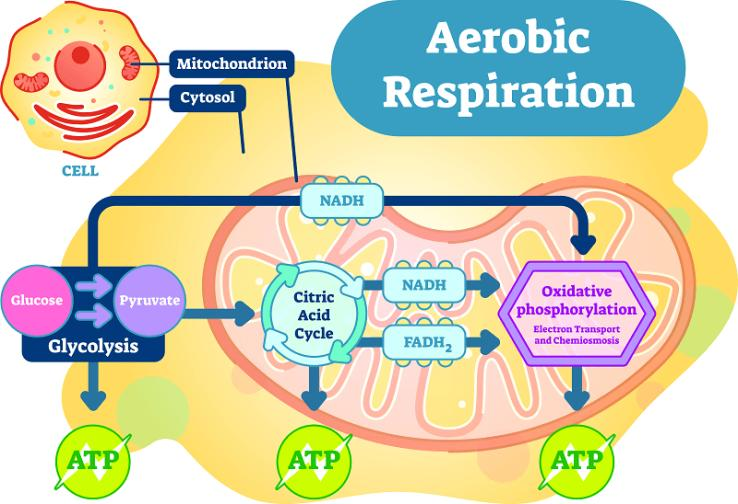
Where does it occur? Aerobic respiration occurs partly in the cytoplasm (in the initial stage called glycolysis) and predominantly in the mitochondria of the cell – often called the cell’s “powerhouses” because they generate most of the ATP.
Significance: This process is highly efficient and produces a higher yield of ATP compared to anaerobic respiration. In humans, plants, and most animals, aerobic respiration is the primary mode of breaking down glucose to produce energy.
Did you know? The net gain of ATP in aerobic respiration can be around 36-38 ATP molecules per glucose molecule, which is significantly higher than in anaerobic respiration.
What is Anaerobic Respiration?
“Anaerobic” means “without air” or “in the absence of oxygen.” During anaerobic respiration, glucose is not fully broken down because there is insufficient or no oxygen. Instead, the partial breakdown of glucose releases energy alongside other by-products. The two main types of anaerobic respiration are:
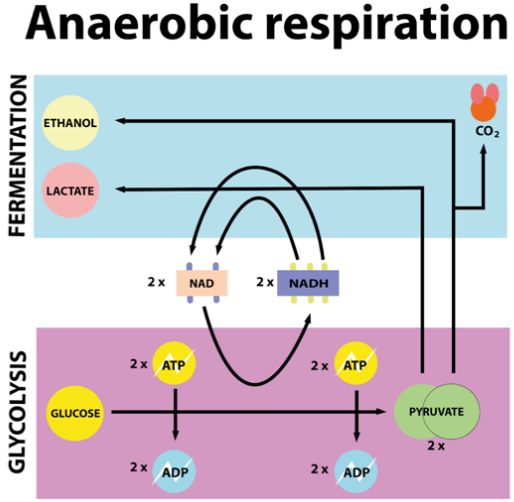
Alcoholic Fermentation (common in yeast and some bacteria)
Glucose → Ethanol (Alcohol) + Carbon dioxide + Energy (ATP)
Lactic Acid Fermentation (occurs in certain bacteria, and temporarily in human muscle cells)
Glucose → Lactic acid + Energy (ATP)
Where does it occur?
Anaerobic respiration mainly occurs in the cytoplasm, as the mitochondria require oxygen for most of their functions.
Significance: Many microorganisms (e.g., yeast) rely primarily on anaerobic respiration. Even human muscles can switch to anaerobic respiration briefly when oxygen is in short supply, for instance, during very intense exercise.
Did you know? Anaerobic respiration yields far fewer ATP (around 2 ATP molecules per glucose) compared to aerobic respiration. However, it is a quick way to produce energy, which can be crucial during emergencies, such as intense bursts of activity.
Difference between Aerobic and Anaerobic Respiration
Similarities Between Aerobic and Anaerobic Respiration
While the difference between aerobic respiration and anaerobic respiration is often emphasised, it’s equally important to note their similarities:
Both Breakdown Glucose: In both processes, glucose is the primary substrate that is broken down to release energy.
ATP Production: Whether oxygen is present or not, both types of respiration aim to generate ATP, the energy currency of the cell.
Initial Step – Glycolysis: In both aerobic and anaerobic respiration, glycolysis (in the cytoplasm) is the first stage where glucose is converted into pyruvate, producing a small amount of ATP.
Enzymatic Control: Both processes are regulated by enzymes, ensuring that the conversion of glucose to by-products is controlled and efficient.
Understanding these similarities can provide a holistic view of aerobic and anaerobic respiration and why living organisms might switch between these two methods under certain conditions.
Anaerobic Respiration in Humans
A common misconception is that humans rely solely on aerobic respiration. While it’s true that our bodies predominantly use aerobic pathways under normal conditions, muscles can engage in anaerobic respiration during short, intense bursts of activity.
Muscle Cramps and Lactic Acid
During vigorous exercise (e.g., sprinting, weight lifting), muscle cells may not receive enough oxygen to meet the energy demand.
As a result, muscles switch to lactic acid fermentation, producing lactic acid as a by-product.
The accumulation of lactic acid can cause muscle fatigue and the familiar burning sensation or cramps.
Resting, stretching, or taking a warm bath can improve blood flow, delivering more oxygen to these muscles and helping to clear lactic acid.
Additional Insights & Unique Facts
Here are some extra bits of information to make your understanding of aerobic and anaerobic respiration even more robust:
Industrial Uses:
Yeast (using alcoholic fermentation) helps produce bread, beer, and wine.
Certain bacteria (using lactic acid fermentation) are used in producing yoghurt, cheese, and pickles.
Adaptations in Nature:
Some aquatic organisms (e.g., certain fungi and bacteria in waterlogged soils) thrive purely on anaerobic respiration.
Seeds in waterlogged soil or plant roots in very wet conditions can switch to anaerobic pathways until oxygen levels become adequate again.
Krebs Cycle & Electron Transport Chain:
Aerobic respiration includes more complex stages such as the Krebs cycle and electron transport chain (ETC) in mitochondria, maximising energy extraction from glucose.
Energy Efficiency:
Aerobic respiration is highly efficient in converting glucose into usable energy (ATP). In contrast, anaerobic respiration is less efficient but faster in providing ATP when oxygen levels are low.
Role of Oxygen in Mitochondria:
Oxygen in aerobic respiration serves as the final electron acceptor in the ETC, enabling more ATP to be produced.
By exploring these unique and extra details, you not only understand what is the difference between aerobic and anaerobic respiration but also appreciate the critical roles both processes play in diverse environments and industrial applications.


FAQs on Aerobic vs Anaerobic Respiration: Core Differences for Students
1. What is Respiration?
Respiration is a vital biochemical process common to all living organisms. It involves taking in oxygen (in aerobic respiration) and expelling carbon dioxide, although some organisms can respire anaerobically without oxygen.
2. List the Similarities and Differences between Aerobic and Anaerobic Respiration.
Similarities: Both processes break down glucose, produce ATP, start with glycolysis, and are enzyme-controlled.
Differences: Aerobic uses oxygen and yields more ATP; anaerobic does not require oxygen, produces less ATP, and generates by-products like lactic acid or ethanol.
3. What is the Overall Equation of Aerobic Cellular Respiration?
$C_6H_{12}O_6 + 6O_2 \rightarrow 6CO_2 + 6H_2O + ATP$
4. What are the Types of Anaerobic Respiration?
Alcoholic fermentation (common in yeast)
Lactic acid fermentation (occurs in certain bacteria and temporarily in human muscle cells)
5. What are the Stages of Aerobic Respiration?
Aerobic respiration can be broadly divided into:
Glycolysis (in the cytoplasm)
Krebs Cycle (in the mitochondrial matrix)
Electron Transport Chain or Oxidative Phosphorylation (on the mitochondrial inner membrane)
6. Where Do Aerobic and Anaerobic Respiration Occur?
Aerobic respiration: Primarily in the mitochondria (after the initial glycolysis in the cytoplasm).
Anaerobic respiration: Entirely in the cytoplasm.










