
How Can NCERT Solution Class 12 Chemistry Chapter 8 Help With Aldehydes Ketones And Carboxylic Acids Questions And Answers Class 12 For Exam Preparation
NCERT Solutions for aldehydes ketones and carboxylic acids class 12 questions and answers help students master organic chemistry concepts. This chapter covers important functional groups and their reactions. Aldehydes ketones and carboxylic acids ncert pdf solutions make learning simple and clear.
 Table of Content
Table of ContentVedantu provides easy explanations for Class 12 Chemistry Chapter 8. Students can understand each reaction step by step. You can also check NCERT Solutions Class 12 Chemistry for complete chapter coverage.
These solutions help students solve problems quickly and correctly. Every answer follows NCERT guidelines perfectly. Download the NCERT Solutions PDF for free and improve your chemistry scores today.
How Can NCERT Solution Class 12 Chemistry Chapter 8 Help With Aldehydes Ketones And Carboxylic Acids Questions And Answers Class 12 For Exam Preparation
NCERT Exercise
1. What is meant by the following terms? Give an example of the reaction in each case.
(i) Cyanohydrin
Ans: Cyanohydrins are organic, RR′(OH)CN chemicals, where R and R′s may be either alkyl or aryl.
In the presence of excess sodium cyanide (NaCN) as a catalyst in the field of cyanohydrin, aldehydes and ketones react with hydrogen cyanide (HCN). These are called cyanohydrins reactions. The reaction is given below:
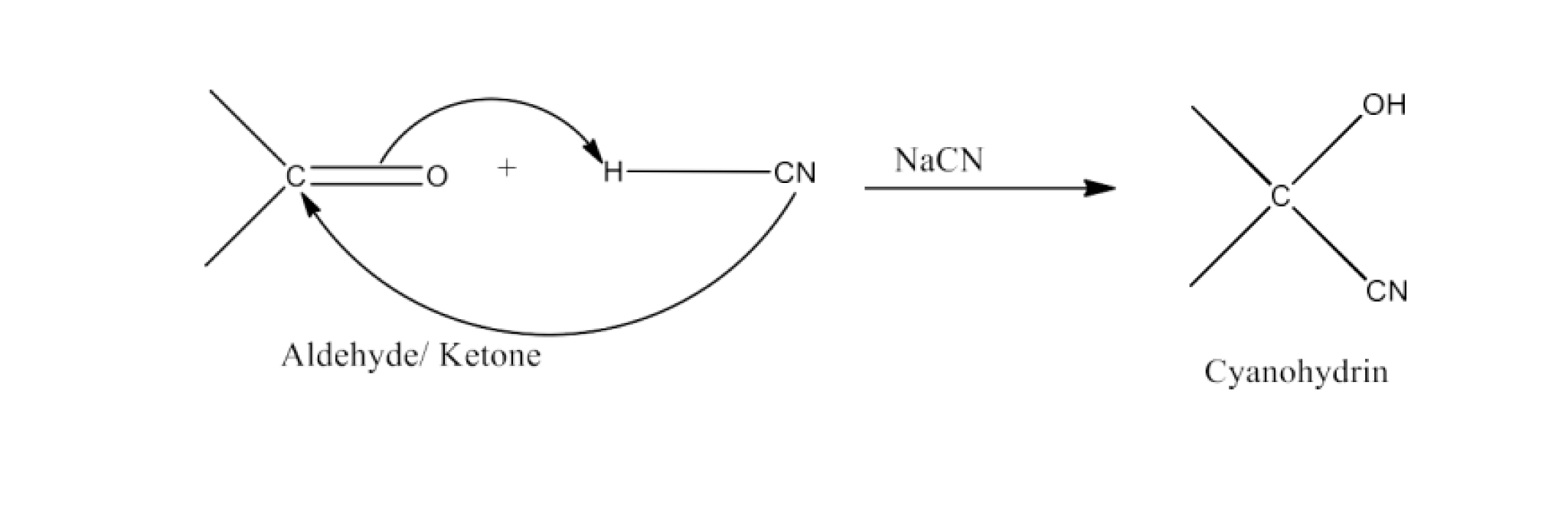
(ii) Acetal
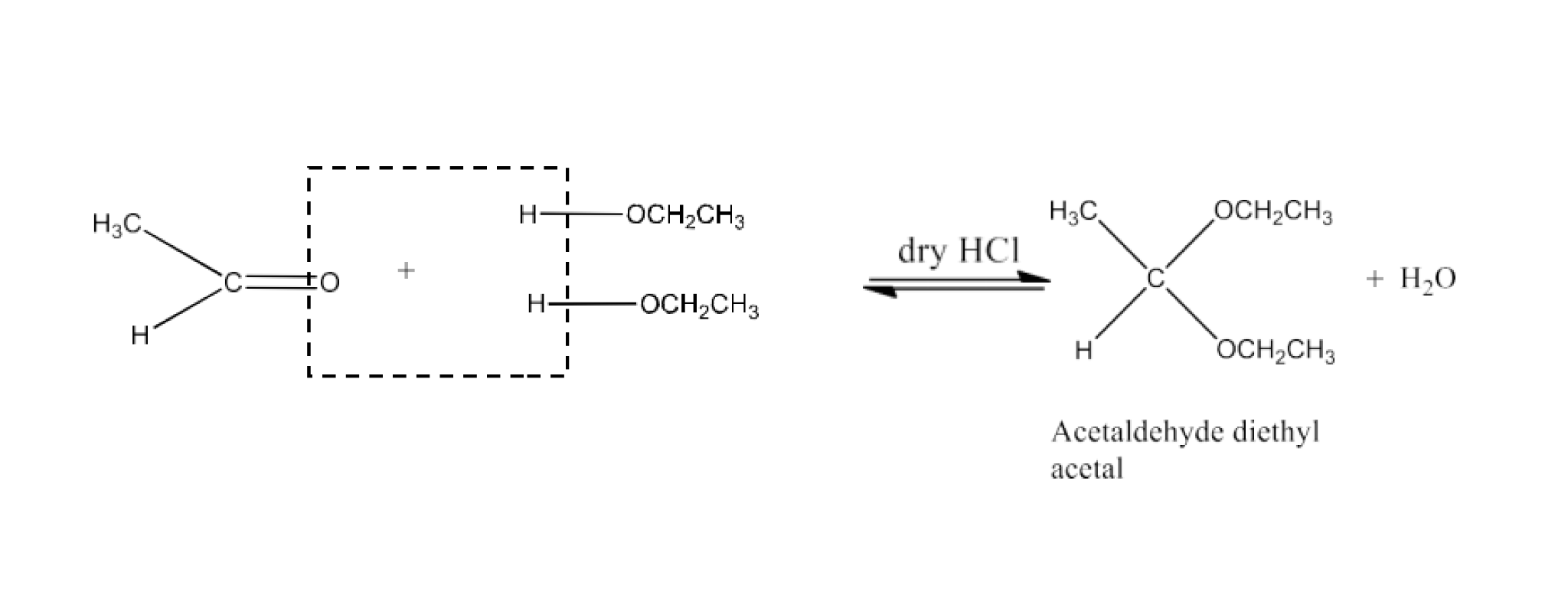
Ans: Acetals are gem-dialcoxy alkanes in which the terminal carbon atom consists of two alcohol groups. There are connections between one bond and an alkyl group and the other with a hydrogen atom.
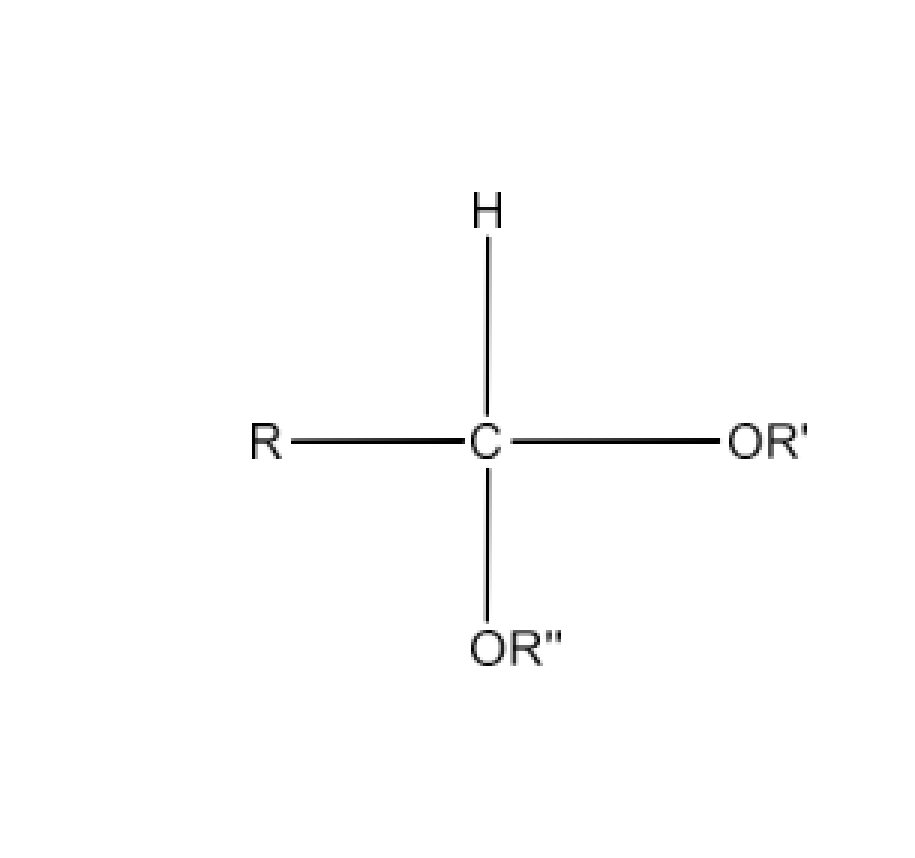
When two monohydric alcohol equivalents are treated with dry HCl gas, the hemiacetals are generated which react further with another alcohol molecule to form acetal. The reaction is given below:
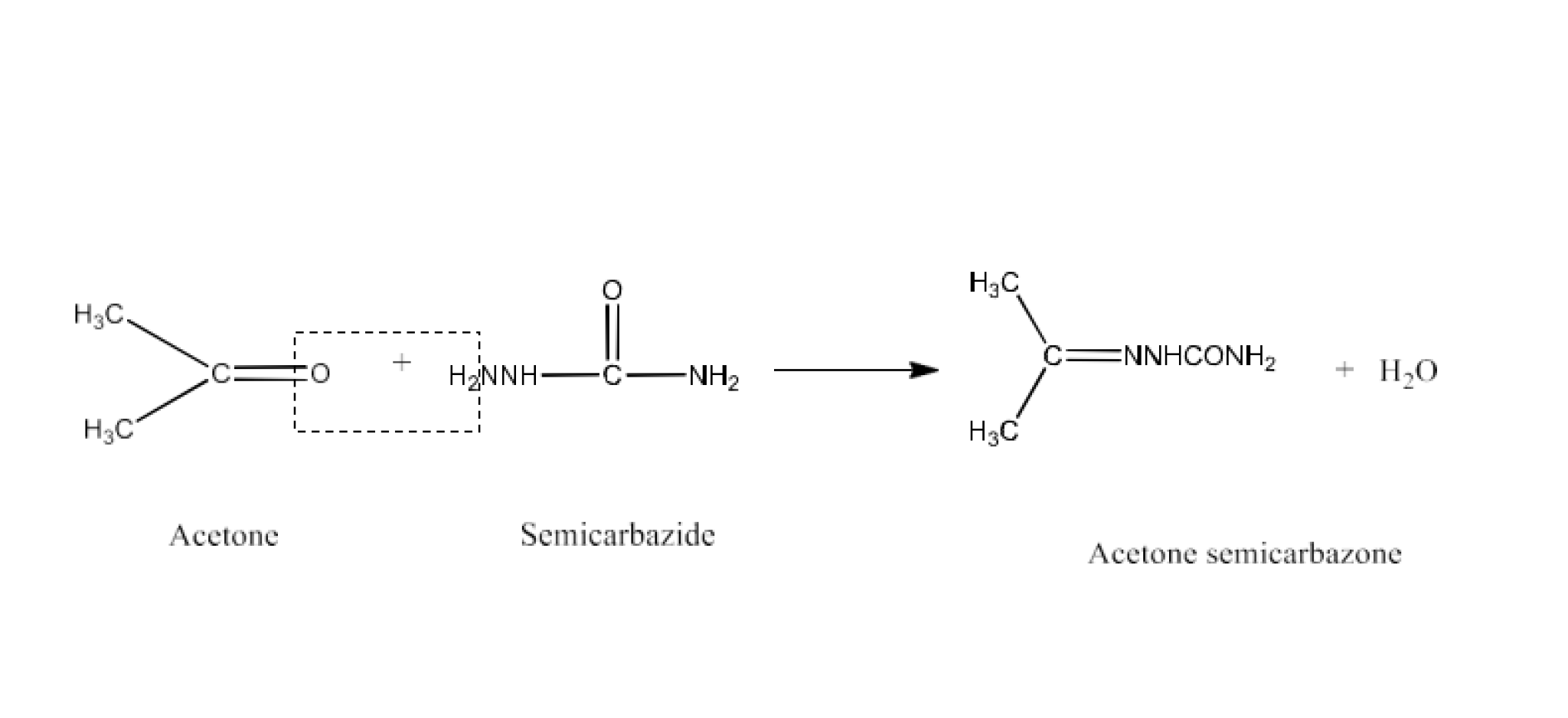
(iii) Semicarbazone
Ans: Semicarbazones are products of the condensation process of aldehydes and ketones generated by ketone or aldehyde and semicarbazide. An example of the formation of semicarbazone is given below:
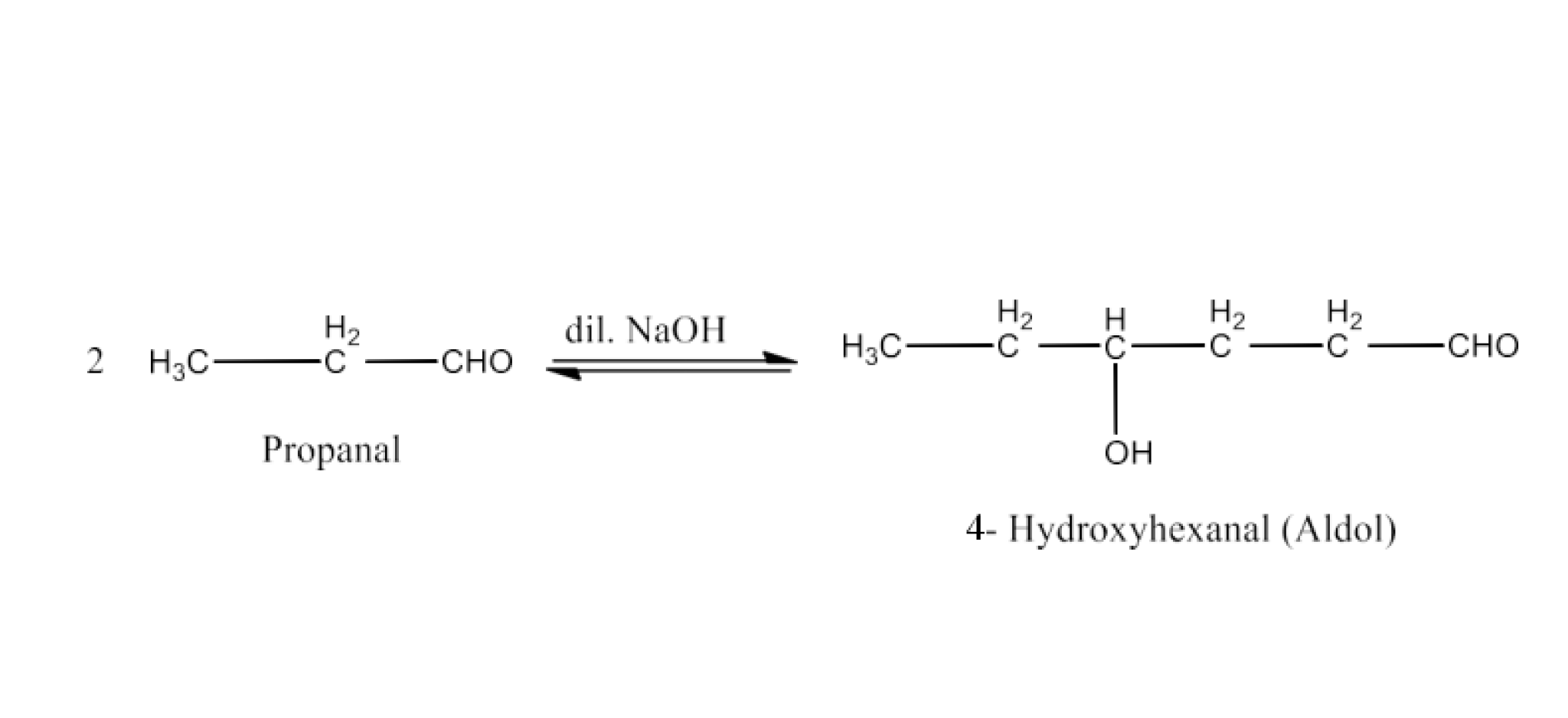
(iv) Aldol
Ans: An aldol is recognised to be a $\text{ }\!\!\beta\!\!\text{ -hydroxy}$ aldehyde or ketone. The condensation process in the presence of a base is generated between two molecules of one or two distinct aldehydes or ketones. An example of the formation of aldol is given below:
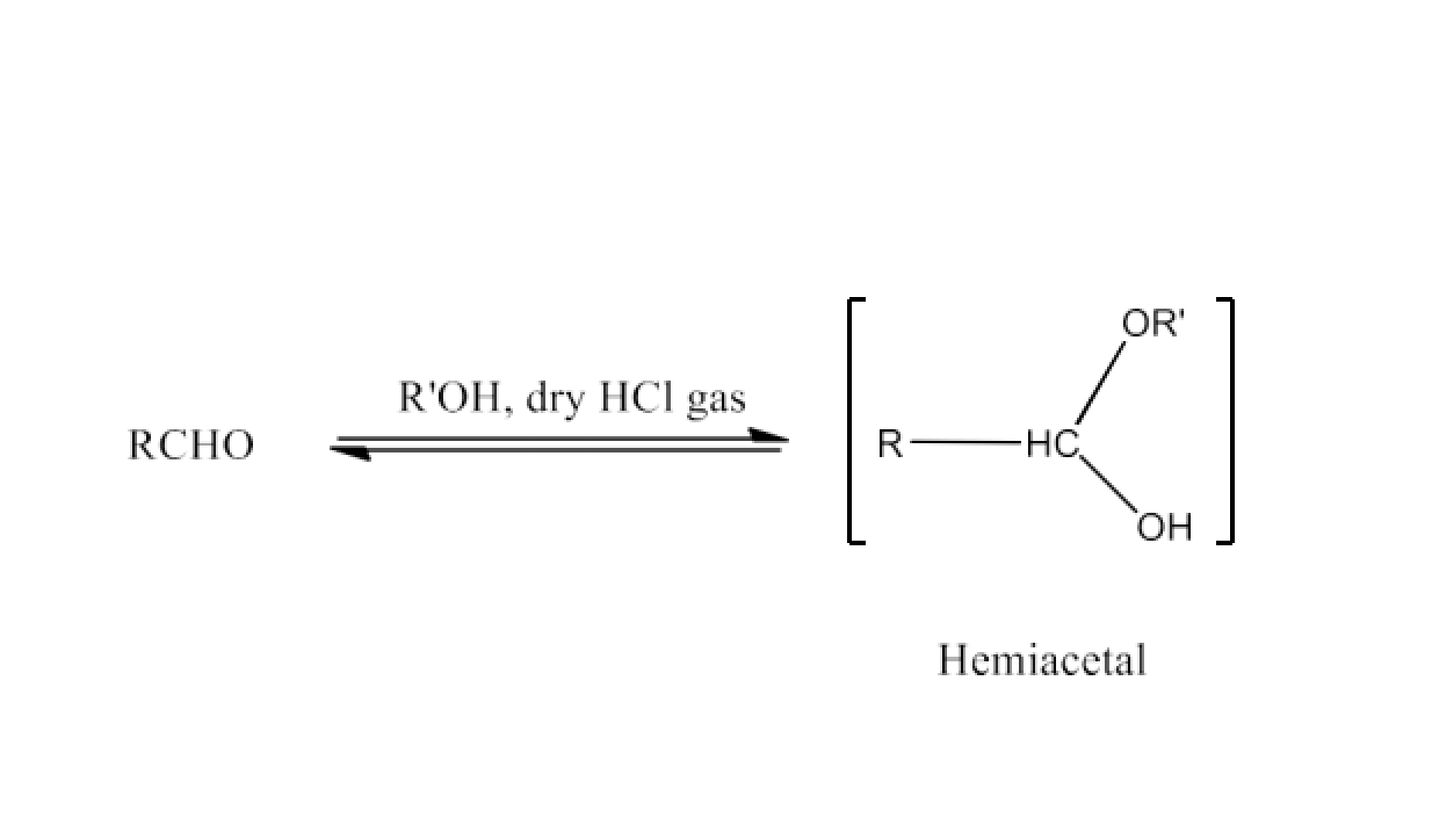
(v) Hemiacetal
Ans: We can say that $\text{ }\!\!\alpha\!\!\text{ -alkoxyalcohols}$ are known as hemiacetals. In the presence of dry HCl gas, aldehyde interacts with a single molecule of monohydric alcohol. The reaction is given below:
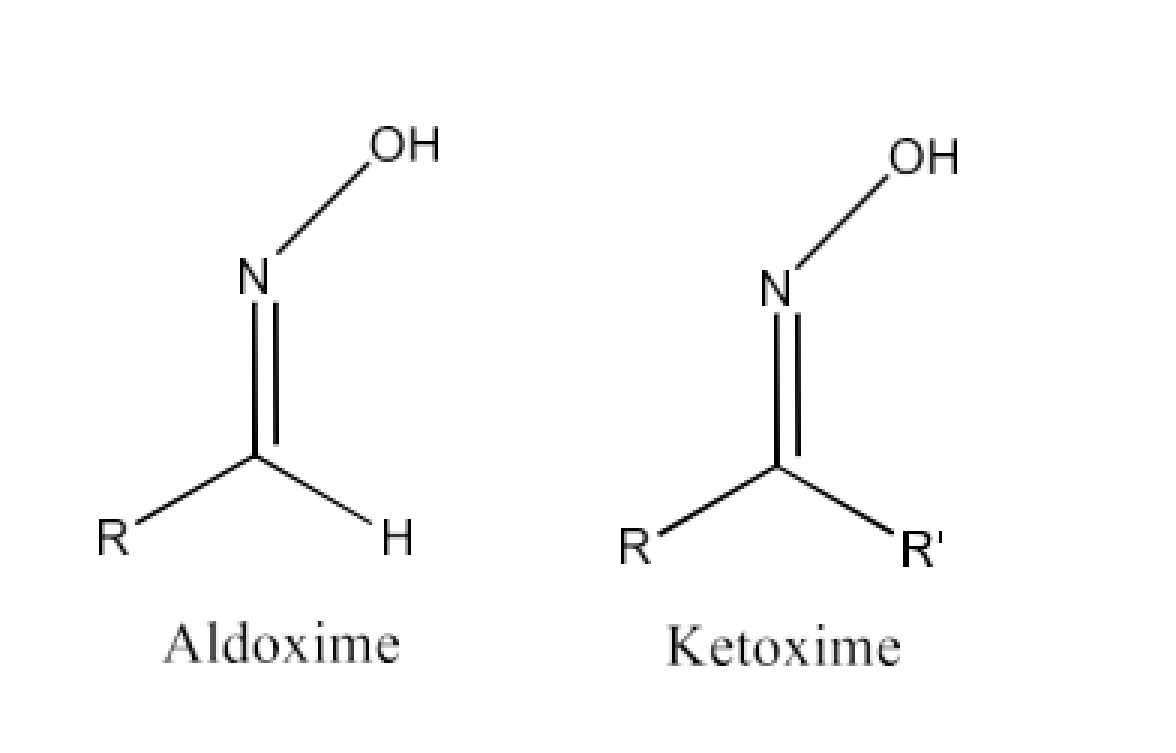
(vi) Oxime
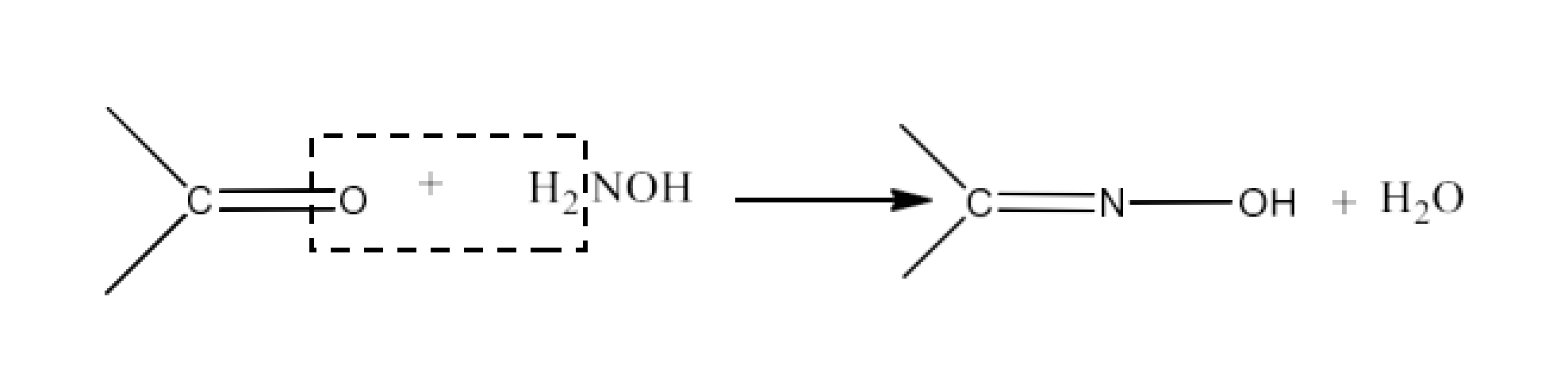
Ans: Oximes have the common formula of RR′CNOH where R is an organic side chain, and R′ is hydrogen or organic side chain. Oximes are organic chemicals. If R′ is H, the aldoxime is known and R′ is known as ketoxime if it is an organic side chain.
Aldehydes or ketones from oximes are treated with hydroxylamine in a slightly acidic media. The reaction is given below:
(vii) Ketal
Ans: Ketals are gemodioxyalcanes in which the chain contains two alcoholic groups on the same carbon atom. The other two carbon bonds are linked to two groups of alkyles. In the presence of HCl dry gas, ketones react with ethylene glycol to a cyclic compound called ethylene glycol ketals. An example of the formation of a ketal is given below:
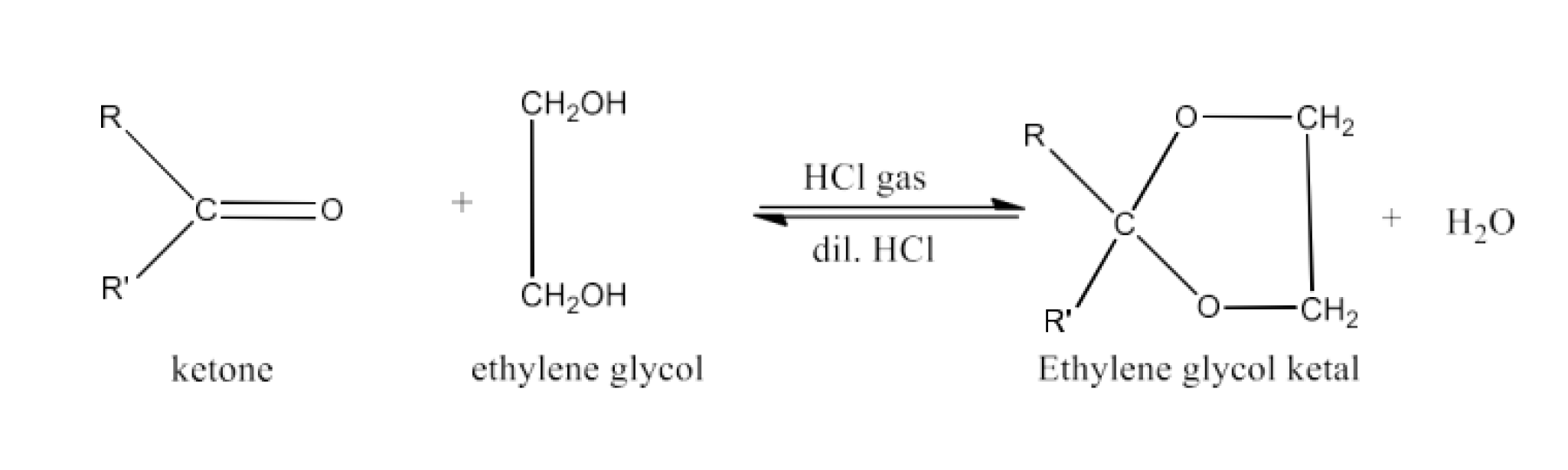
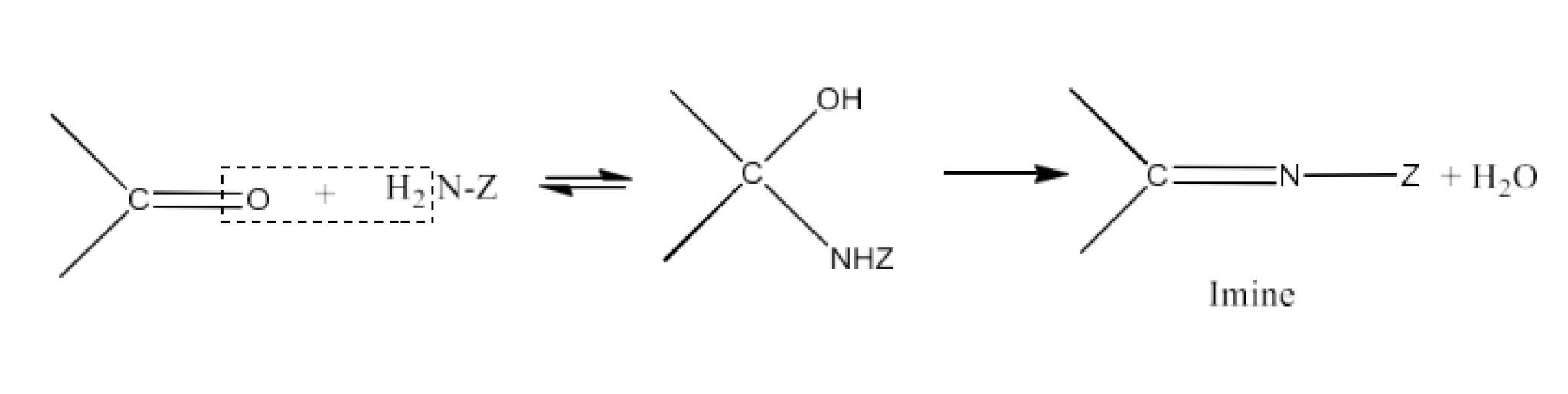
(viii) Imine
Ans: Imines are chemicals that have a double bond of carbon-nitrogen. Imines are generated when ammonia and its derivatives are reacted by aldehydes and ketones. The reaction is given below:
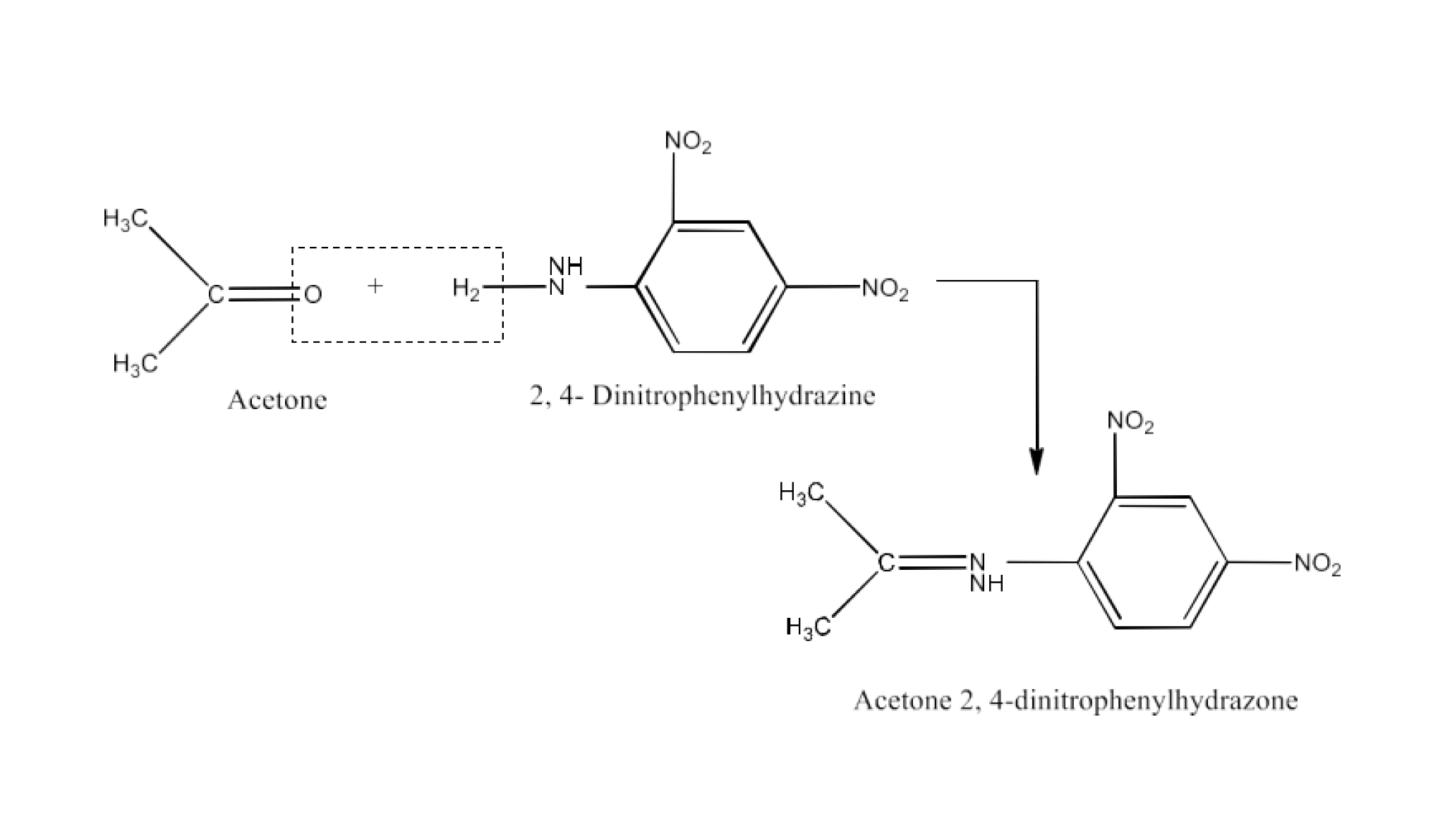
(ix) 2,4-DNP-derivative
Ans: 2, 4−dinitrophenylhydrazones 2, 4−DNP− derivatives that are made using 2, 4−dinitrophenylhydrazine in a low acidic medium in the case of aldehydes or ketones. The reaction is given below:
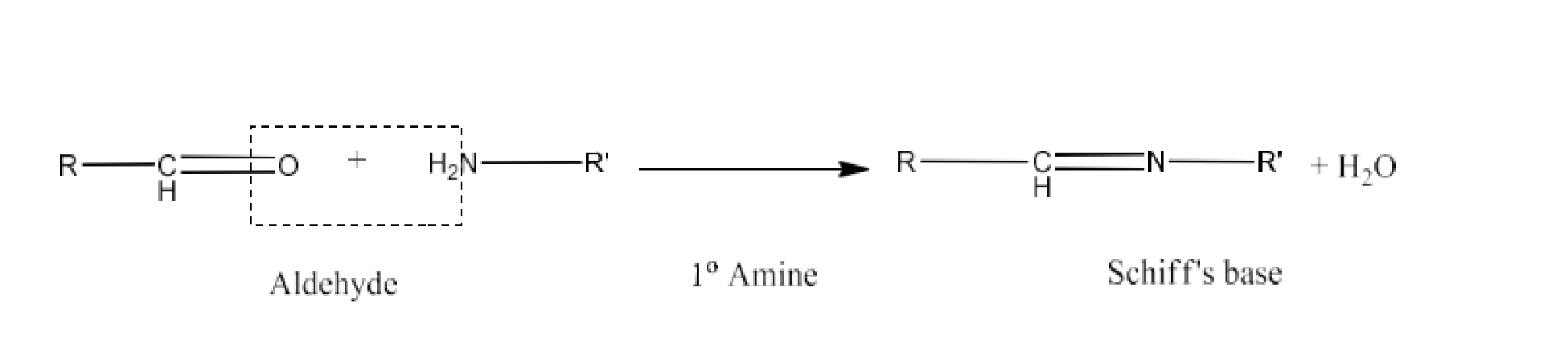
(x) Schiff’s base
Ans: The Schiff's base (or azomethine) is a chemical molecule that has an aryl or alkyl group, but not hydrogen, carbon-nitrogen twice the atom of nitrogen. They have the usual R1R2C=NR3 formulation. As it is an imine and it is named after Hugo Schiff, a physicist. The reaction is given below:
2. Name the following compounds according to IUPAC system of nomenclature:
(i) $\text{C}{{\text{H}}_{\text{3}}}\text{CH(C}{{\text{H}}_{\text{3}}}\text{)C}{{\text{H}}_{\text{2}}}\text{C}{{\text{H}}_{\text{2}}}\text{CHO}$
Ans: The IUPAC name of the given compound is 4-Methylpentanal.
(ii) $\text{C}{{\text{H}}_{\text{3}}}\text{C}{{\text{H}}_{\text{2}}}\text{COCH(}{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}\text{)C}{{\text{H}}_{\text{2}}}\text{C}{{\text{H}}_{\text{2}}}\text{Cl}$
Ans: The IUPAC name of the given compound is 6-Chloro-4-ethylhexan-3-one.
(iii) $\text{C}{{\text{H}}_{\text{3}}}\text{CH=CHCHO}$
Ans: The IUPAC name of the given compound is But-2-en-1-al.
(iv) $\text{C}{{\text{H}}_{\text{3}}}\text{COC}{{\text{H}}_{\text{2}}}\text{COC}{{\text{H}}_{\text{3}}}$
Ans: The IUPAC name of the given compound is Pentan-2,4-dione.
(v) $\text{C}{{\text{H}}_{\text{3}}}\text{CH(C}{{\text{H}}_{\text{3}}}\text{)C}{{\text{H}}_{\text{2}}}\text{C(C}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{2}}}\text{COC}{{\text{H}}_{\text{3}}}$
Ans: The IUPAC name of the given compound is 3,3,5-Trimethylhexan-2-one.
(vi) ${{\text{(C}{{\text{H}}_{\text{3}}}\text{)}}_{\text{3}}}\text{CC}{{\text{H}}_{\text{2}}}\text{COOH}$
Ans: The IUPAC name of the given compound is 3, 3-Dimethylbutanoic acid.
(vii) $\text{OHC}{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{4}}}\text{CHO-p}$
Ans: The IUPAC name of the given compound is Benzene-1,4-dicarbaldehyde.
3. Draw the structures of the following compounds:
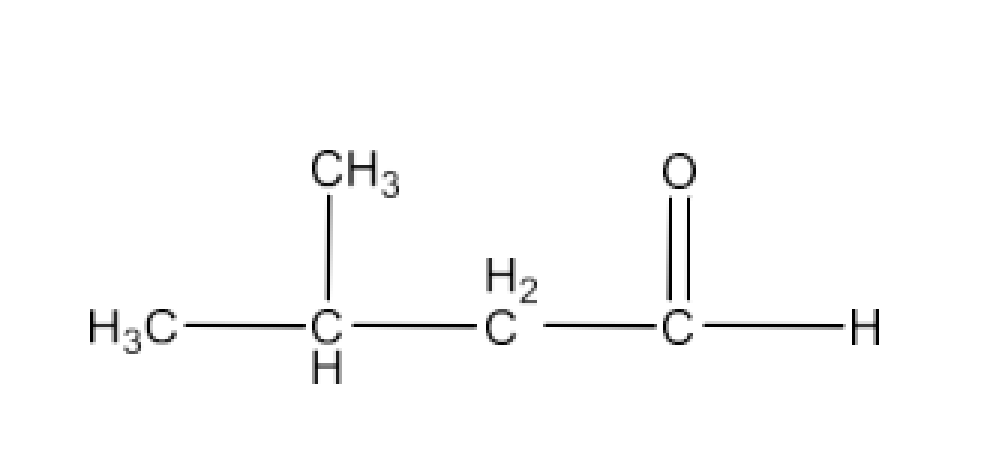
(i) 3-Methylbutanal
Ans: The structure of 3-Methylbutanal is given below:
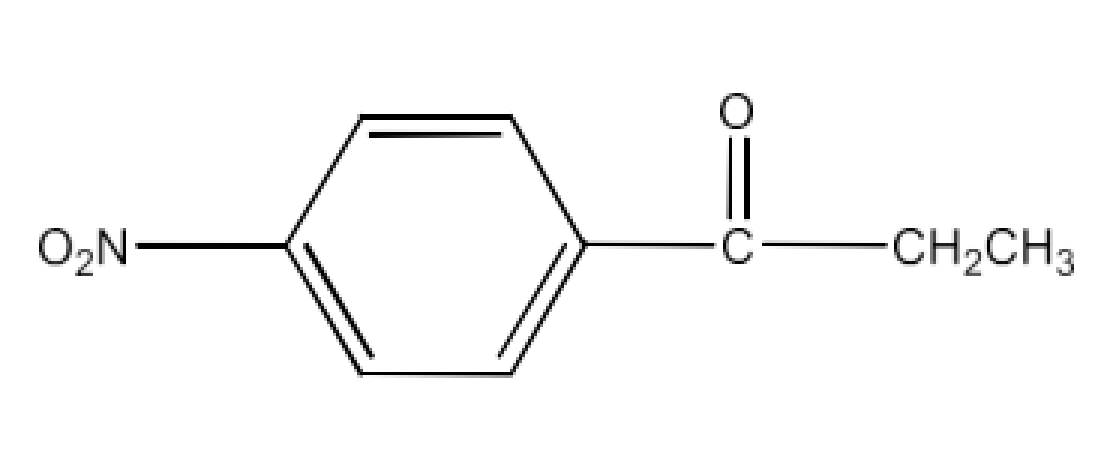
(ii) p-Nitropropiophenone
Ans: The structure of p-Nitropropiophenone is given below:
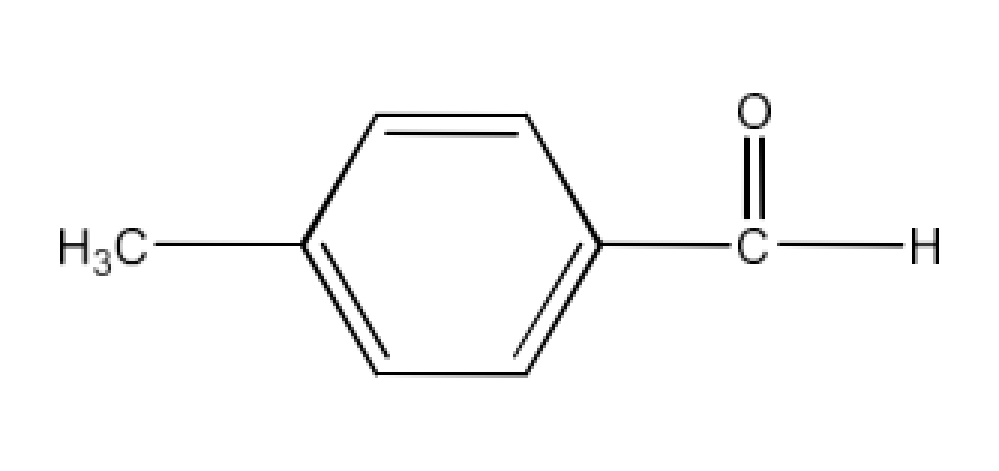
(iii) p-Methylbenzaldehyde
Ans: The structure of p-Methylbenzaldehyde is given below:
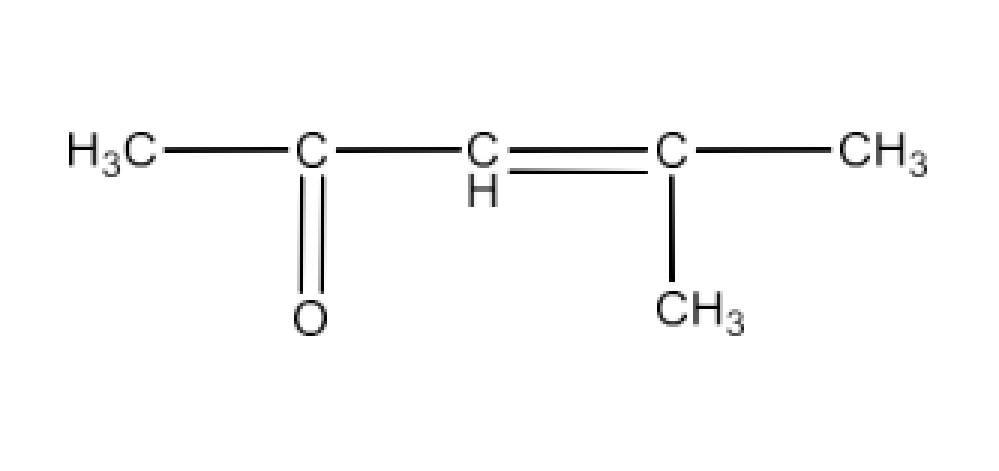
(iv) 4-Methylpent-3-en-2-one
Ans: The structure of 4-Methykpent-3-en-2-one is given below:
(v) 4-Chloropentan-2-one
Ans: The structure of 4-Chloropentan-2-one is given below:
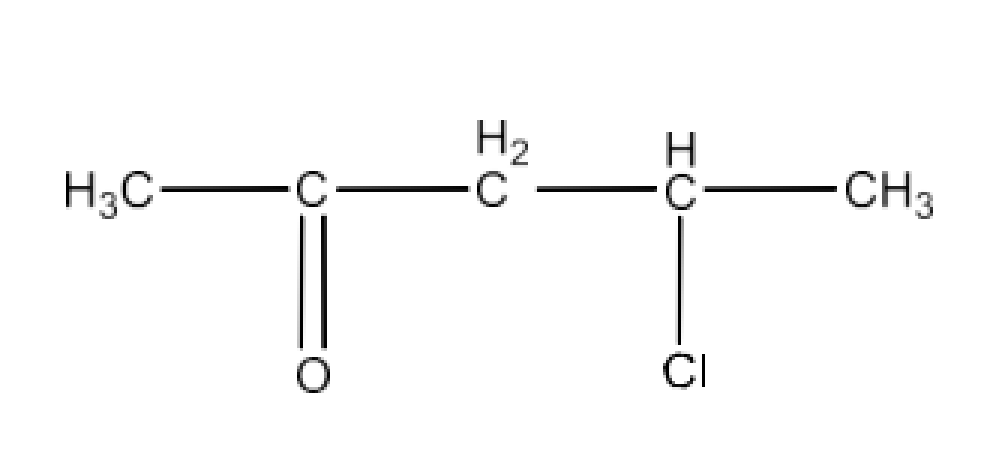
(vi) 3-Bromo-4-phenylpentanoic acid
Ans: The structure of 3-Bromo-4-phenylpentanoic acid is given below:
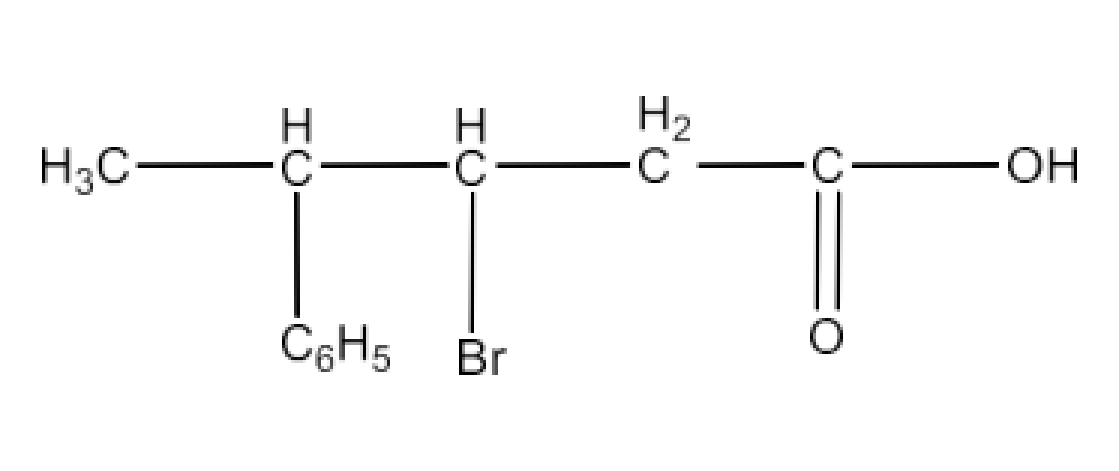
(vii) p,p’-Dihydroxybenzophenone
Ans: The structure of p,p’-Dihydroxybenzophenone is given below:
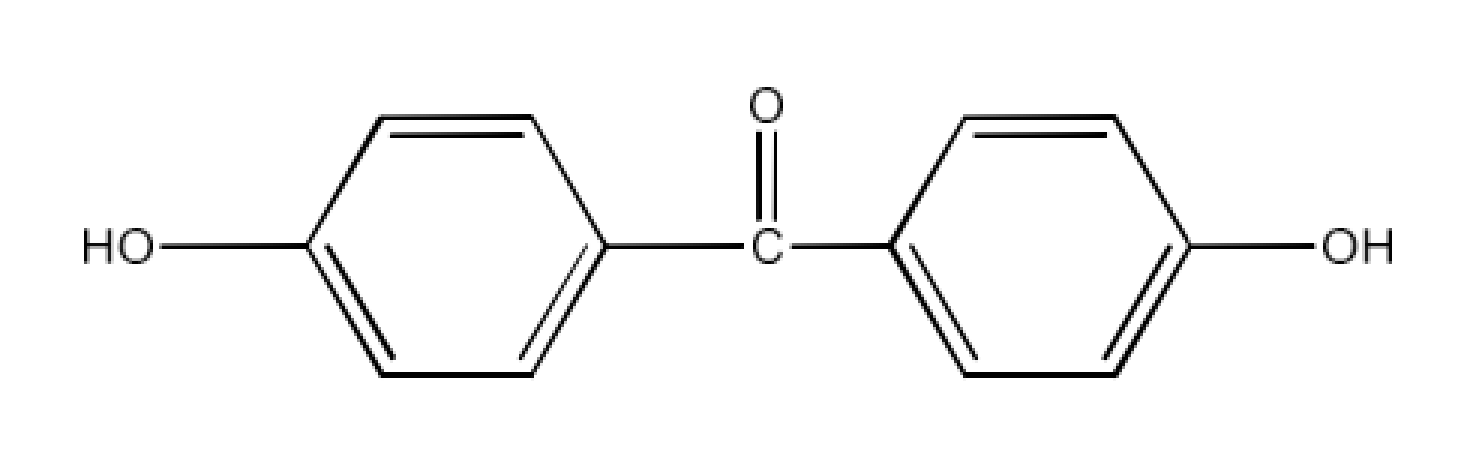
(viii) Hex-2-en-4-ynoic acid
Ans: The structure of Hex-2-en-4-ynoic acid is given below:
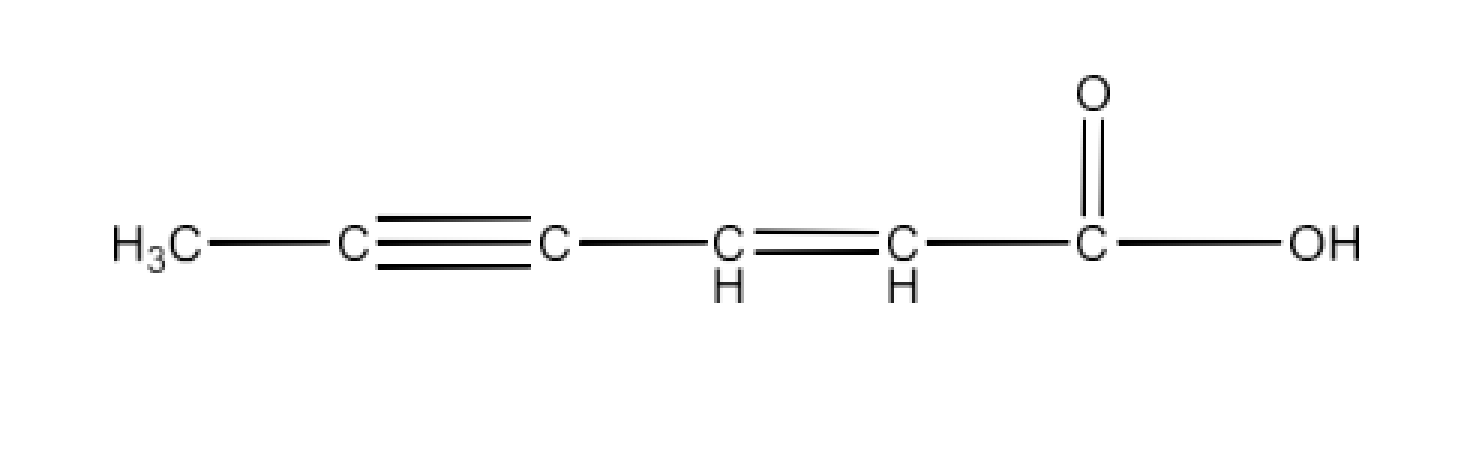
4. Write the IUPAC names of the following ketones and aldehydes. Whenever possible, give also common names.
(i) $\text{C}{{\text{H}}_{\text{3}}}\text{CO(C}{{\text{H}}_{\text{2}}}{{\text{)}}_{\text{4}}}\text{C}{{\text{H}}_{\text{3}}}$
Ans: The IUPAC name of the given compound is Heptan-2-one and its common name is Methyl n-pentyl ketone.
(ii) $\text{C}{{\text{H}}_{\text{3}}}\text{C}{{\text{H}}_{\text{2}}}\text{CHBrC}{{\text{H}}_{\text{2}}}\text{CH(C}{{\text{H}}_{\text{3}}}\text{)CHO}$
Ans: The IUPAC name of the given compound is 4-Bromo-2-methylhexanal and its common name is $\text{ }\!\!\gamma\!\!\text{ -Bromo- }\!\!\alpha\!\!\text{ -methylcaproaldehyde}$
(iii) $\text{C}{{\text{H}}_{\text{3}}}{{\text{(C}{{\text{H}}_{\text{2}}}\text{)}}_{\text{5}}}\text{CHO}$
Ans: The IUPAC name of the given compound is Heptanal.
(iv) $\text{Ph-CH=CH-CHO}$
Ans: The IUPAC name of the given compound is 3-Phenylprop-2-enal and its common name is beta-phenylacrolein.
(v)
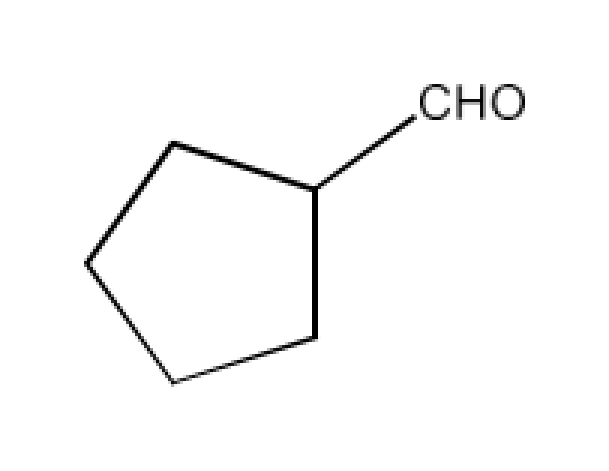
Ans: The IUPAC name of the given compound is Cyclopentanecarbaldehyde and its common name is also Cyclopentanecarbaldehyde.
(vi) PhCOPh
Ans: The IUPAC name of the given compound is Diphenylmetthanone and its common name is Benzophenone.
5. Draw structures of the following derivatives.
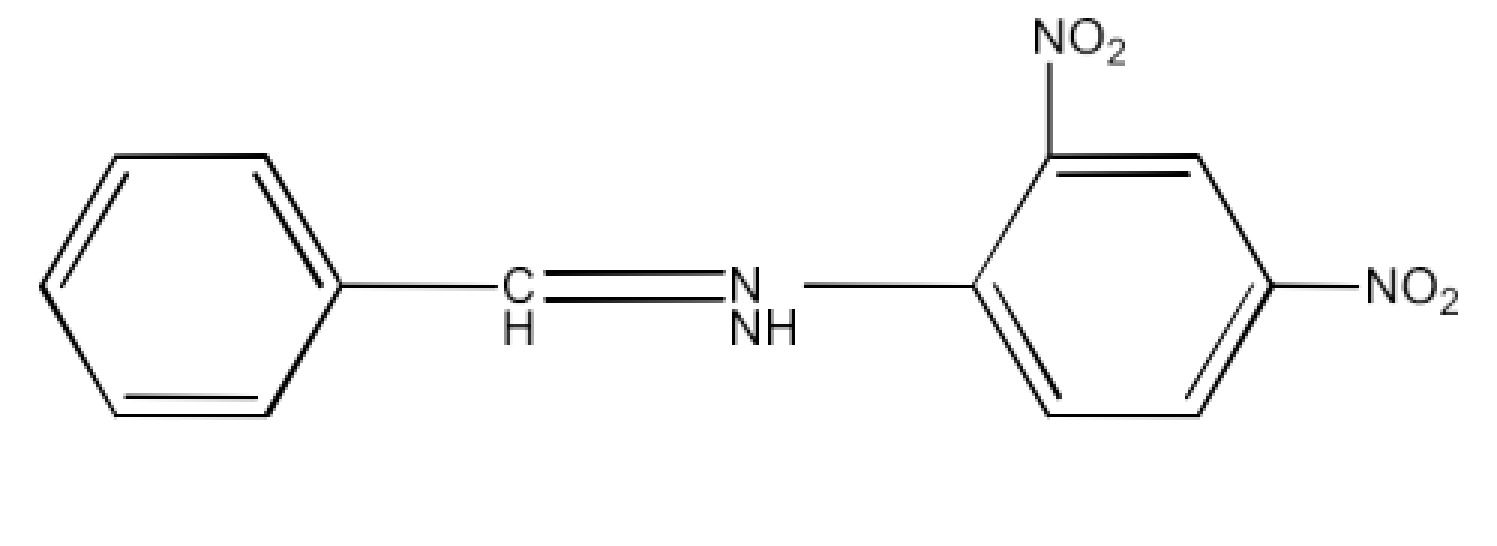
(i) The 2,4-dinitrophenylhydrazone of benzaldehyde
Ans: The structure of derivative of 2,4-dinitrophenylhydrazone of benzaldehyde will be:
(ii) Cyclopropanone oxime
Ans: The structure of derivative of Cyclopropanone oxime is given below:

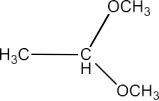
(iii) Acetaldehyde Dimethyl Acetal
Ans: The structure of derivative of Acetaldehyde Dimethyl Acetal is given below:
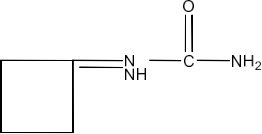
(iv) The semicarbazone of cyclobutanone
Ans: The structure of derivative of the semicarbazone of cyclobutanone is given below:
(v) The ethylene ketal of hexan-3-one
Ans: The structure of derivative of the ethylene ketal of hexan-3-one is given below:
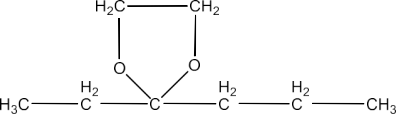
(vi) The methyl hemiacetal of formaldehyde
Ans: The structure of derivative of the methyl hemiacetal of formaldehyde is given below:
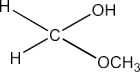
6. Predict the products formed when cyclohexanecarbaldehyde reacts with following reagents.
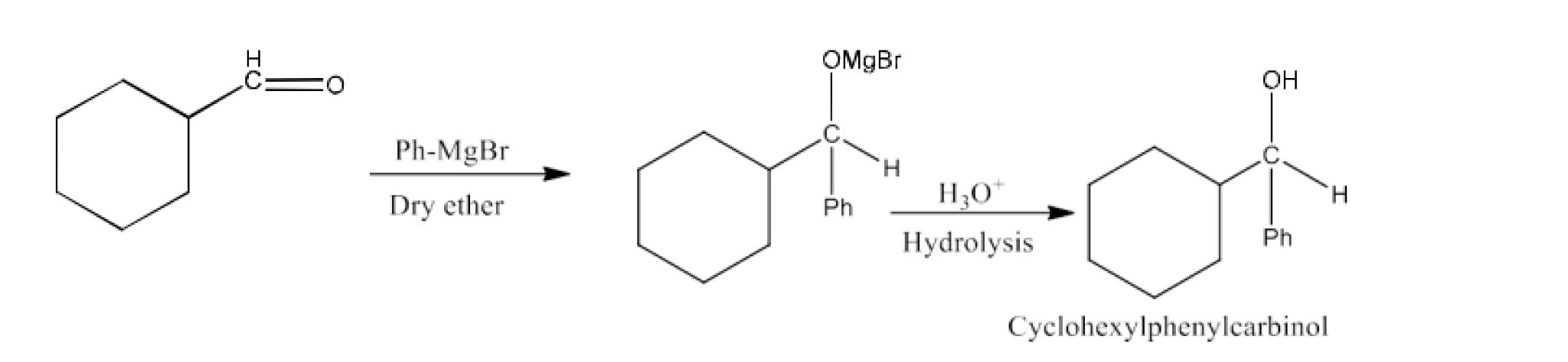
(i) PhMgBr and then ${{\text{H}}_{\text{3}}}{{\text{O}}^{\text{+}}}$
Ans: The product in this reaction will be Cyclohexyl Phenyl Carbinol. The reaction is given below:
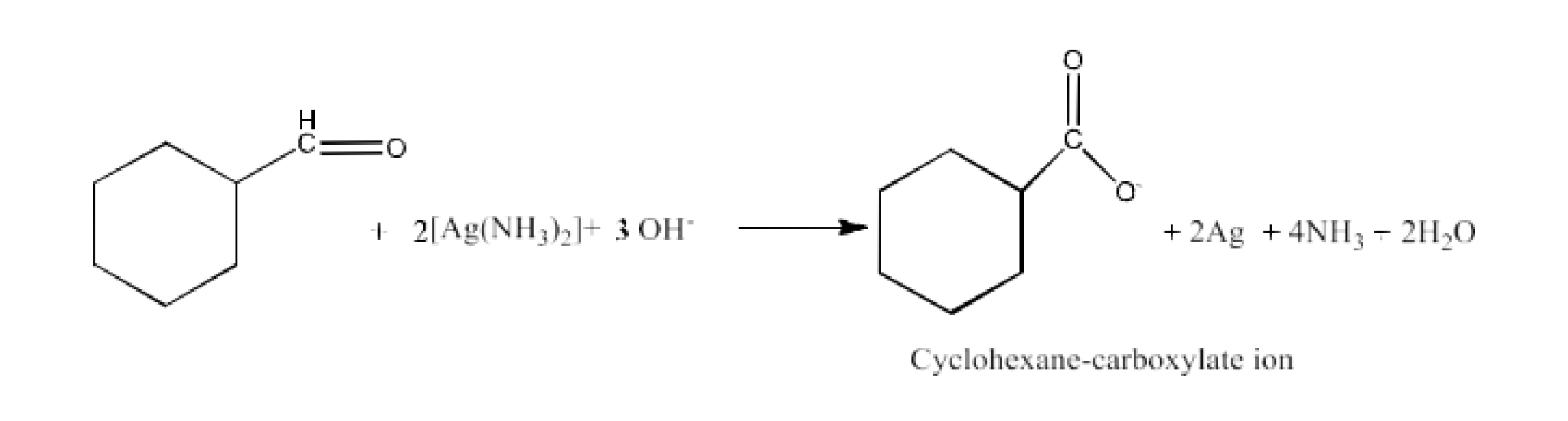
(ii) Tollens’ reagent
Ans: The product formed in this reaction is Cyclohexane-carboxylate ion. The reaction is given below:
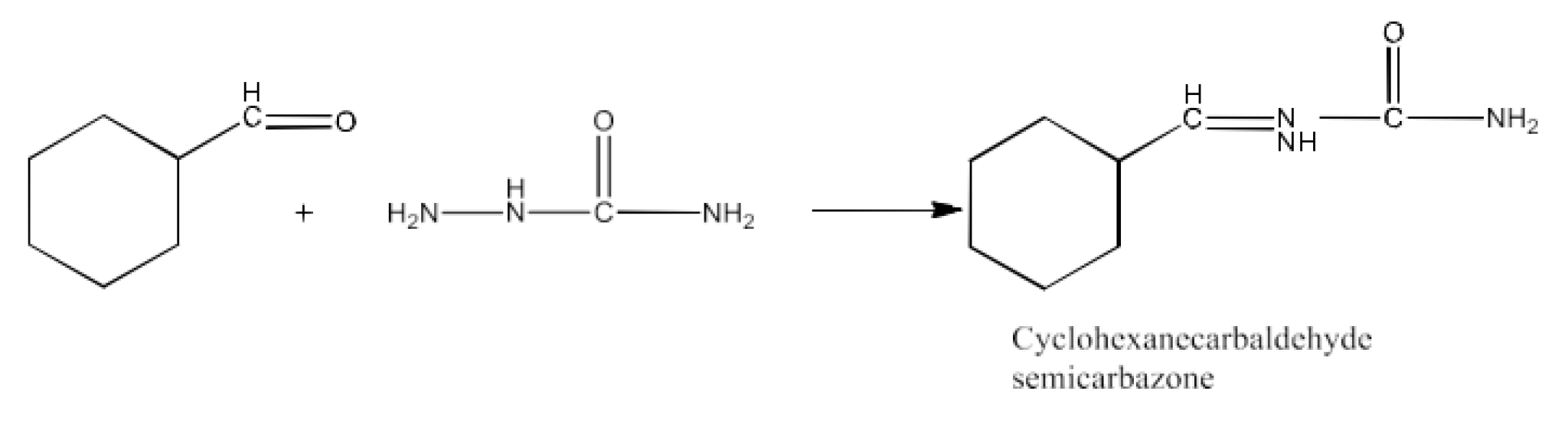
(iii) Semicarbazide and weak acid
Ans: The product formed in this reaction is Cyclohexanecarbaldehyde semicarbazone. The reaction is given below:
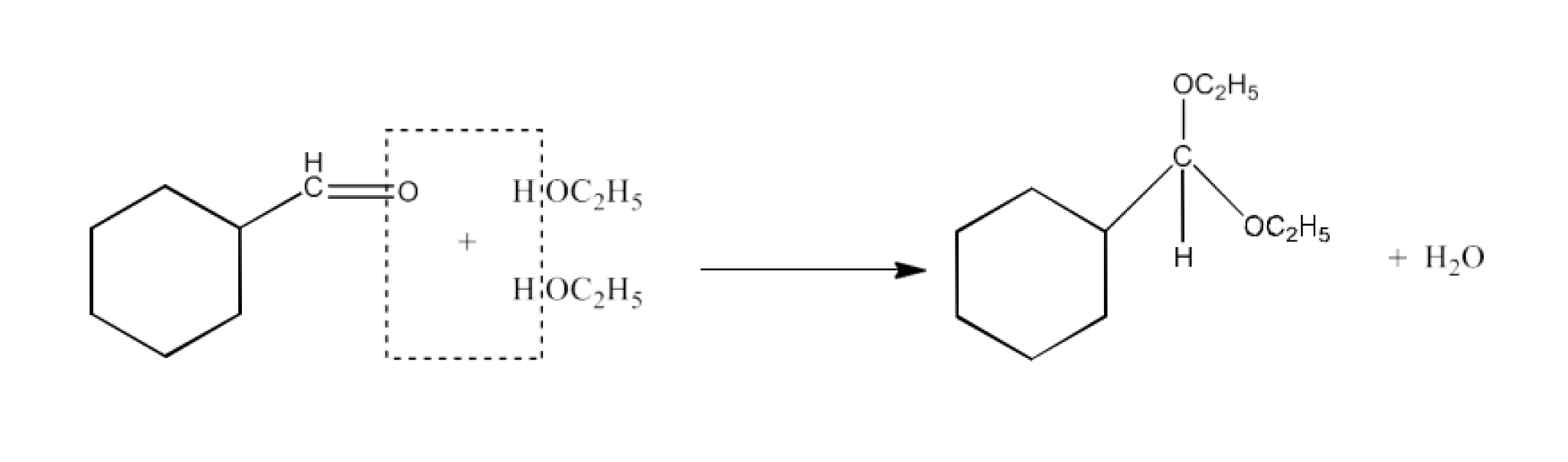
(iv) Excess ethanol and acid
Ans: The product formed in this reaction is Cyclohexanecarbaldehyde diethyl acetal. The reaction is given below:
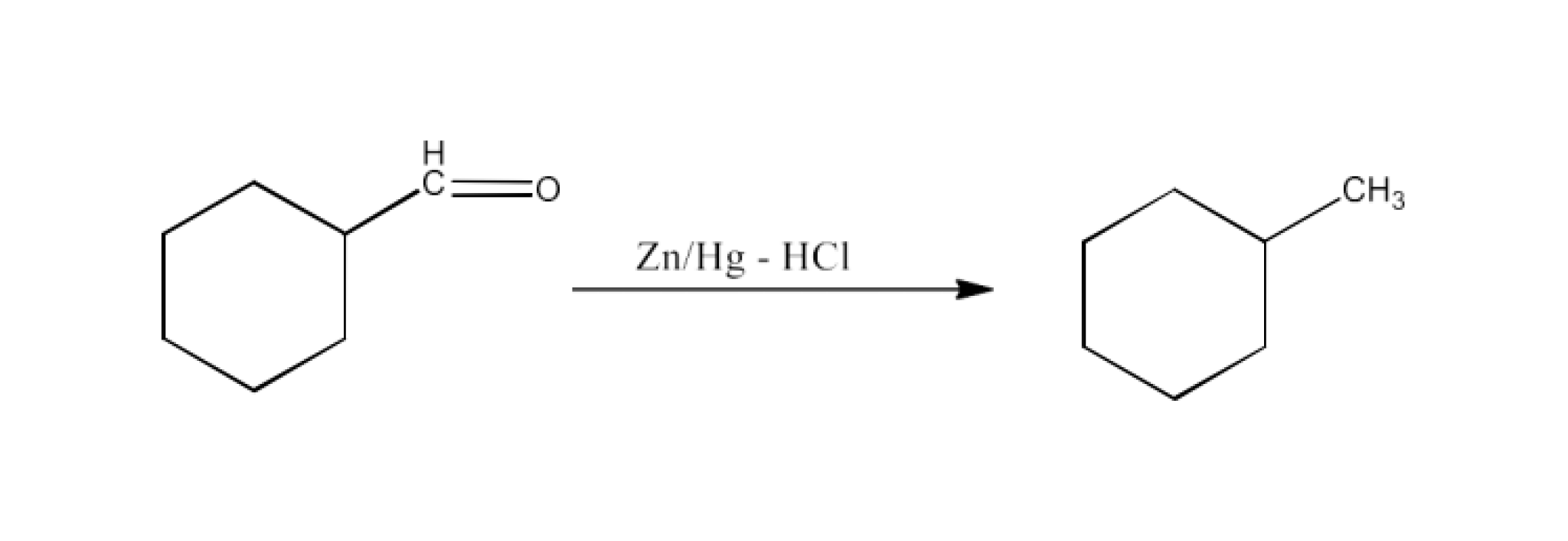
(v) Zinc amalgam and dilute hydrochloric acid.
Ans: The product formed in this reaction will be Methylcyclohexane. The reaction is given below:
7. Which of the following compounds would undergo aldol condensation, which the Cannizzaro reaction and which neither? Write the structures of the expected products of aldol condensation and Cannizzaro reaction.
Methanal
2-Methylpentanal
Benzaldehyde
Benzophenone
Cyclohexanone
1-Phenylpropanone
Phenylacetaldehyde
Butan-1-ol
2, 2-Dimethylbutanal
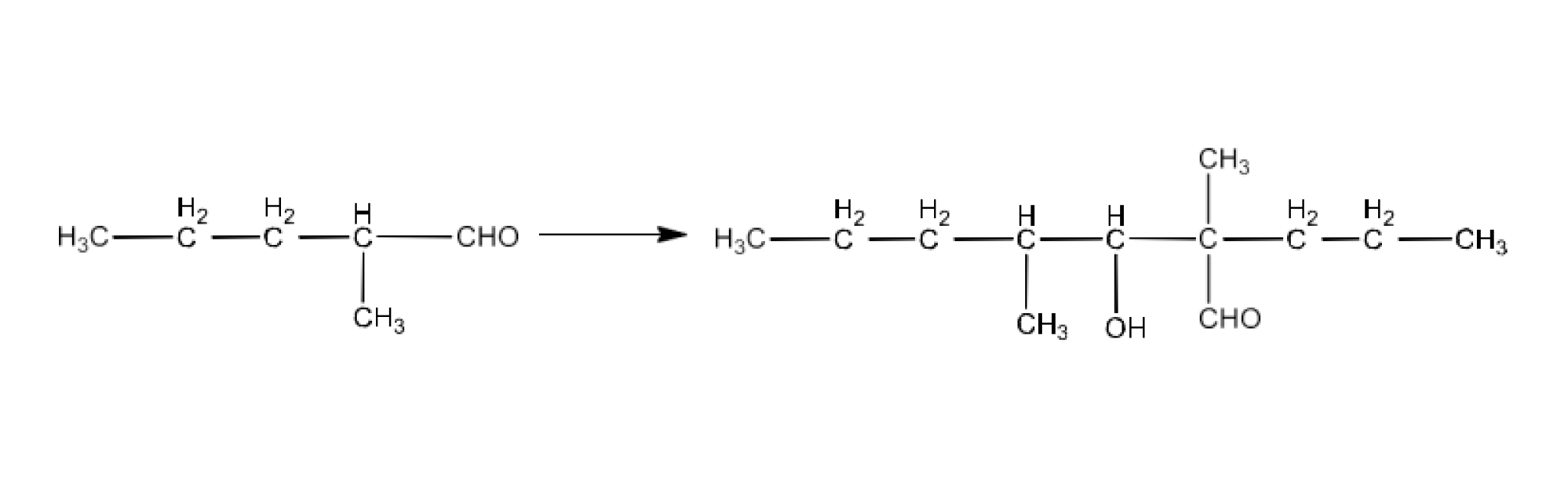
Ans: Aldehydes and ketones having at least one $\text{ }\!\!\alpha\!\!\text{ -hydrogen}$ undergo aldol condensation. The compounds (ii) 2−methylpentanal, (v) cyclohexanone, (vi) 1-phenylpropanone, and (vi) phenylacetaldehyde contain one or more $\text{ }\!\!\alpha\!\!\text{ -hydrogen}$atoms. Therefore, these undergo aldol condensation.
The product formed by the aldol condensation of 2-Methylpentanal is 3-Hydroxy-2,4-dimethyl-2-propylheptanal. The reaction is given below:
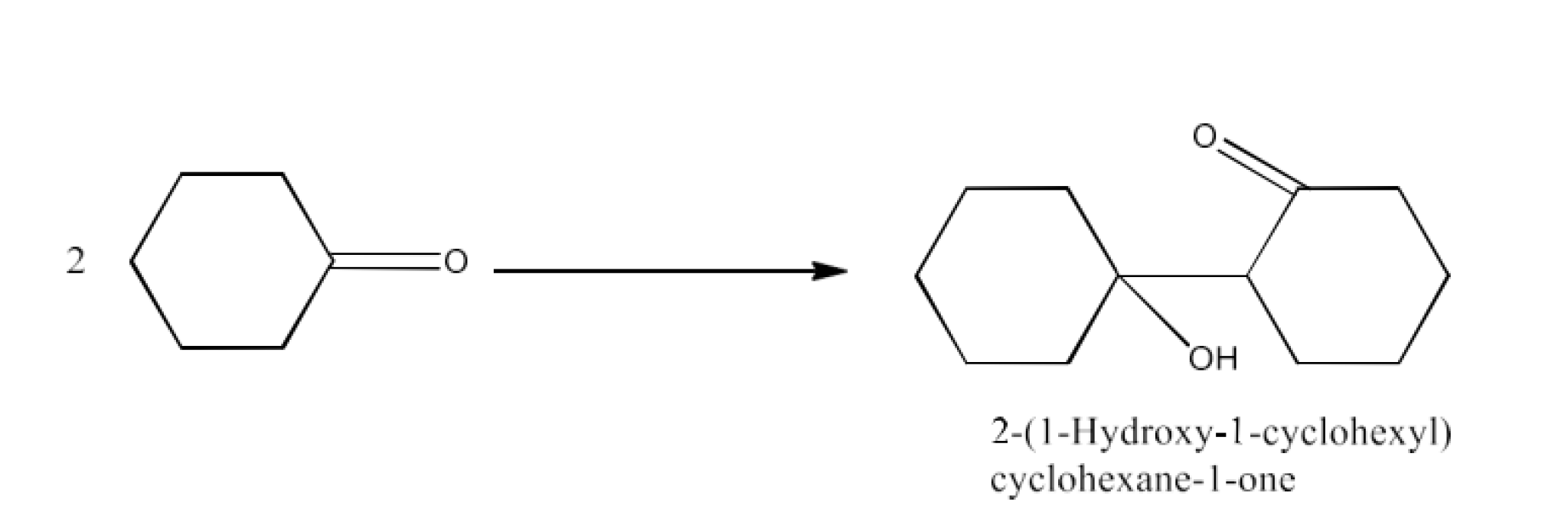
The product formed by the aldol condensation of cyclohexanone is 2-(1-Hydroxy-1-cyclohexyl)cyclohexane-1-one. The reaction is given below:
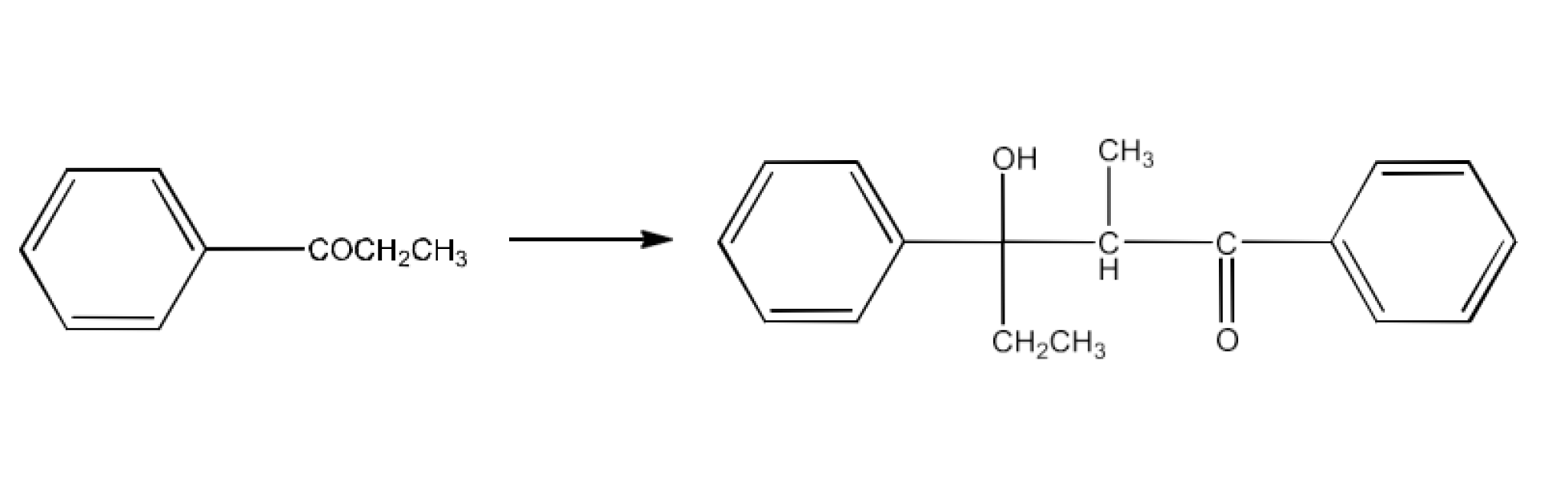
The product formed by the aldol condensation of 1-Phenylpropanone is 3-Hydroxy-2-methyl-1,3-diphenylpentan-1-one. The reaction is given below:
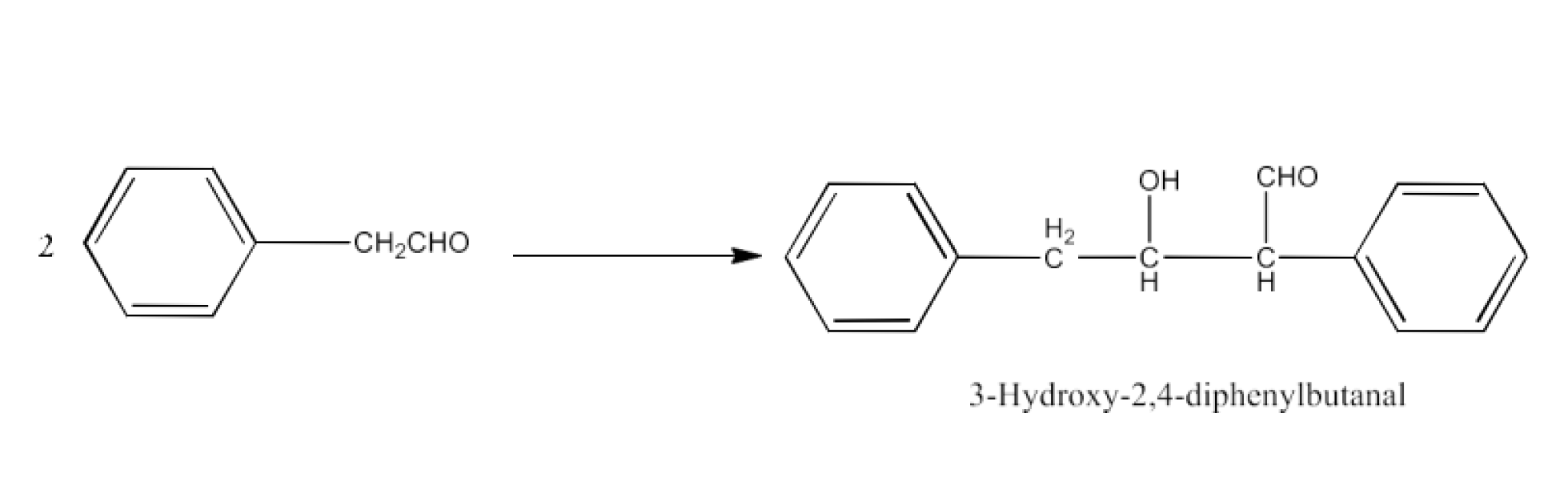
The product formed by the aldol condensation of Phenylacetyladehyde is 3-Hydroxy-2,4-diphenylbutanal. The reaction is given below:
Aldehydes having no $\text{ }\!\!\alpha\!\!\text{ -hydrogen}$ atoms undergo Cannizzaro reactions. The compounds (i) Methanal, (iii) Benzaldehyde, and (ix) 2, 2-dimethylbutanal do not have any $\text{ }\!\!\alpha\!\!\text{ -hydrogen}$.Therefore, these undergo cannizzaro reactions.
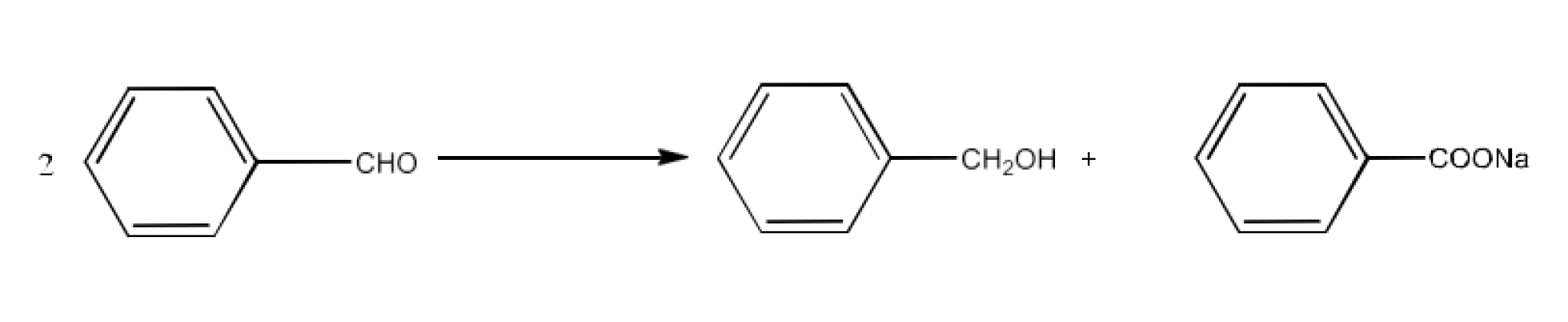
The products formed by the Cannizaro reaction of methanal are methanol and sodium methanoate. The reaction is given below:
\[\text{2 HCHO }\xrightarrow{\text{conc}\text{. NaOH}}\text{ C}{{\text{H}}_{\text{3}}}\text{OH + HCOONa}\]
The products formed by the Cannizaro reaction of benzaldehyde are benzyl alcohol and sodium benzoate. The reaction is given below:
The products formed by the Cannizaro reaction of 2,2-Dimethylbutanol are benzyl alcohol and sodium benzoate. The reaction is given below:
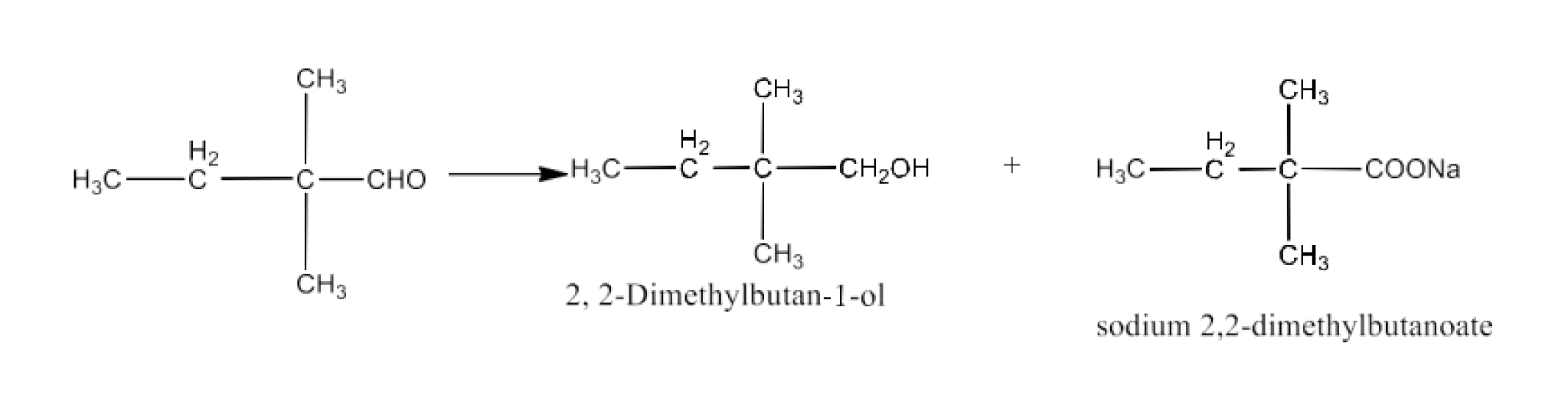
Compound (iv) Benzophenone is a ketone having no α-hydrogen atom and compound (viii) Butan-1-ol is an alcohol. Hence, these compounds do not undergo either aldol condensation or cannizzaro reactions.
8. How will you convert ethanal into the following compounds?
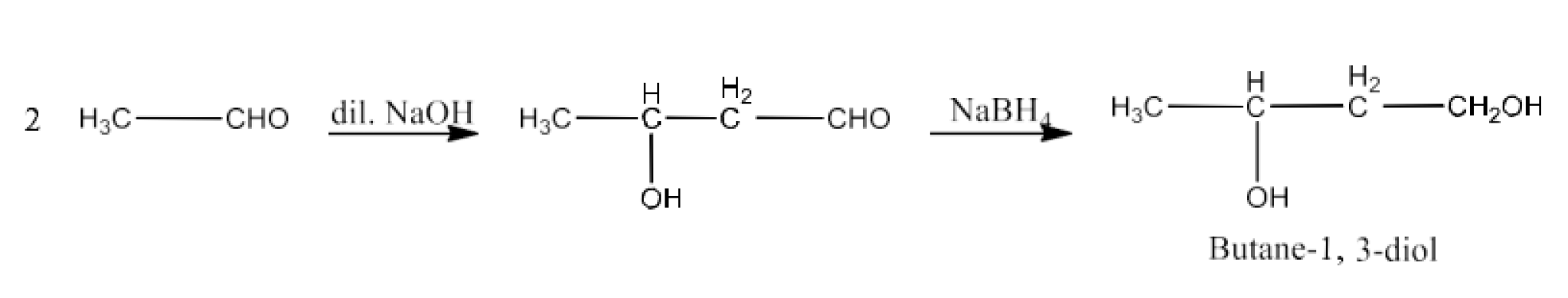
Butane-1, 3-diol
Ans: When ethanal reacts with dilute sodium hydroxide to form 3-Hydroxybutanal. Now, 3-Hydroxybutanal reacts with $\text{NaB}{{\text{H}}_{\text{4}}}$ to form butane-1, 3-diol. The reaction is given below:
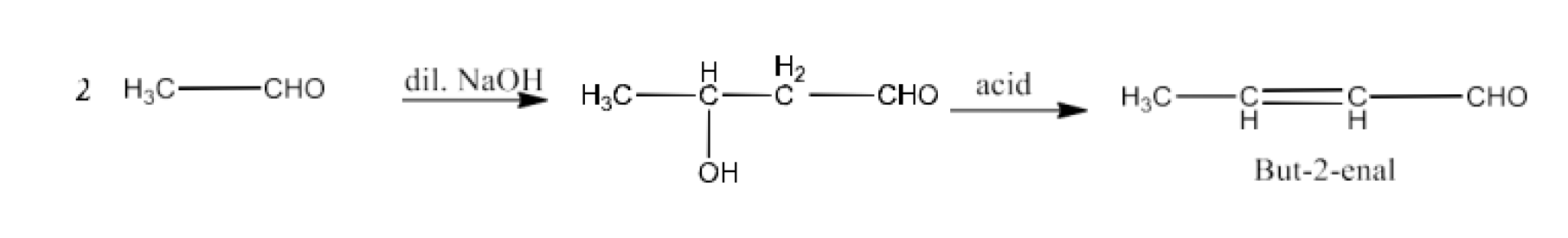
But-2-enal
Ans: When ethanal reacts with dilute sodium hydroxide to form 3-Hydroxybutanal. Now, 3-Hydroxybutanal reacts with acid to form But-2-enal. The reaction is given below:
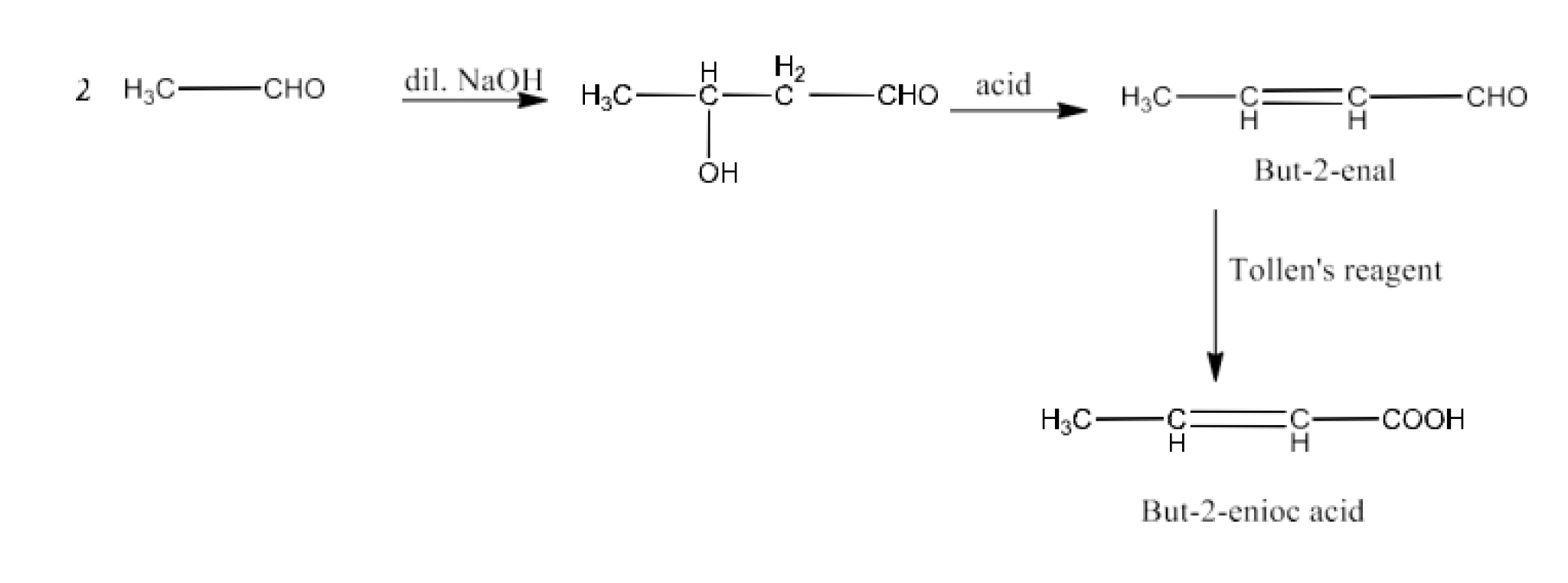
But-2-enoic acid
Ans: When ethanal reacts with dilute sodium hydroxide to form 3-Hydroxybutanal. Now, 3-Hydroxybutanal reacts with acid to form But-2-enal. Now, but-2-enal reacts with Tollen’s reagent to But-2-enoic acid. The reaction is given below:
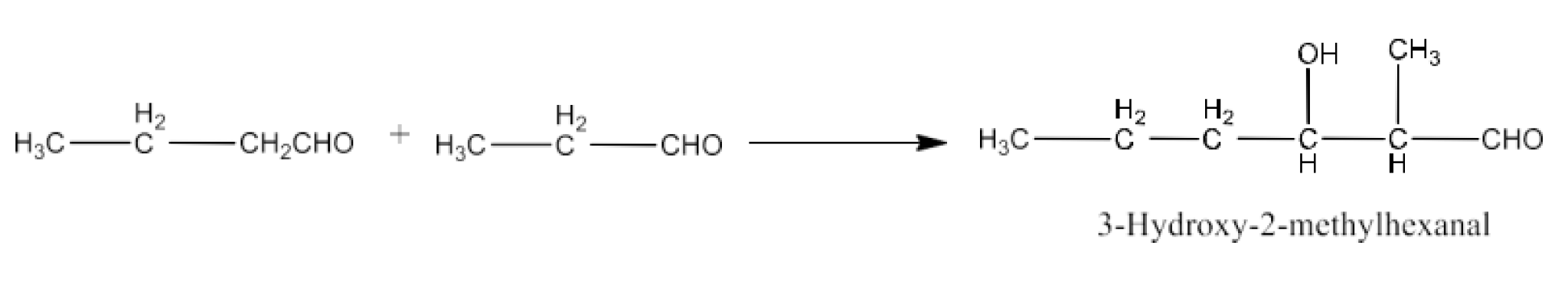
9. Write structural formulas and names of four possible aldol condensation products from propanal and butanal. In each case, indicate which aldehyde acts as nucleophile and which as electrophile.
Ans:
Taking propanal as nucleophile as well as electrophile. The product will be 3-Hydroxy-2-methylpentanal. The reaction is given below:
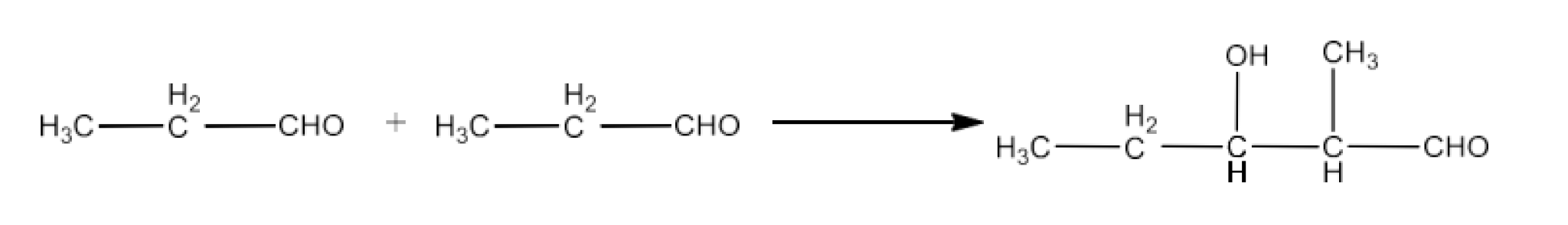
Taking propanal as electrophile and butanal as nucleophile. The product will be 2-Ethyl-3-hydroxypentanal. The reaction is given below:
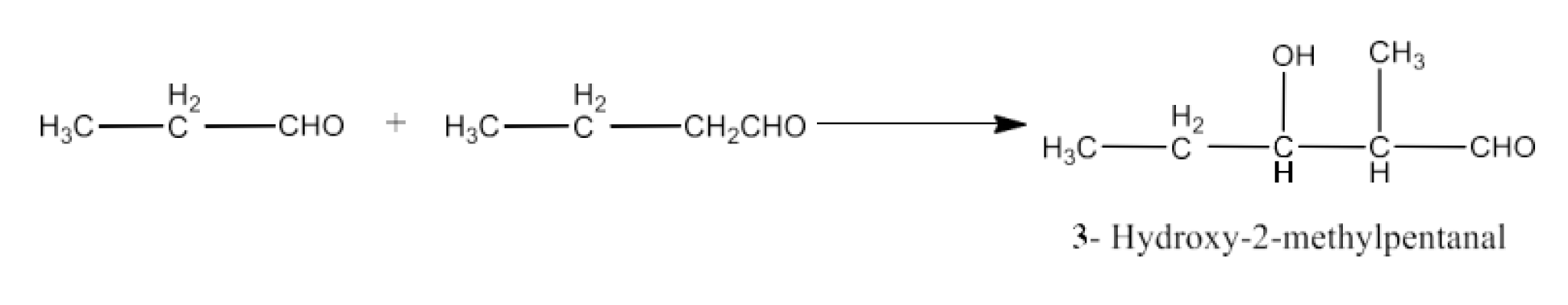
Taking butanal as electrophile and propanal as nucleophile. The product will be 3-Hydroxy-2-methylhexanal. The reaction is given below:
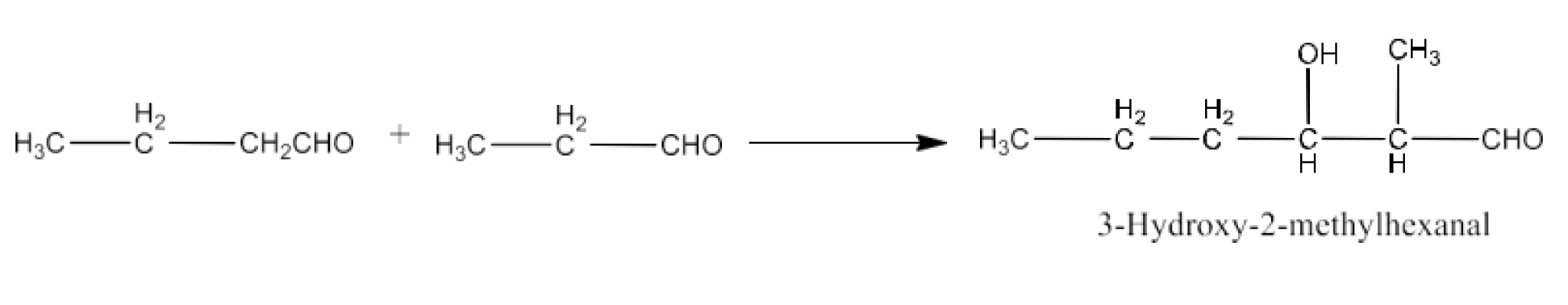
Taking butanal as nucleophile as well as electrophile. The product will be 2-Ethyl-3-hydroxyhexanal. The reaction is given below:
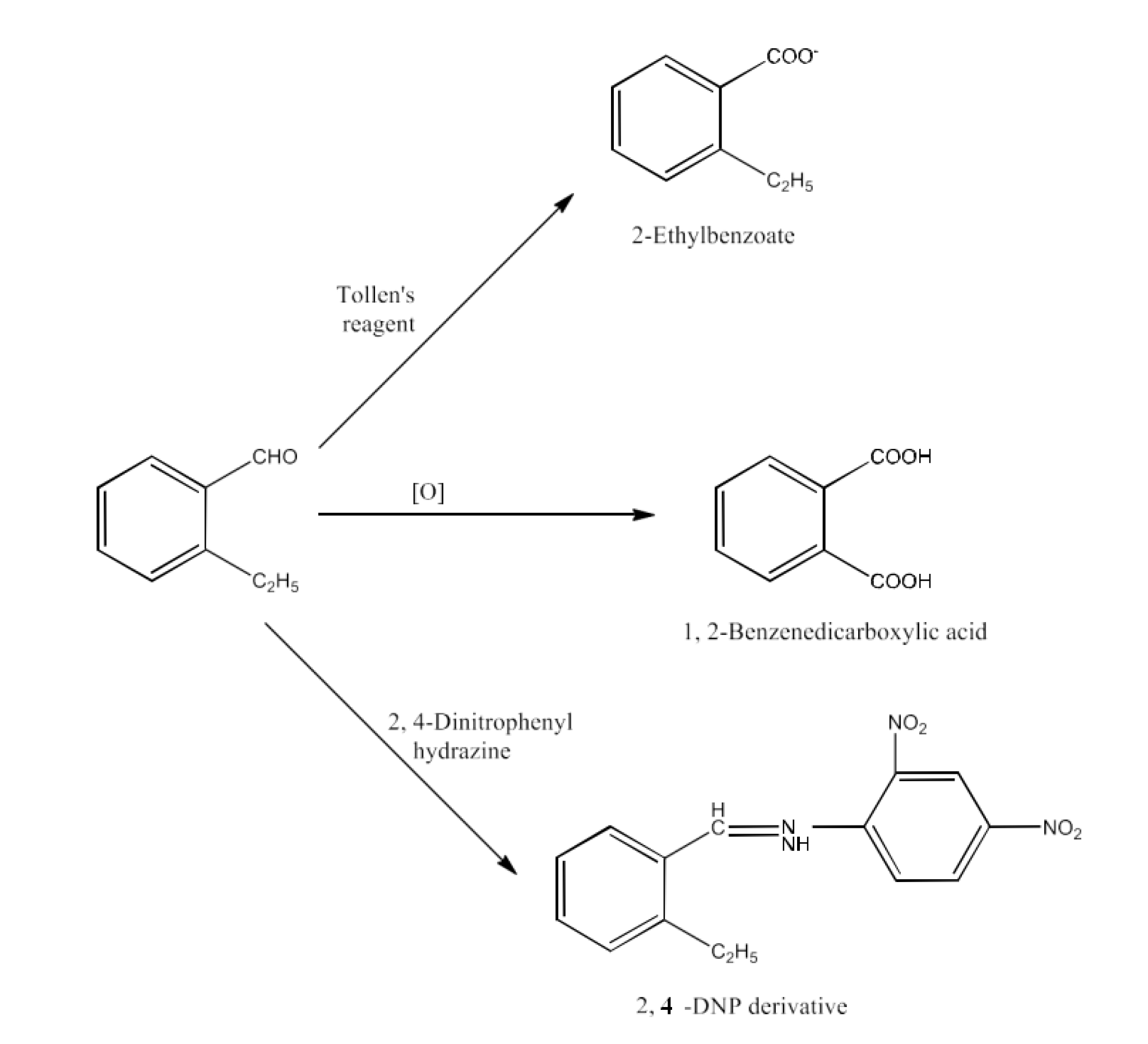
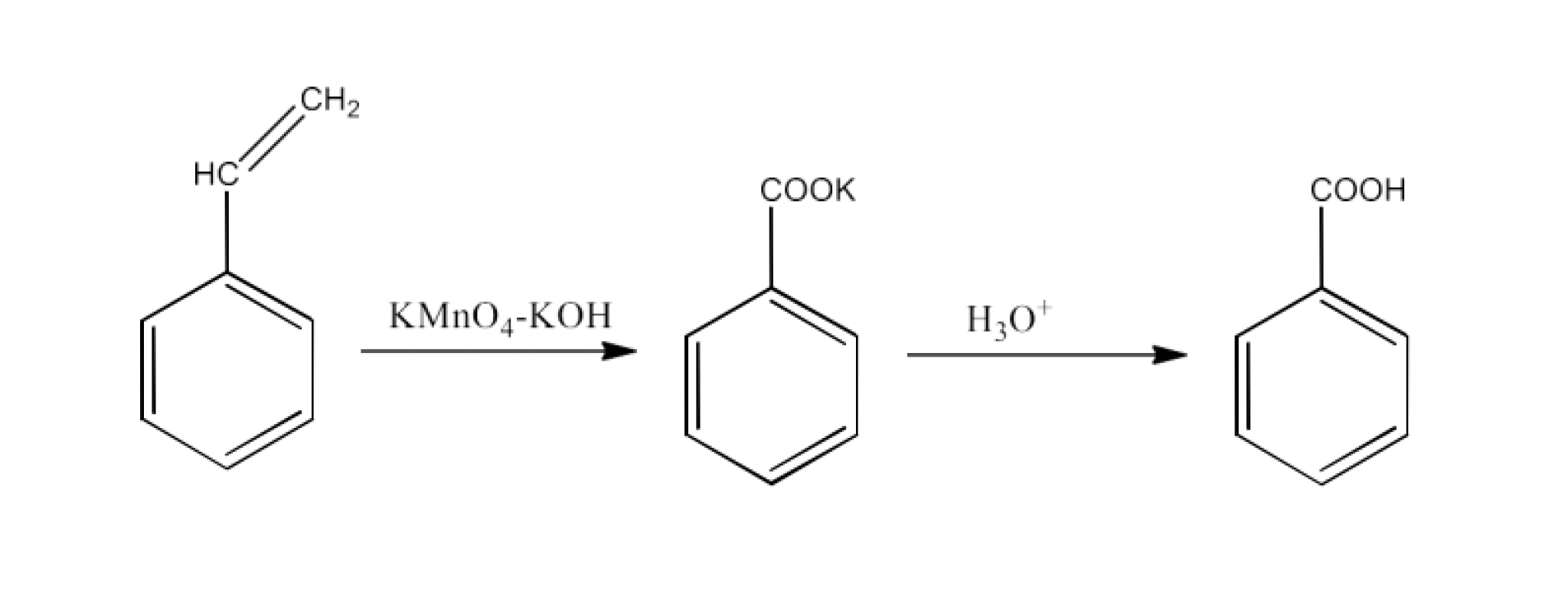
10. An organic compound with the molecular formula ${{\text{C}}_{\text{9}}}{{\text{H}}_{\text{10}}}\text{O}$ forms 2, 4-DNP derivative, reduces Tollens’ reagent and undergoes Cannizzaro reaction. On vigorous oxidation, it gives 1, 2-benzenedicarboxylic acid. Identify the compound.
Ans: It is because the chemical (${{\text{C}}_{\text{9}}}{{\text{H}}_{\text{10}}}\text{O}$) generates a derivative of 2, 4-dnp and reduces the reagent of Tollen. The chemical must thus be an aldehyde. The product is again subjected to cannizzaro's reaction and 1,2-benzenedicarboxylic acid is provided by oxidation. The −CHO group is therefore immediately connected to a benzene ring and ortho-substituted for this benzaldehyde. Thus, 2-ethylbenzaldehyde is the chemical.
The reactions given in the questions are given below:
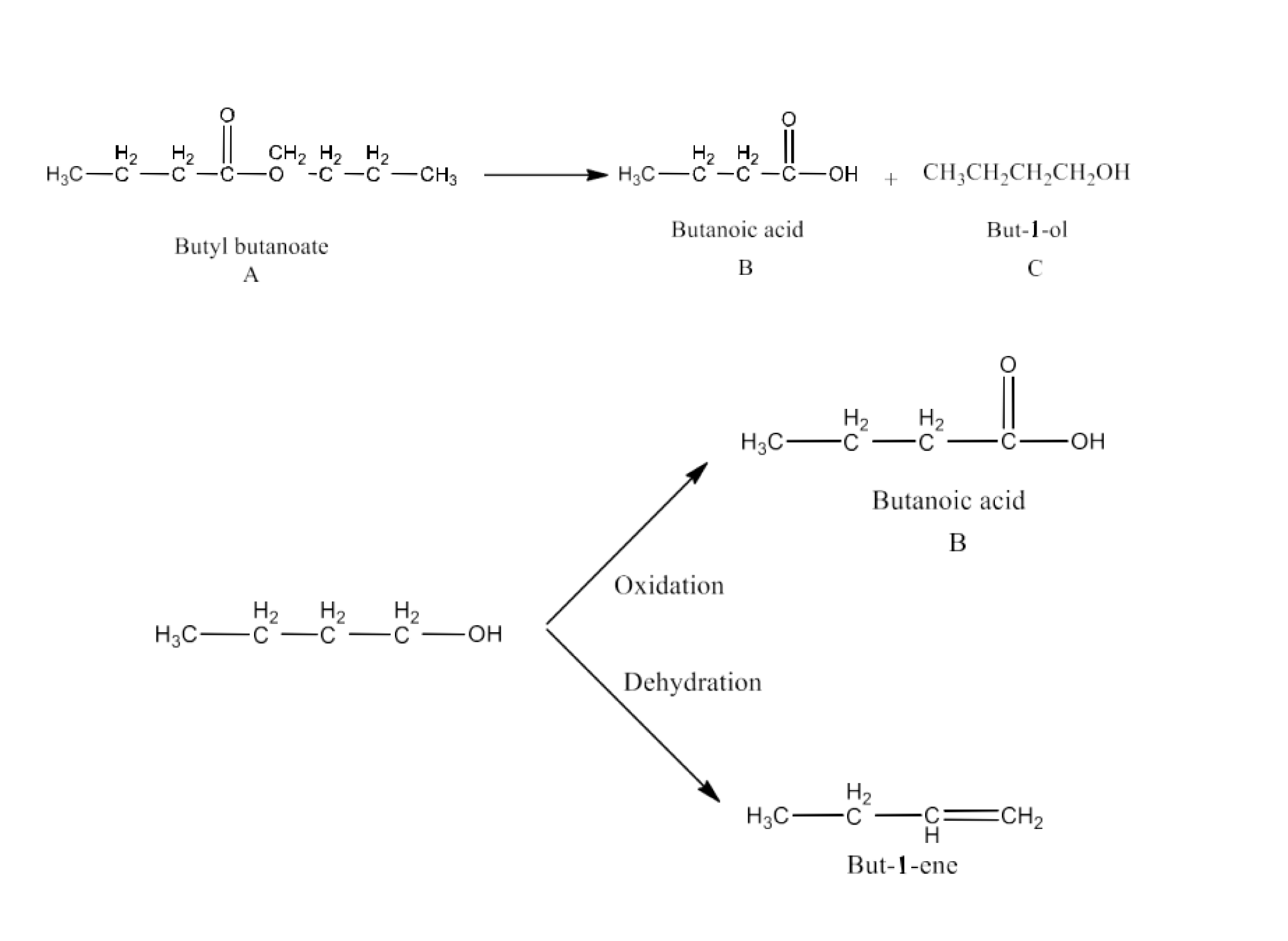
11. An organic compound (A) (molecular formula ${{\text{C}}_{\text{8}}}{{\text{H}}_{\text{16}}}{{\text{O}}_{\text{2}}}$) was hydrolyzed with dilute sulphuric acid to give a carboxylic acid (B) and an alcohol (C). Oxidation of (C) with chromic acid produced (B). (C) on dehydration gives but-1-ene.Write equations for the reactions involved.
Ans: A molecular molecular formula A molecule ${{\text{C}}_{\text{8}}}{{\text{H}}_{\text{16}}}{{\text{O}}_{\text{2}}}$ provides hydrolysis with dilute sulphuric acid carboxylic acid (B) and alcohol (C) Compound A therefore has to be an ester. Alcohol C also provides acid B for chromic acid oxidation. Therefore, the number of carbon atoms must be identical in B and C. Compound A has 8 carbon atoms, while 4 carbon atoms are included in each of B and C. Alcohol C again produces but-1-ene when it is dehydrated. C is thus straight and hence butane-1-ol. Butan-1-ol produces butanoic acid when oxidised. Therefore, butanoic acid is B acid. Thus, butyl butanoate is the molecular ester ${{\text{C}}_{\text{8}}}{{\text{H}}_{\text{16}}}{{\text{O}}_{\text{2}}}$.
All the reactions in questions are given below:
12. Arrange the following compounds in increasing order of their property as indicated:
Acetaldehyde, Acetone, Di-tert-butyl ketone, Methyl tert-butyl ketone (reactivity towards HCN)
Ans: When HCN interacts with a molecule, the attacking species is nucleophile, CN- Consequently, its reactivity to HCN diminishes as the negative charge of the compound rises. The +I effect rises in the given compounds as indicated below. The steric impediment also rises in this. It may be noticed.
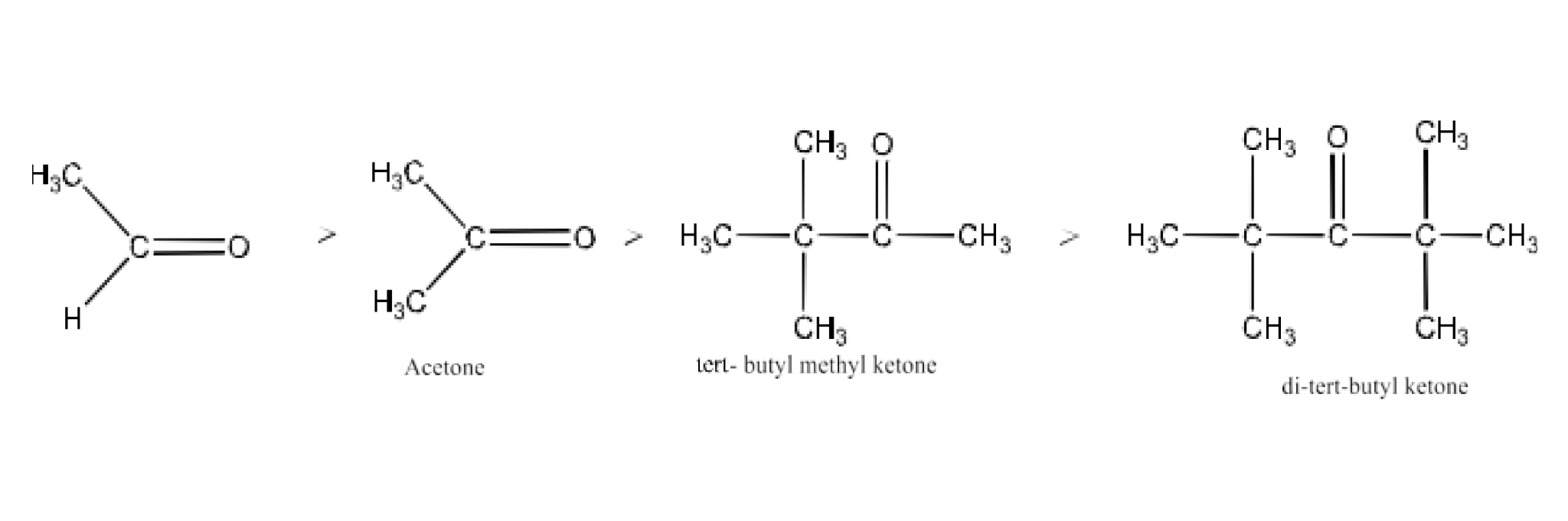
Hence, the given compounds can be arranged according to their increasing reactivities toward HCN as:
Di-tert-butyl ketone < Methyl tert-butyl ketone < Acetone < Acetaldehyde.
$\text{C}{{\text{H}}_{\text{3}}}\text{C}{{\text{H}}_{\text{2}}}\text{CH(Br)COOH, C}{{\text{H}}_{\text{3}}}\text{CH(Br)C}{{\text{H}}_{\text{2}}}\text{COOH, (C}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{2}}}\text{CHCOOH, }$
$\text{C}{{\text{H}}_{\text{3}}}\text{C}{{\text{H}}_{\text{2}}}\text{C}{{\text{H}}_{\text{2}}}\text{COOH (acid strength)}$
Ans: Carboxylic acids are negatively charged after losing a proton, as illustrated:
\[\text{R-COOH }\to \text{ R-CO}{{\text{O}}^{\text{-}}}\text{+}{{\text{H}}^{\text{+}}}\]
Now any group which helps stabilise the negative load will enhance the carboxylic ion's stability and hence improve the acid strength. This will reduce the strength of acids in groups with +I effect and raise the strength of acids in groups with −I effect. The group $\text{-C}{{\text{H}}_{\text{3}}}$ exhibits +I and $\text{B}{{\text{r}}^{\text{-}}}$ action in the following compounds. $\text{B}{{\text{r}}^{\text{-}}}$ containing acids are hence stronger. Now, +I is more than the n-propyl isopropyl group effect.
Therefore, ${{\text{(C}{{\text{H}}_{\text{3}}}\text{)}}_{\text{2}}}\text{CHCOOH}$ is a weaker acid than $\text{C}{{\text{H}}_{\text{3}}}\text{C}{{\text{H}}_{\text{2}}}\text{C}{{\text{H}}_{\text{2}}}\text{COOH}$.
Also, the –I effect grows weaker as distance increases. Hence, $\text{C}{{\text{H}}_{\text{3}}}\text{CH(Br)C}{{\text{H}}_{\text{2}}}\text{COOH}$is a weaker acid than $\text{C}{{\text{H}}_{\text{3}}}\text{C}{{\text{H}}_{\text{2}}}\text{CH(Br)COOH}$.
Hence, the order the strength is given below:
$\text{C}{{\text{H}}_{\text{3}}}\text{CH(C}{{\text{H}}_{\text{3}}}\text{)COOH}$ < $\text{C}{{\text{H}}_{\text{3}}}\text{C}{{\text{H}}_{\text{2}}}\text{C}{{\text{H}}_{\text{2}}}\text{COOH}$ < $\text{C}{{\text{H}}_{\text{3}}}\text{CH(Br)C}{{\text{H}}_{\text{2}}}\text{COOH}$ < $\text{C}{{\text{H}}_{\text{3}}}\text{C}{{\text{H}}_{\text{2}}}\text{CH(Br)COOH}$
Benzoic acid, 4-Nitrobenzoic acid, 3,4-Dinitrobenzoic acid, 4-Methoxybenzoic acid (acid strength)
Ans: As we saw in the past case, electron-donor groups reduce acid strengths whereas electron-donor groups enhance acid strengths. Benefits of 4-methoxybenzoic acid is a lesser acid than benzoic acid as a group of electron-donor. Nitro group is a retracting electron group and increases acid strength. As it includes 2 nitrogen groups of 3,4-dinitrobenzoic acid, it is a little stronger than 4-nitrobenzoic acid.
Hence, the strengths of the given acids increase as:
4-Methoxybenzoic acid < Benzoic acid < 4-Nitrobenzoic acid < 3,4-Dinitrobenzoic acid
13. Give simple chemical tests to distinguish between the following pairs of compounds.
Propanal and Propanone
Ans:
Tollen’s test
An aldehyde is Propanal. It reduces the reagent of Tollen. But, propanone being a ketone does not reduces Tollen’s reagent.
\[\text{C}{{\text{H}}_{\text{3}}}\text{C}{{\text{H}}_{\text{2}}}\text{CHO + 2 }\!\![\!\!\text{ Ag(N}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{2}}}{{\text{ }\!\!]\!\!\text{ }}^{\text{+}}}\text{+3O}{{\text{H}}^{\text{-}}}\text{ }\to \text{ C}{{\text{H}}_{\text{3}}}\text{C}{{\text{H}}_{\text{2}}}\text{CO}{{\text{O}}^{\text{-}}}\text{+Ag}\downarrow \text{+4N}{{\text{H}}_{\text{3}}}\text{+2}{{\text{H}}_{\text{2}}}\text{O}\]
Fehling’s test
Aldehydes are answering the test of Fehling, while ketones are not. Aldehyde-propanal converts the solution to a red-brown Cu2O precipitate, although propanone does not.
\[\text{C}{{\text{H}}_{\text{3}}}\text{C}{{\text{H}}_{\text{2}}}\text{CHO + 2C}{{\text{u}}^{\text{2+}}}\text{+5O}{{\text{H}}^{\text{-}}}\text{ }\to \text{ C}{{\text{H}}_{\text{3}}}\text{C}{{\text{H}}_{\text{2}}}\text{CO}{{\text{O}}^{\text{-}}}\text{+C}{{\text{u}}_{\text{2}}}\text{O+3}{{\text{H}}_{\text{2}}}\text{O}\]
Iodoform test
As one methyl group associated with the carbonyl carbon atom reacts to the iodoform test, with aldehydes and ketones. They are oxidised to yield iodoform using sodium hypoiodite (NaOI). Propanone is a methyl ketone, while propanal is not.
\[\text{C}{{\text{H}}_{\text{3}}}\text{COC}{{\text{H}}_{\text{3}}}\text{+3NaOI }\to \text{ C}{{\text{H}}_{\text{3}}}\text{COONa+CH}{{\text{I}}_{\text{3}}}\text{+2NaOH}\]
Acetophenone and Benzophenone
Ans: Iodoform test
Acetophenone will give the Iodoform test but benzophenone will not.
\[{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}\text{COC}{{\text{H}}_{\text{3}}}\text{+3NaOI }\to \text{ }{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}\text{COONa+CH}{{\text{I}}_{\text{3}}}\text{+2NaOH}\]
Phenol and Benzoic acid
Ans: Ferric chloride test
Phenol will give the violet coloration with neutral ferric chloride.
\[\text{6}{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}\text{OH+FeC}{{\text{l}}_{\text{3}}}\text{ }\to \text{ }\underset{\text{Violet color}}{\mathop{{{\text{ }\!\![\!\!\text{ Fe(O}{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}{{\text{)}}_{\text{6}}}\text{ }\!\!]\!\!\text{ }}^{\text{3-}}}}}\,\text{+3}{{\text{H}}^{\text{+}}}\text{+3C}{{\text{l}}^{\text{-}}}\]
Benzoic acid will give the buff coloration with neutral ferric chloride.
\[\text{3}{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}\text{COOH+FeC}{{\text{l}}_{\text{3}}}\text{ }\to \text{ }\underset{\text{buff color}}{\mathop{{{\text{ }\!\![\!\!\text{ Fe(OO}{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}{{\text{)}}_{3}}\text{ }\!\!]\!\!\text{ }}^{\text{3-}}}}}\,\text{+3}{{\text{H}}^{\text{+}}}\text{+3C}{{\text{l}}^{\text{-}}}\]
Benzoic acid and Ethyl benzoate
Ans: Sodium bicarbonate test
Benzoic acid will react with sodium bicarbonate and will evolve carbon dioxide.
\[{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}\text{COOH+NaHC}{{\text{O}}_{\text{3}}}\text{ }\to \text{ }{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}\text{COONa+C}{{\text{O}}_{\text{ 2}}}\text{+}{{\text{H}}_{\text{2}}}\text{O}\]
Pentan-2-one and Pentan-3-one
Ans: Iodoform test
Pentan-2-one will react to the Iodoform test but Pentan-3-one will not react.\[\text{C}{{\text{H}}_{\text{3}}}\text{C}{{\text{H}}_{\text{2}}}\text{C}{{\text{H}}_{\text{2}}}\text{COC}{{\text{H}}_{\text{3}}}\text{+3NaOI }\to \text{ C}{{\text{H}}_{\text{3}}}\text{C}{{\text{H}}_{\text{2}}}\text{C}{{\text{H}}_{\text{2}}}\text{COONa+CH}{{\text{I}}_{\text{3}}}\text{+2NaOH}\]
Benzaldehyde and Acetophenone
Ans: Iodoform test
Acetophenone will give the Iodoform test but benzaldehyde will not.
\[{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}\text{COC}{{\text{H}}_{\text{3}}}\text{+3NaOI }\to \text{ }{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}\text{COONa+CH}{{\text{I}}_{\text{3}}}\text{+2NaOH}\]
Ethanal and Propanal
Ans: Iodoform test
The iodoform test responds to aldehydes and ketones with a minimum of a methyl group associated with the carbonyl carbon atom. This test is followed by ethanal with one methyl group attached to the carbonyl atom. But propanal doesn't contain a carbonyl carbon dioxide-related methyl group and does not thus respond to this condition.
\[\text{C}{{\text{H}}_{\text{3}}}\text{CHO+3NaOI }\to \text{ HCOONa+CH}{{\text{I}}_{\text{3}}}\text{+2NaOH}\]
14. How will you prepare the following compounds from benzene? You may use any inorganic reagent and any organic reagent having not more than one carbon atom.
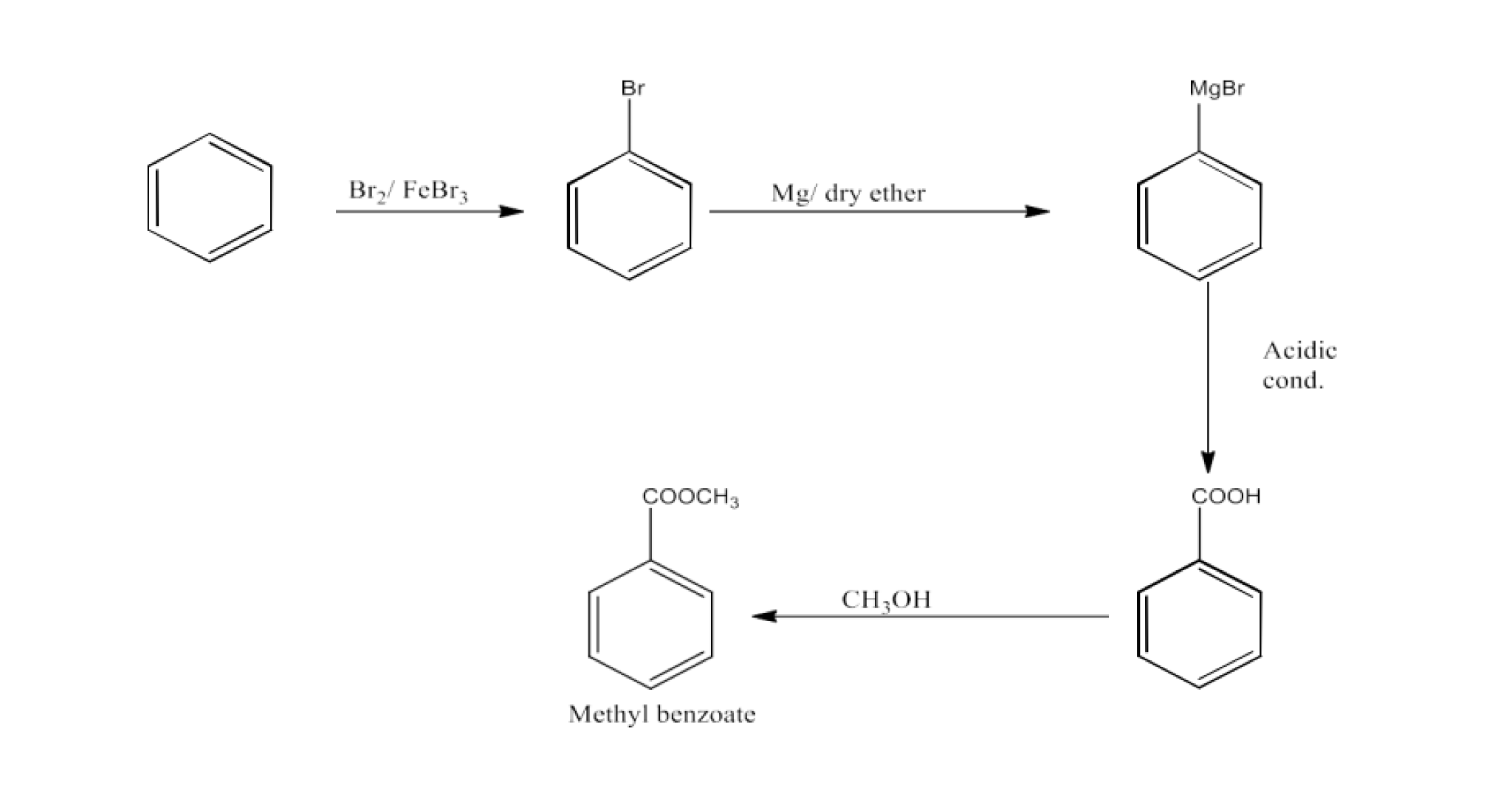
Methyl benzoate
Ans: Benzene will react with bromine to form Bromobenzene. This bromobenzene will be converted into benzoic acid. Now, this benzoic acid will be converted into Methyl benzoate. The reaction is given below:
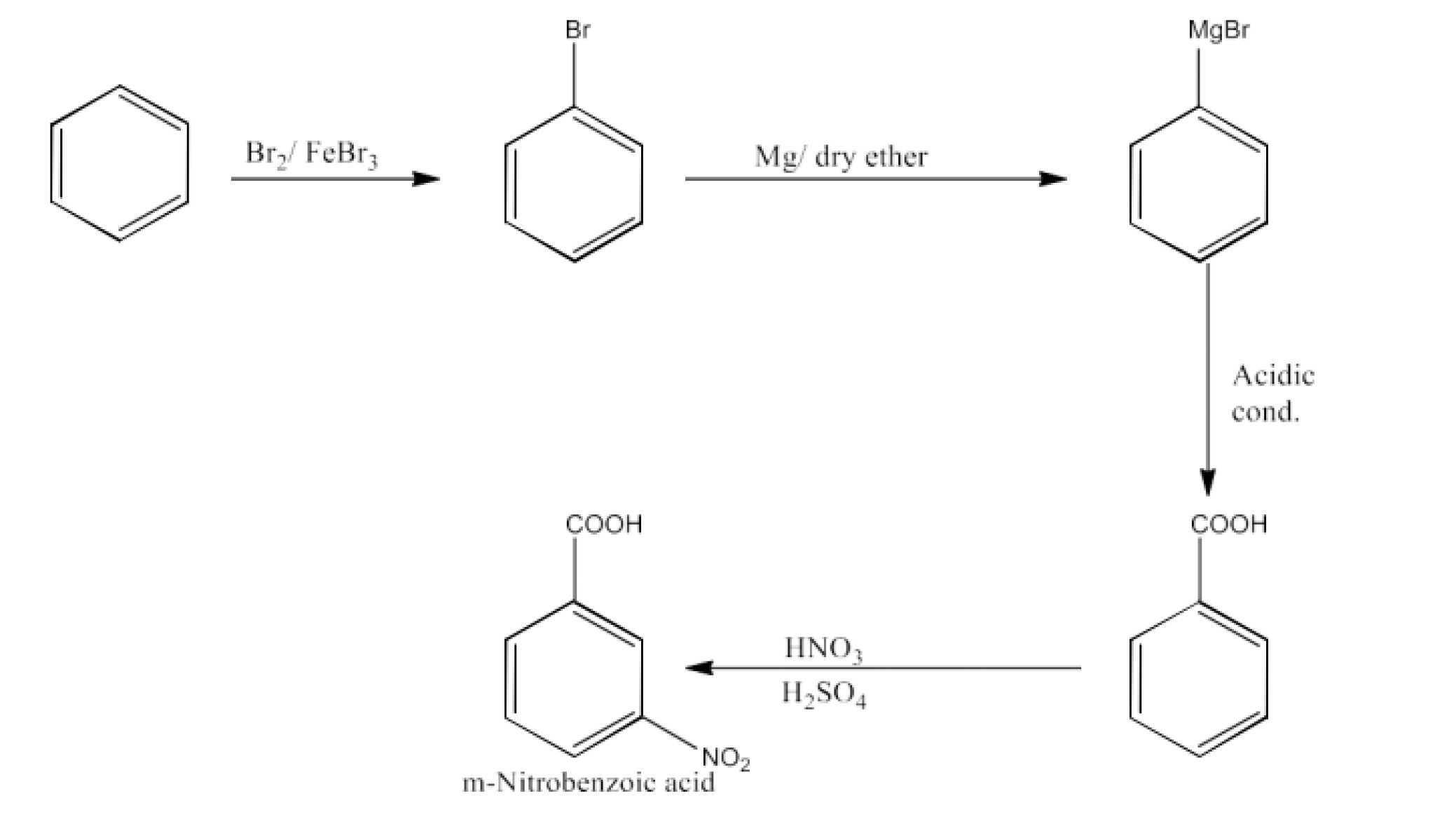
m-Nitrobenzoic acid.
Ans: Benzene will react with bromine to form Bromobenzene. This bromobenzene will be converted into benzoic acid. Now, this benzoic acid will be converted into m-Nitro benzoic acid. The reaction is given below:
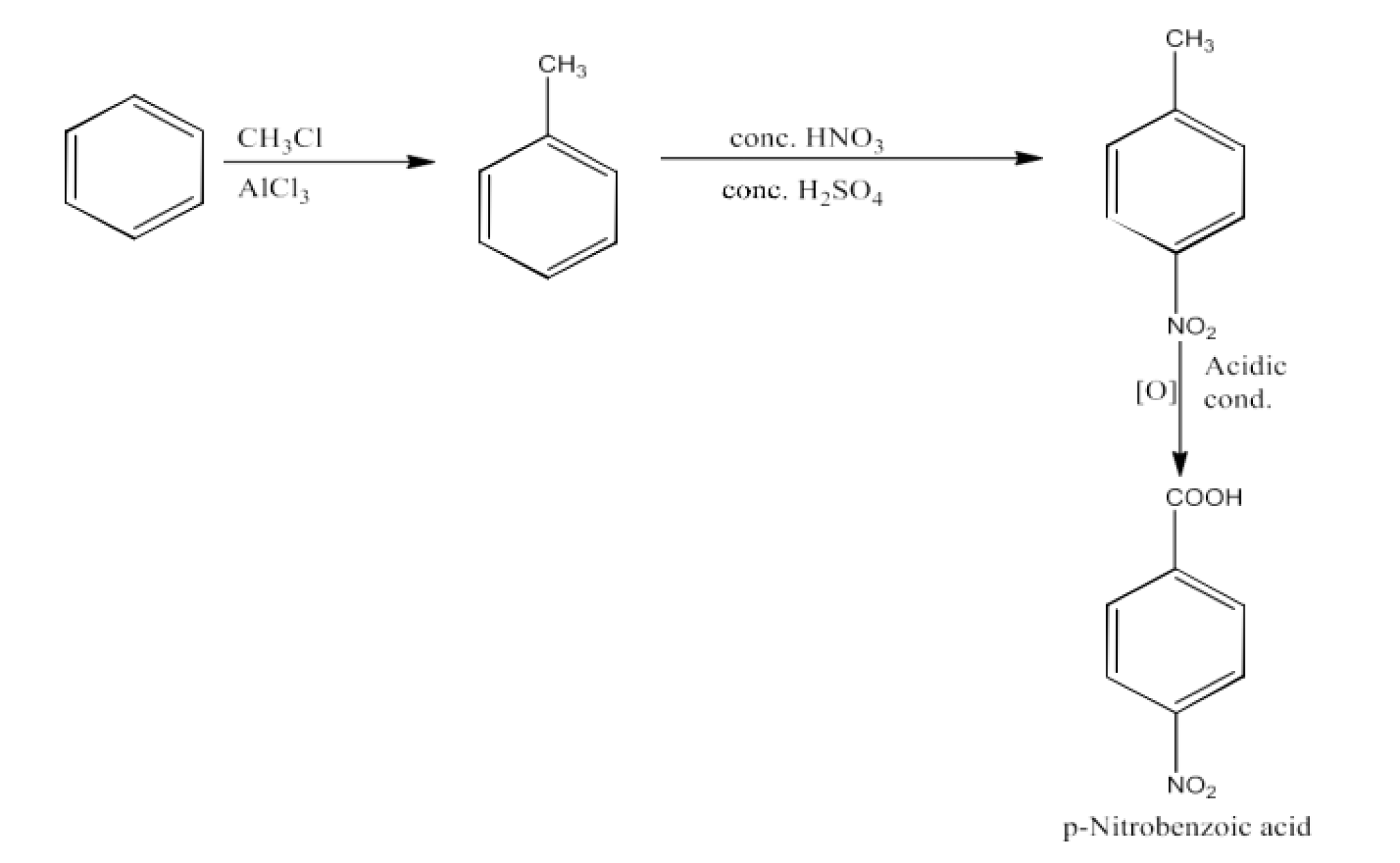
p-Nitrobenzoic acid
Ans: Benzene will be converted into toluene. Now, Toluene will be converted into p-nitrotoluene. Now, this p-nitrotoluene on oxidation will form p-nitrobenzoic acid. The reaction is given below:
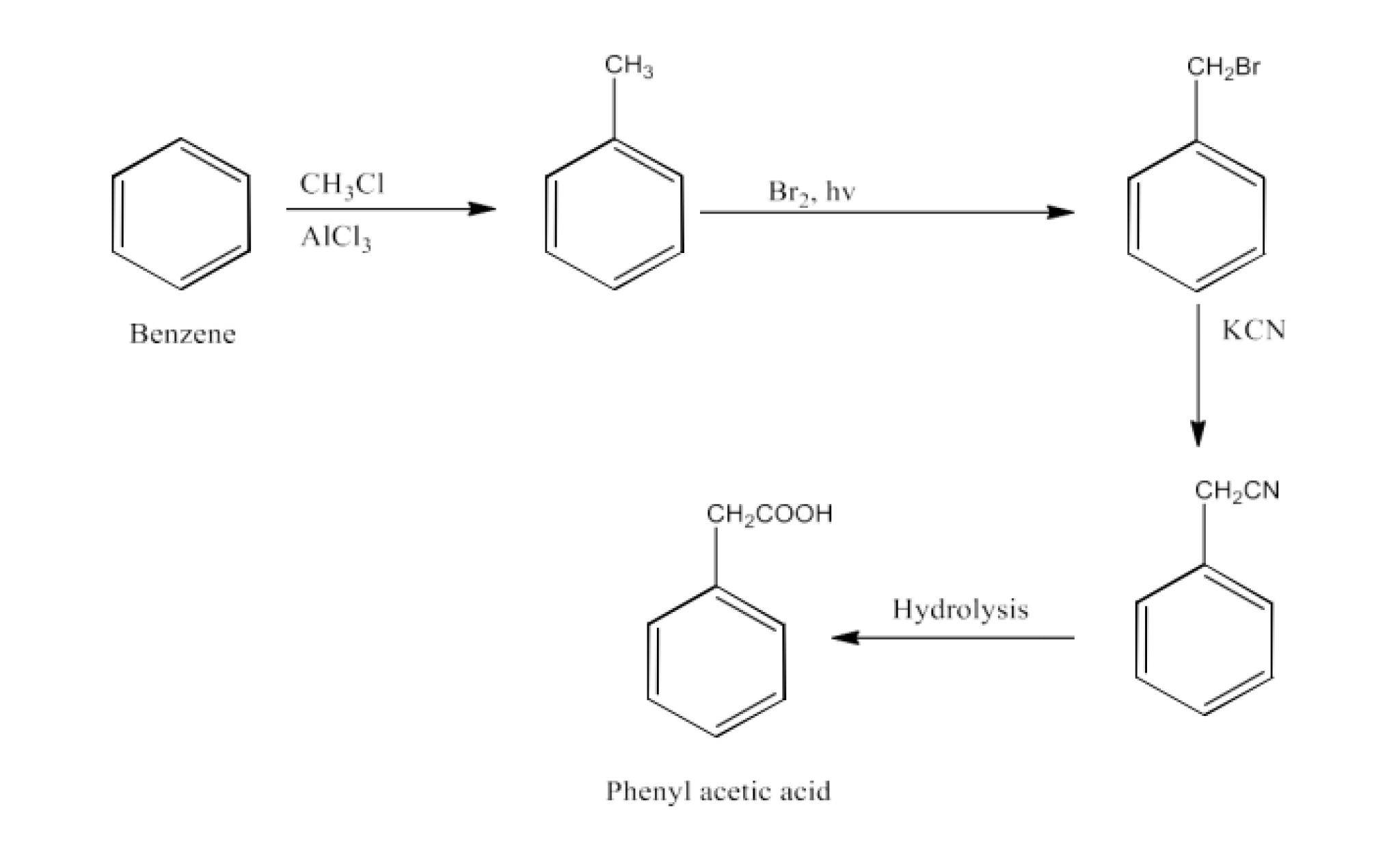
Phenylacetic acid
Ans: Benzene will be converted into toluene. Now, Toluene will be converted into Benzyl bromide; this will be further converted into benzyl cyanide. Benzyl cyanide will be hydrolyzed to Phenylacetic acid. The reaction is given below:
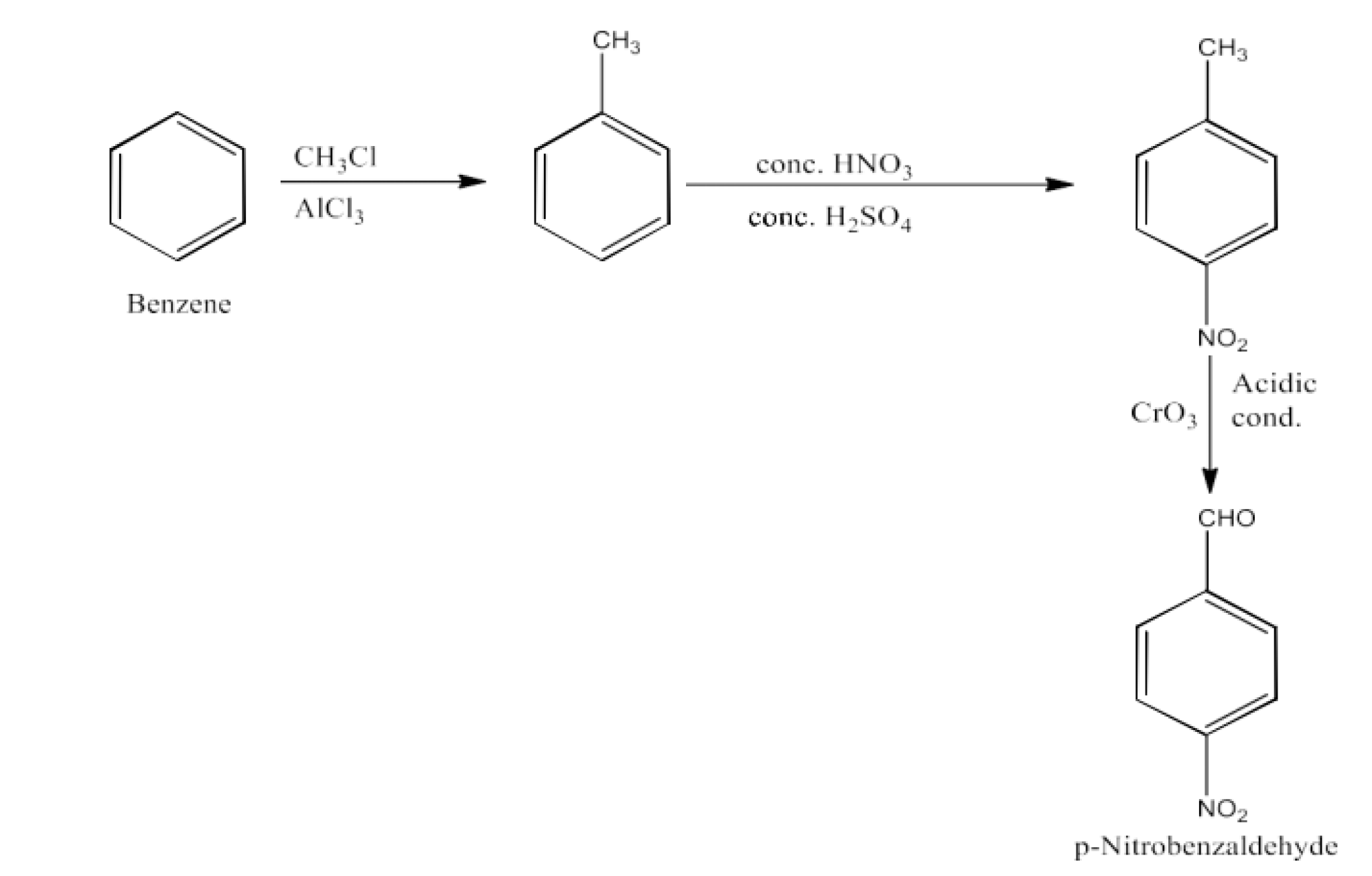
p-Nitrobenzaldehyde
Ans: Benzene will be converted into toluene. Now, Toluene will be converted into p-nitrotoluene. Now, this p-nitrotoluene on oxidation will form p-nitrobenzaldehyde. The reaction is given below:
15. How will you bring about the following conversions in not more than two steps?
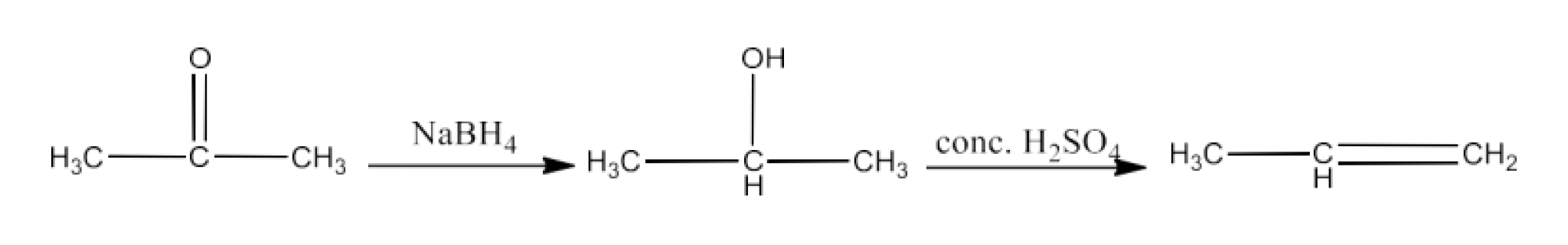
Propanone to Propene
Ans: Propanone will react with $\text{NaB}{{\text{H}}_{\text{3}}}$ to form 2-Propanol. When 2-propanol will react with concentrated sulfuric acid to form propene. The reaction is given below:
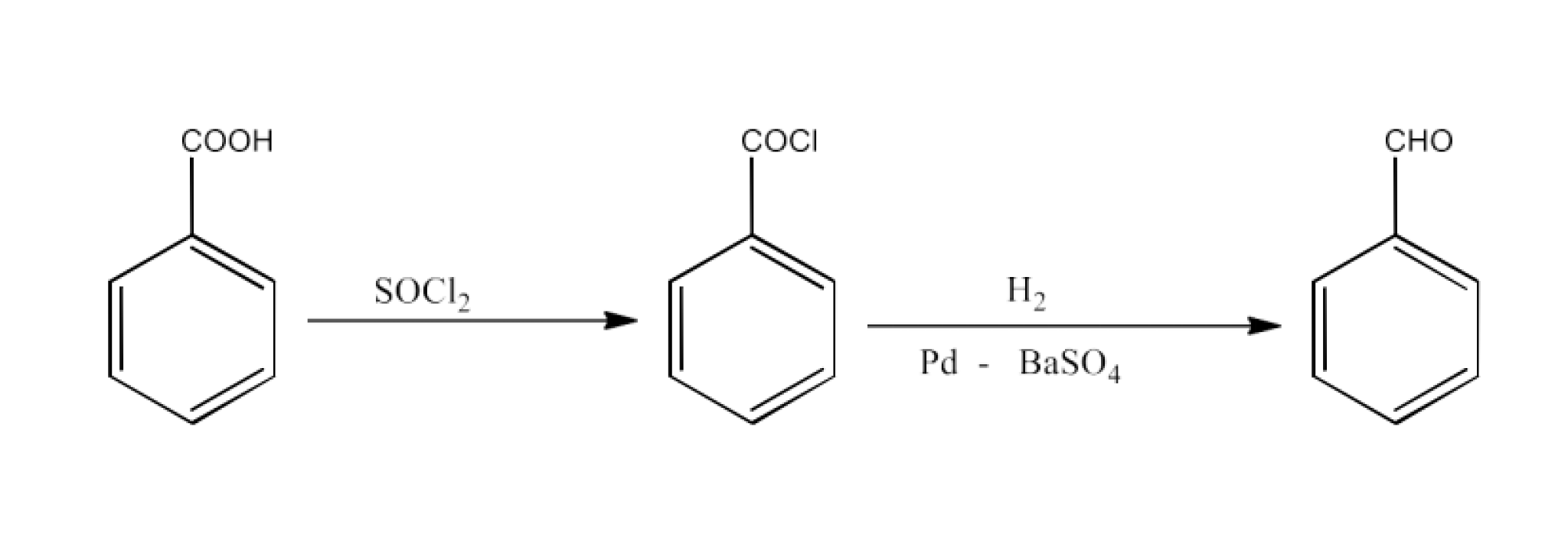
Benzoic acid to Benzaldehyde
Ans: Benzoic acid will be converted into Benzoyl chloride which will react with hydrogen and barium sulphate to give Benzaldehyde. The reaction is given below:
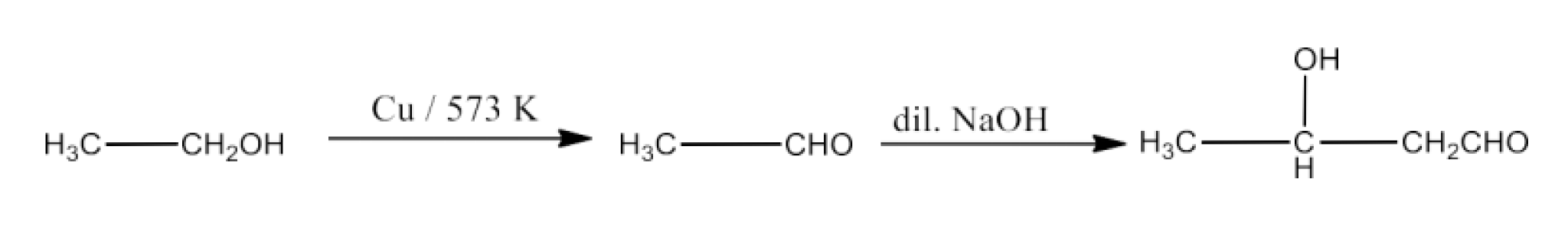
Ethanol to 3-Hydroxybutanal
Ans: Ethanol will be converted into ethanal which will react with dilute NaOH to form 3-Hydroxybutanal. The reaction is given below:
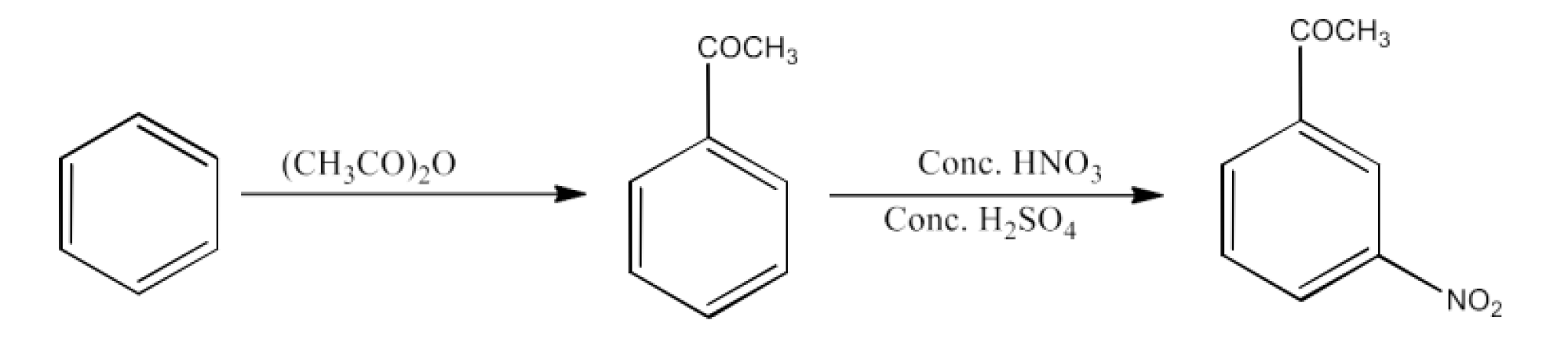
Benzene to m-Nitroacetophenone
Ans: Benzene will be converted into acetophenone which will react with concentrated nitric acid and sulfuric acid to form m-Nitroacetophenone. The reaction is given below:
Benzaldehyde to Benzophenone
Ans: Benzaldehyde will be converted into benzoic acid. The benzoic acid will be converted into benzoyl chloride which will react with benzene to form Benzophenone. The reaction is given below:
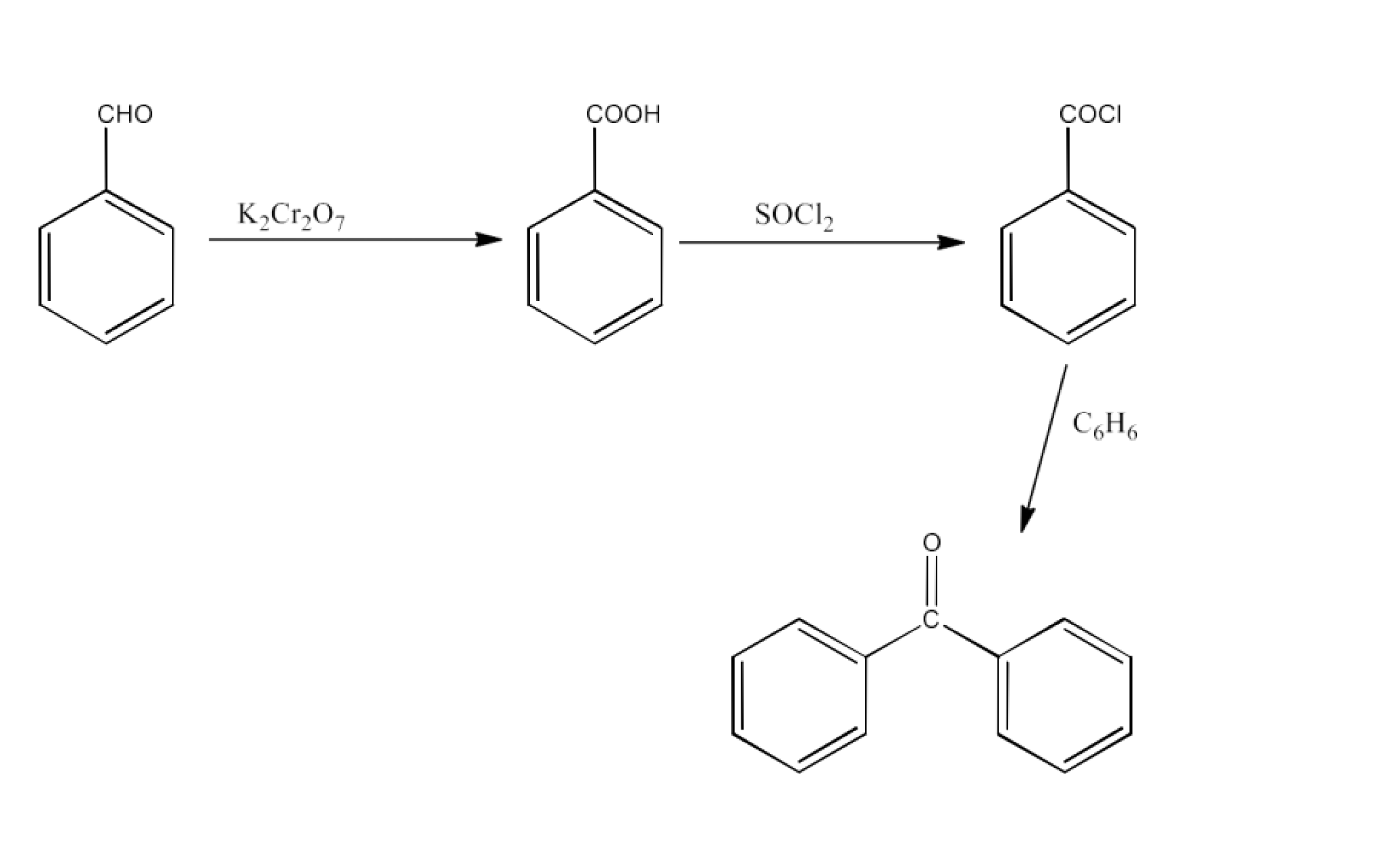
Bromobenzene to 1-Phenylethanol
Ans: Bromobenzene will be converted into Phenyl magnesium bromide which will react with ethanal to form 1-Phenylethanol. The reaction is given below:
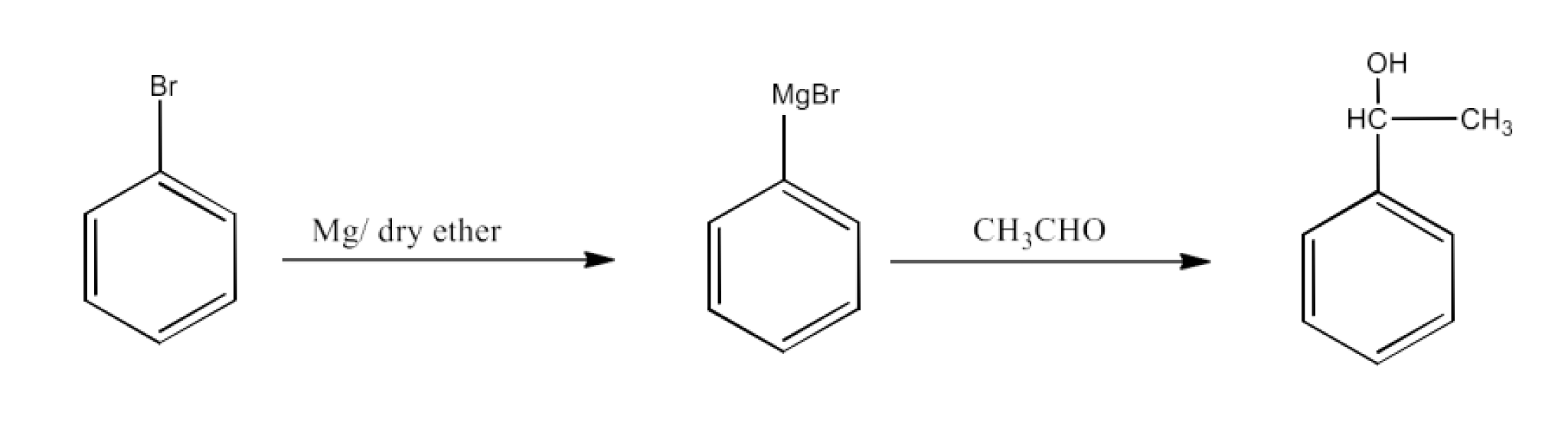
Benzaldehyde to 3-Phenylpropan-1-ol
Ans: Benzaldehyde will be converted into 3-Phenylprop-2-enal which will react with hydrogen to form 3-Phenylpropan-1-ol. The reaction is given below:
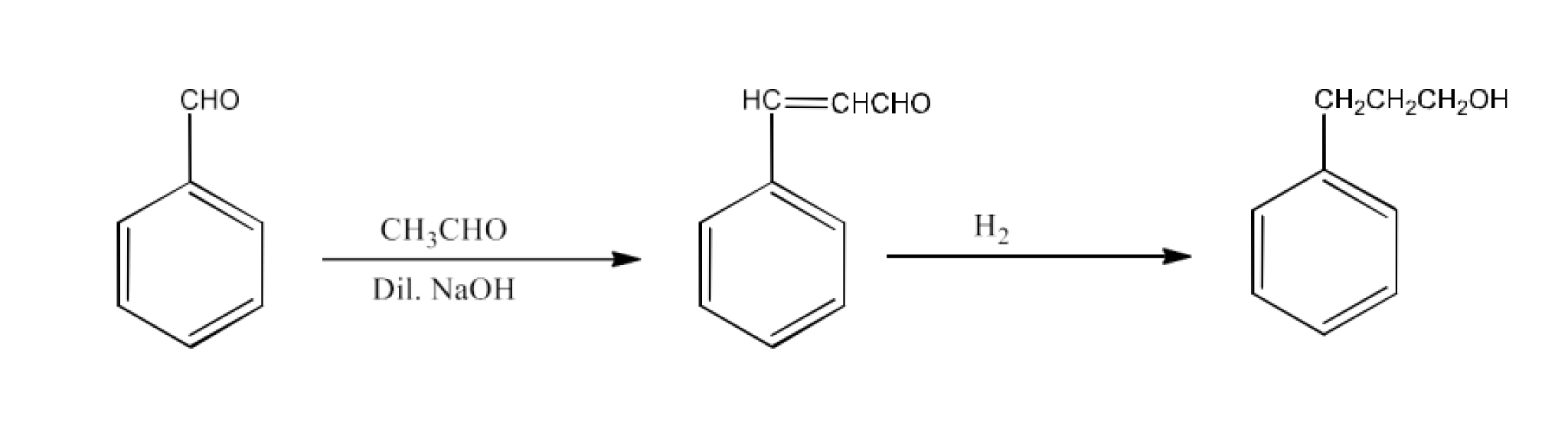
Benzaldehyde to $\text{ }\!\!\alpha\!\!\text{ }$-Hydroxyphenylacetic acid
Ans: Benzaldehyde will be converted into Benzaldehyde cyanohydrin which will react with acidic water to form $\text{ }\!\!\alpha\!\!\text{ -Hydroxyphenylacetic acid}$. The reaction is given below:
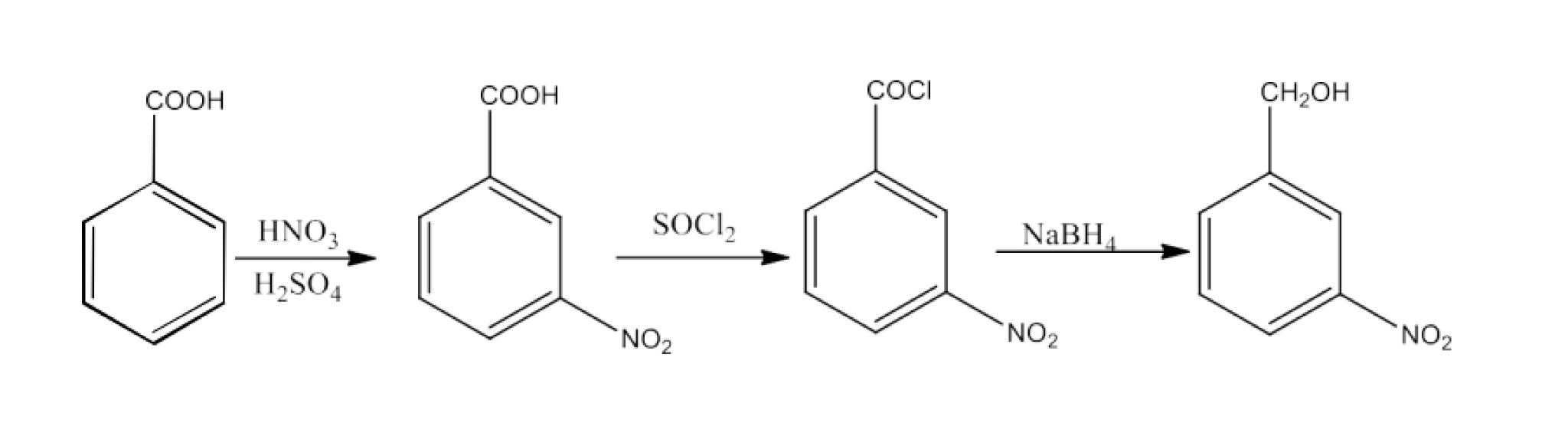
Benzoic acid to m- Nitrobenzyl alcohol
Ans: Benzoic acid will be converted into m-nitrobenzoic acid which will be further converted into m-Nitrobenzoyl chloride. m-Nitrobenzoyl chloride will react with $\text{NaB}{{\text{H}}_{\text{4}}}$ to form m-Nitrobenzyl alcohol. The reaction is given below:
16. Describe the following:
Acetylation
Ans: Acetyl functional group is referred to as acetylation in the organic molecule. It is generally done with a basis such as pyridine, dimethylaniline, etc. This includes replacing an active hydrogen atom with a group of acetyls. The most popular acetylating agents include acetyl chloride and acetic anhydride. For example, ethanol acetylation generates ethyl acetate.
\[\underset{\text{Ethanol}}{\mathop{\text{C}{{\text{H}}_{\text{3}}}\text{C}{{\text{H}}_{\text{2}}}\text{OH}}}\,\text{ + }\underset{\text{Acetyl Chloride}}{\mathop{\text{C}{{\text{H}}_{\text{3}}}\text{COCl}}}\,\xrightarrow{\text{pyridine}}\underset{\text{Ethyl acetate}}{\mathop{\text{ C}{{\text{H}}_{\text{3}}}\text{COO}{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}}}\,\text{+HCl}\]
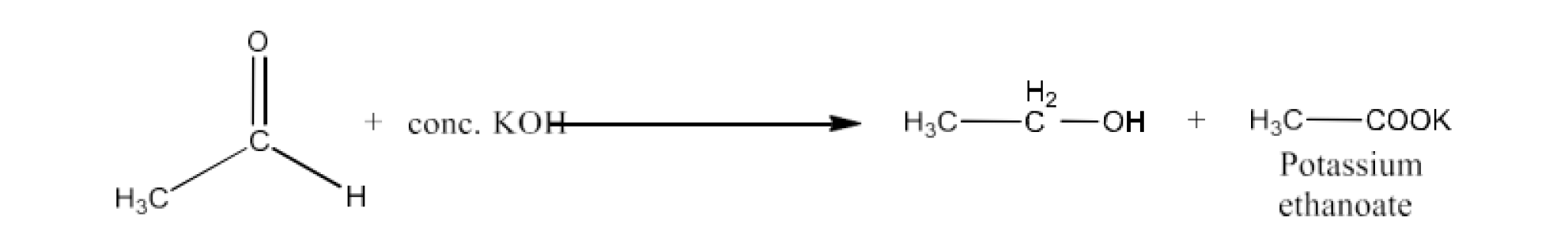
Cannizzaro reaction
Ans: The reaction of aldehydes that have no $\text{ }\!\!\alpha\!\!\text{ }$-hydrogen to concentrated alkalis is sometimes called the Cannizzaro Reaction or Self-oxidation-reduction (disproportion). Two aldehyde molecules are involved in this process, one reduced to alcohol and the other to carboxylic acid oxidised.
Ethanol and potassium ethanoate are, for example, produced when treated with concentrated potassium hydroxide.
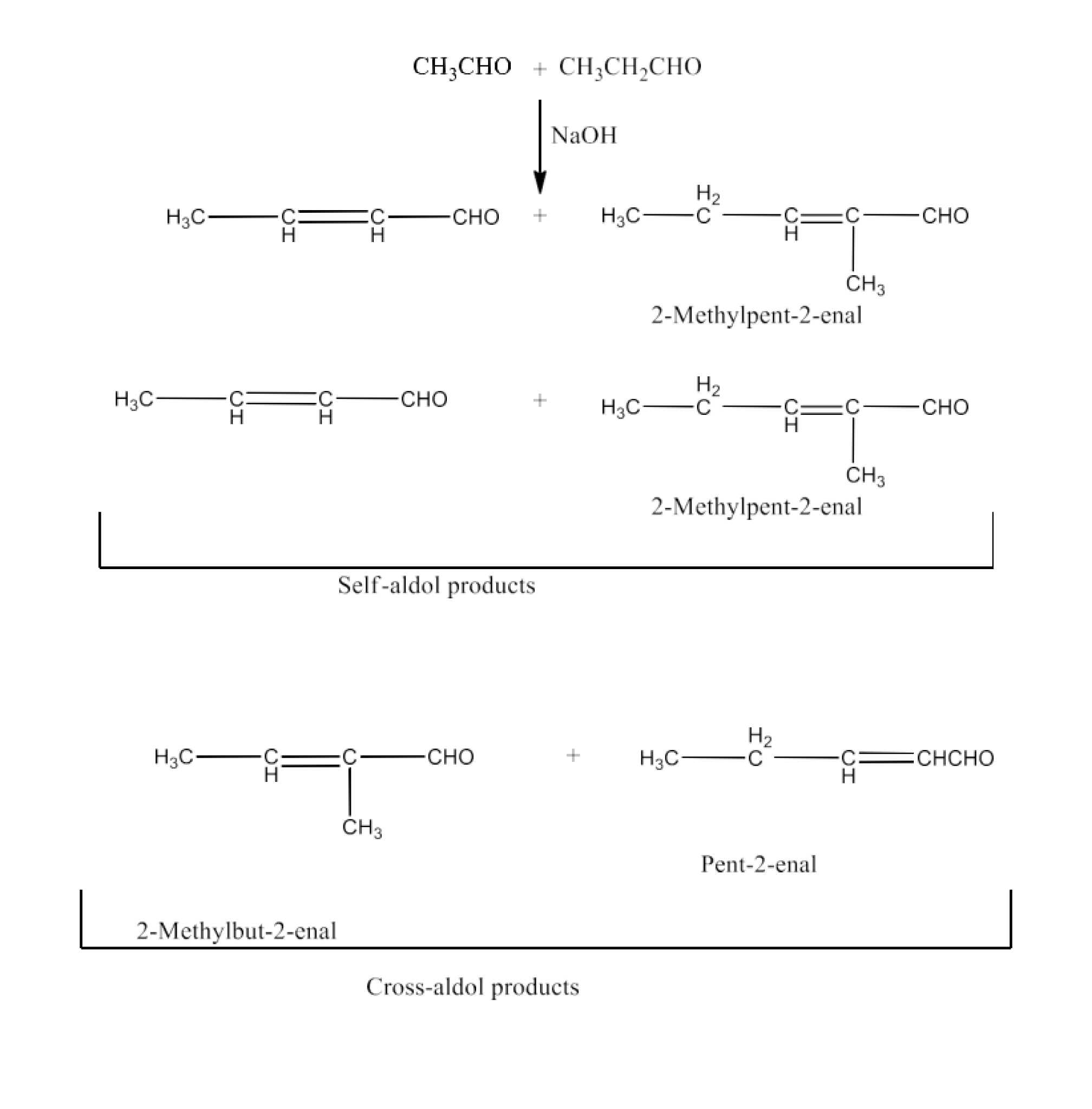
Cross aldol condensation
Ans: The reaction is called a cross-aldol condensation if aldol condensation is carried out by two distinct aldehydes or two separate ketones or an aldehyde and a ketone. If the $\text{ }\!\!\alpha\!\!\text{ }$-hydrogen is present in the two reactants, four compound products are produced.
Ethanal and propanal, for example, respond to four products.
Decarboxylation
Ans: Decarboxylation is the reaction in which carboxylic acids, when their salts are heated by soda-lime, lose carbon dioxide in order to produce hydrocarbons.
\[\text{C}{{\text{H}}_{\text{3}}}\text{-COONa }\xrightarrow{\text{Soda lime}}\text{ C}{{\text{H}}_{\text{4}}}\text{ + N}{{\text{a}}_{\text{2}}}\text{C}{{\text{O}}_{\text{3}}}\]
17. Complete each synthesis by giving missing starting material, reagent or products.
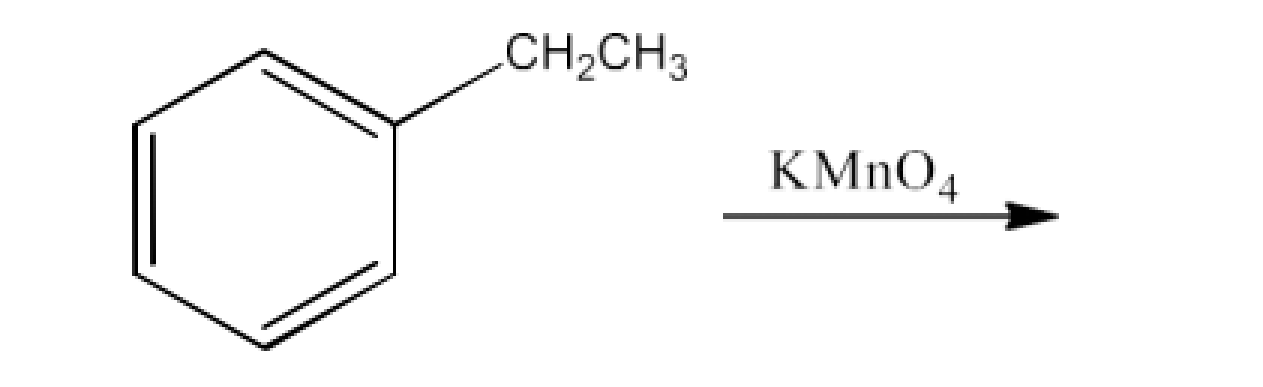
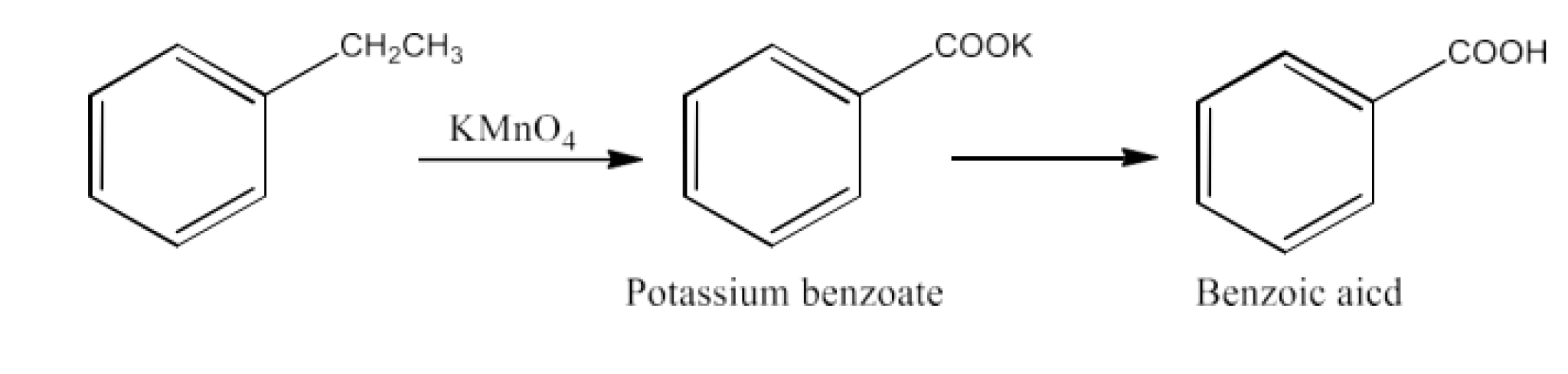
Ans: The products formed in this reaction are potassium benzoate and then benzoic acid. The reaction is given below:
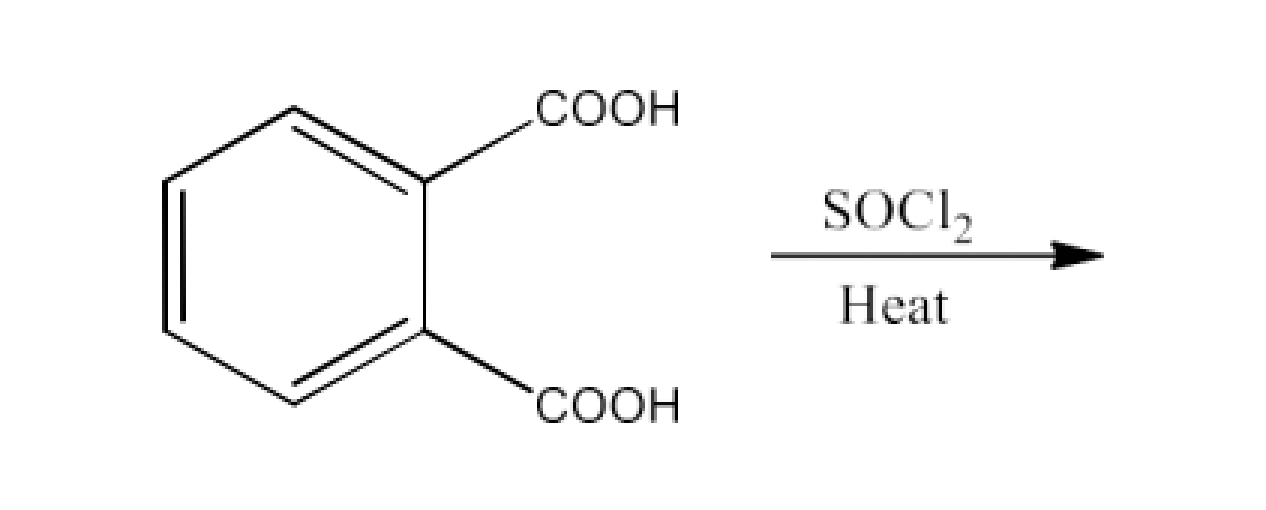
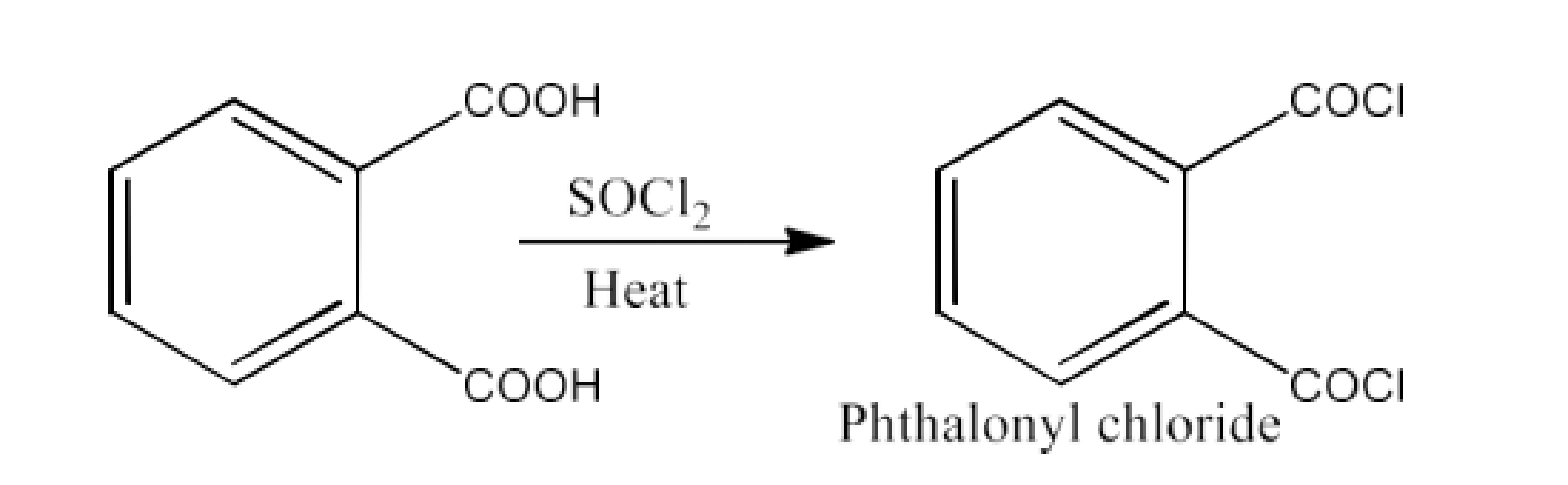
Ans: The product formed in this reaction is Phthaloyl chloride. The reaction is given below:
${{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}\text{CHO}\xrightarrow{{{\text{H}}_{\text{2}}}\text{NCONHN}{{\text{H}}_{\text{2}}}}$
Ans: The product formed in this reaction will be Benzaldehyde semicarbazone. The reaction is given below:
\[{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}\text{CHO}\xrightarrow{{{\text{H}}_{\text{2}}}\text{NCONHN}{{\text{H}}_{\text{2}}}}\text{ }{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}\text{CH=NNHCON}{{\text{H}}_{\text{2}}}\text{ + }{{\text{H}}_{\text{2}}}\text{O}\]
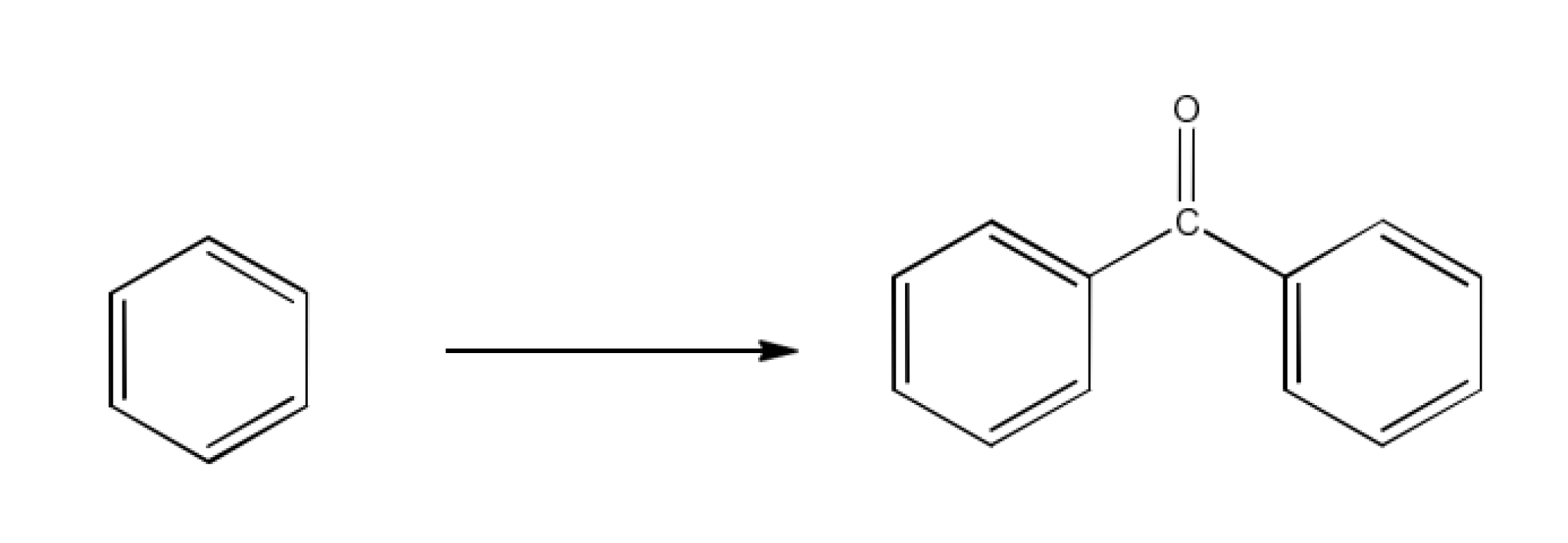
Ans: In this reaction, another reactant is benzoyl chloride and the catalyst is aluminium chloride. The reaction is given below:
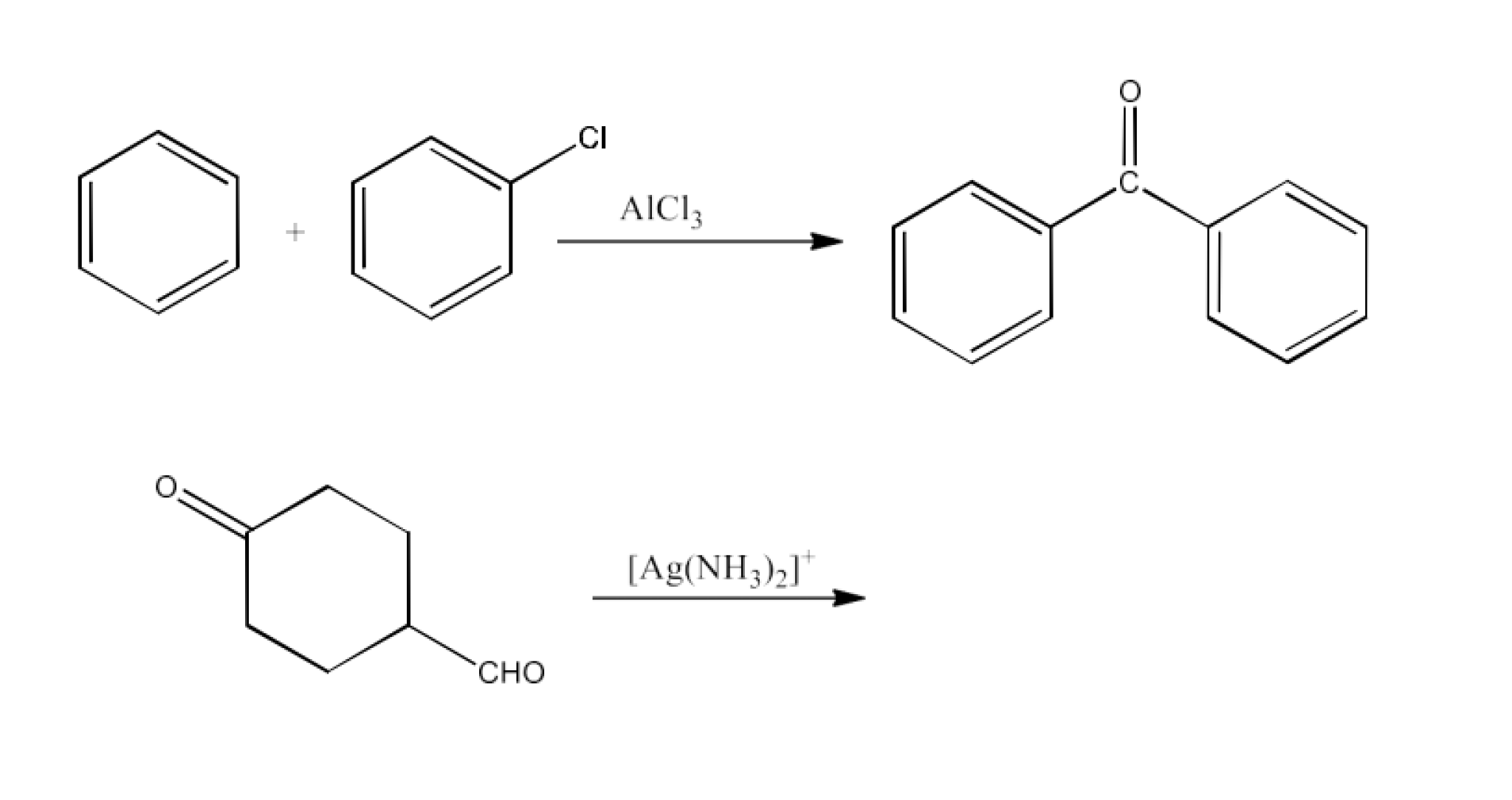
Ans: The tollen’s reagent will only reduce the aldehyde part. The reaction is given below:
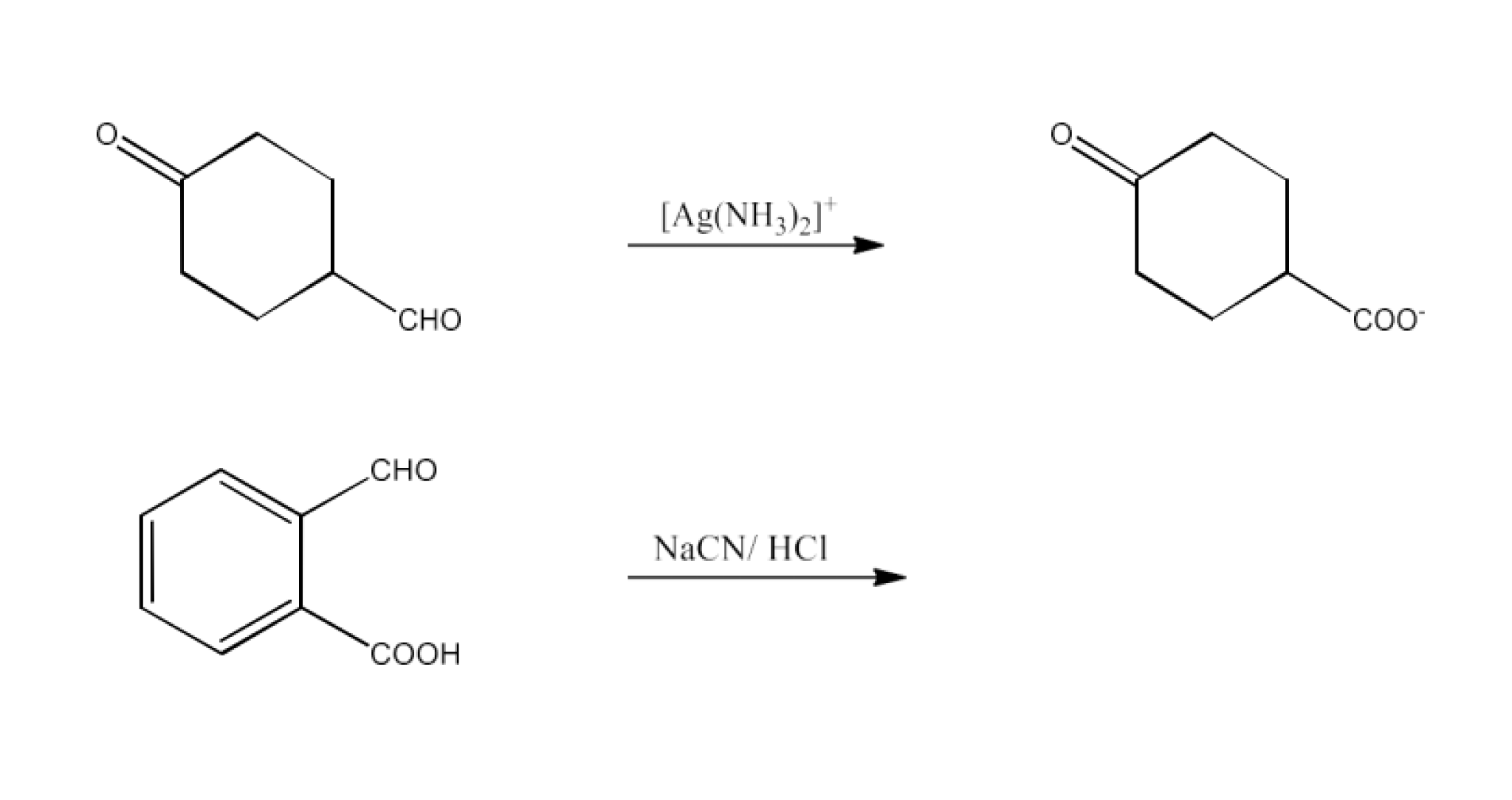
Ans: The product formed in this reaction will be 2-(1-Hydroxy Cyanomethyl) benzoic acid. The reaction is given below:
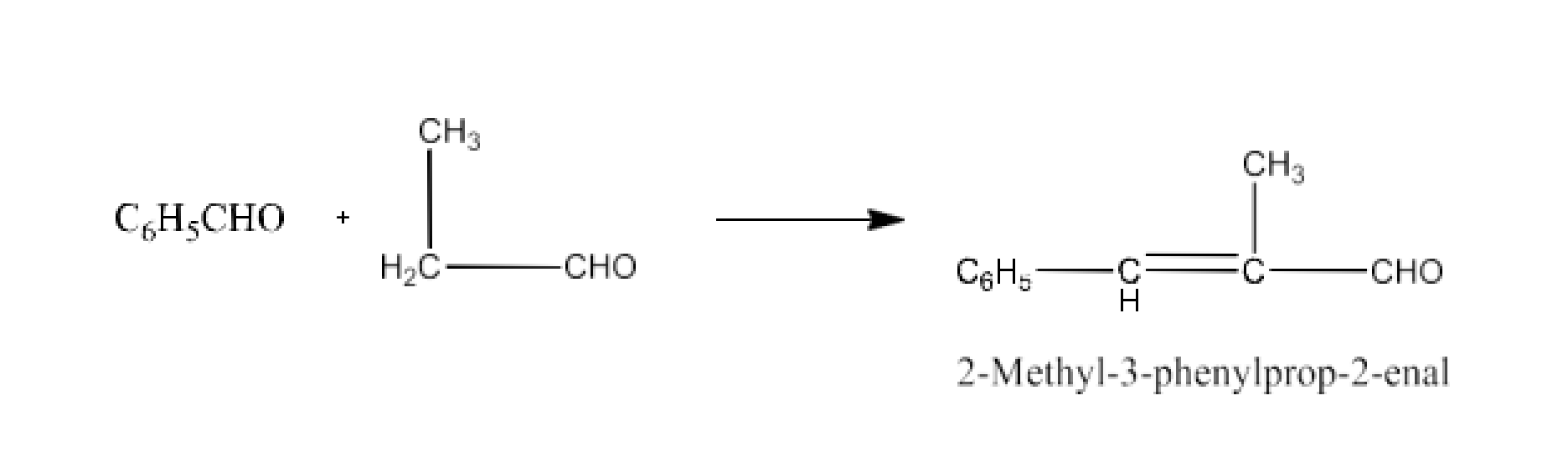
${{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}\text{CHO + C}{{\text{H}}_{\text{3}}}\text{C}{{\text{H}}_{\text{2}}}\text{CHO }\xrightarrow{\text{dil}\text{.NaOH}}$
Ans: The product formed in this reaction will be 2-Methyl-3-phenylprop-2-enal. The reaction is given below:
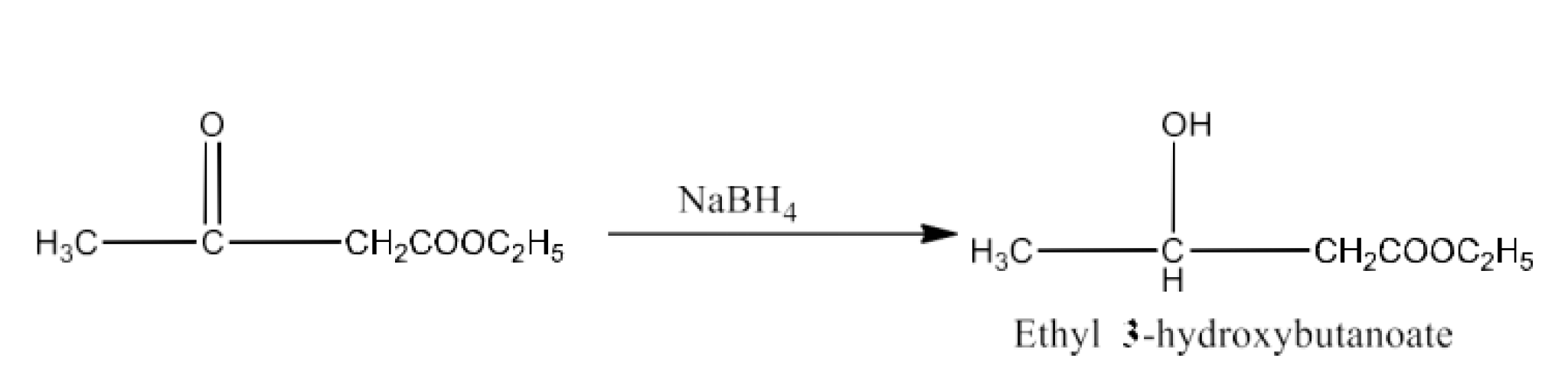
$\text{C}{{\text{H}}_{\text{3}}}\text{COC}{{\text{H}}_{\text{2}}}\text{COO}{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}\xrightarrow[\text{(ii)}{{\text{H}}^{\text{+}}}]{\text{(i)NaB}{{\text{H}}_{\text{4}}}}$
Ans: The product formed in this reaction is Ethyl-3-hydroxybutanoate. The reaction is given below:
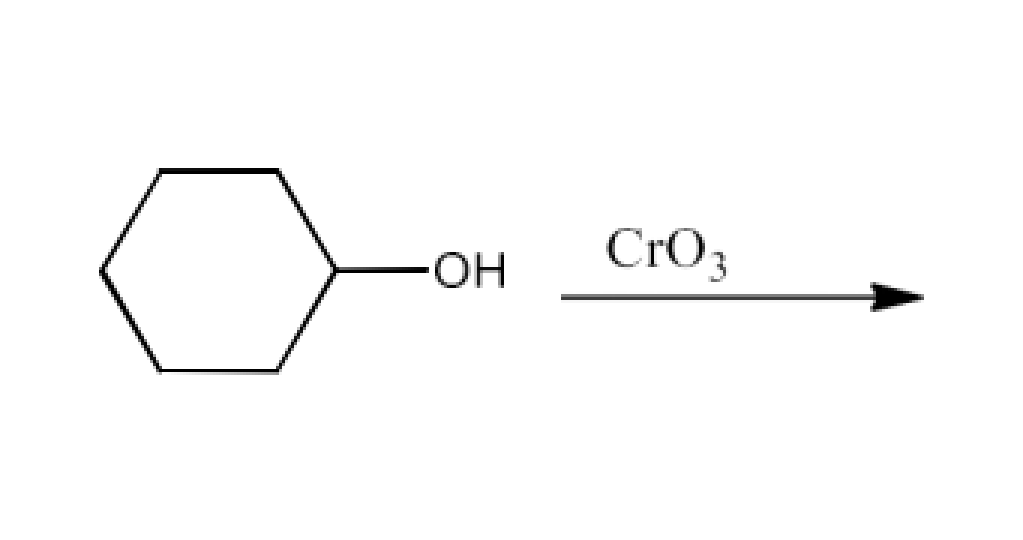
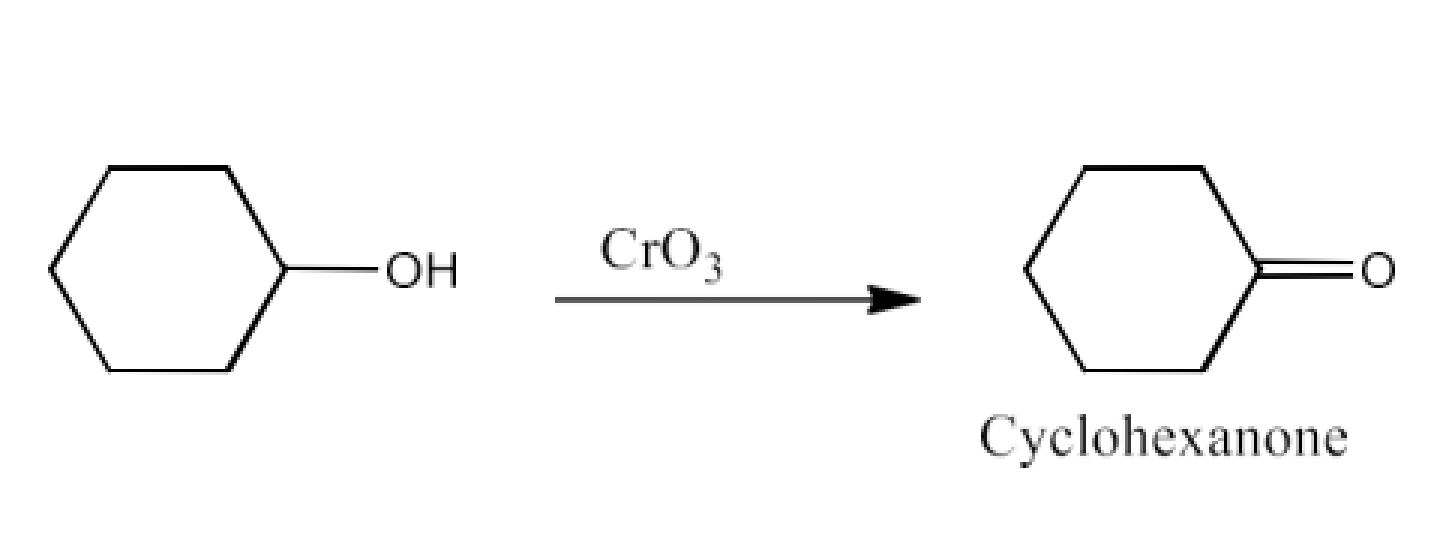
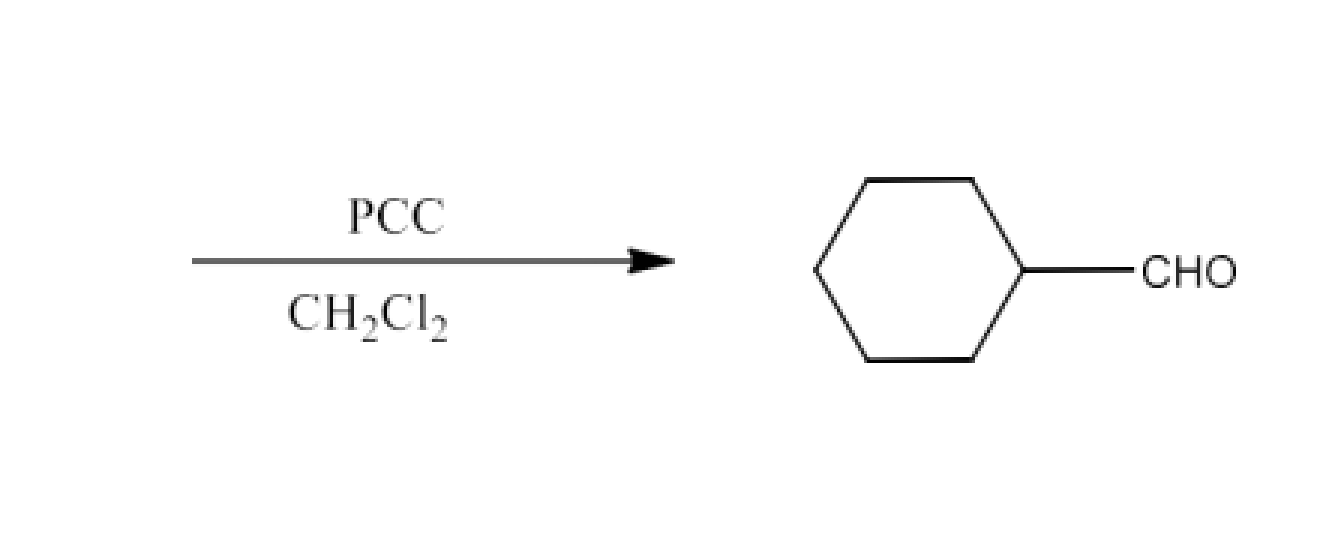
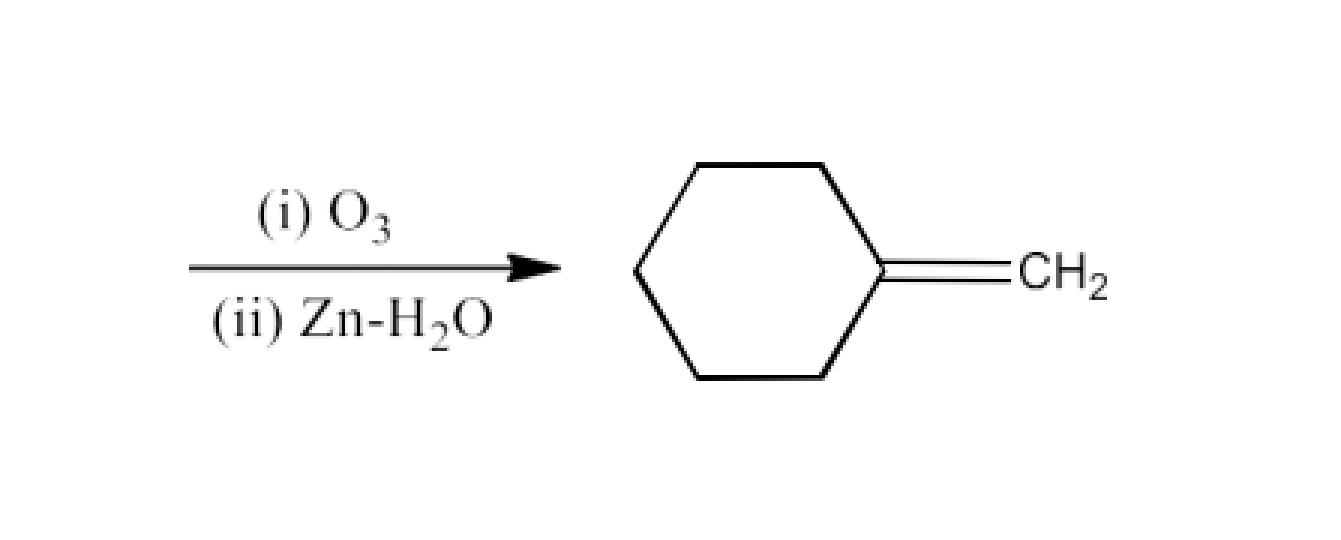
Ans: The product formed in this reaction will be Cyclohexanone. The reaction is given below:
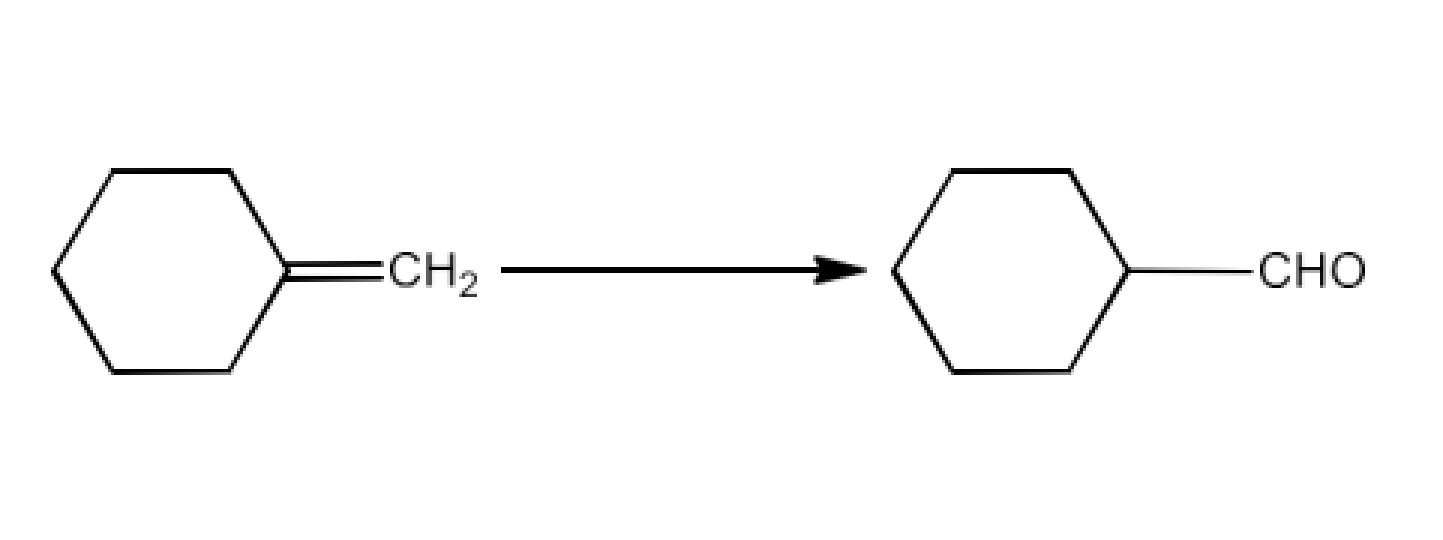
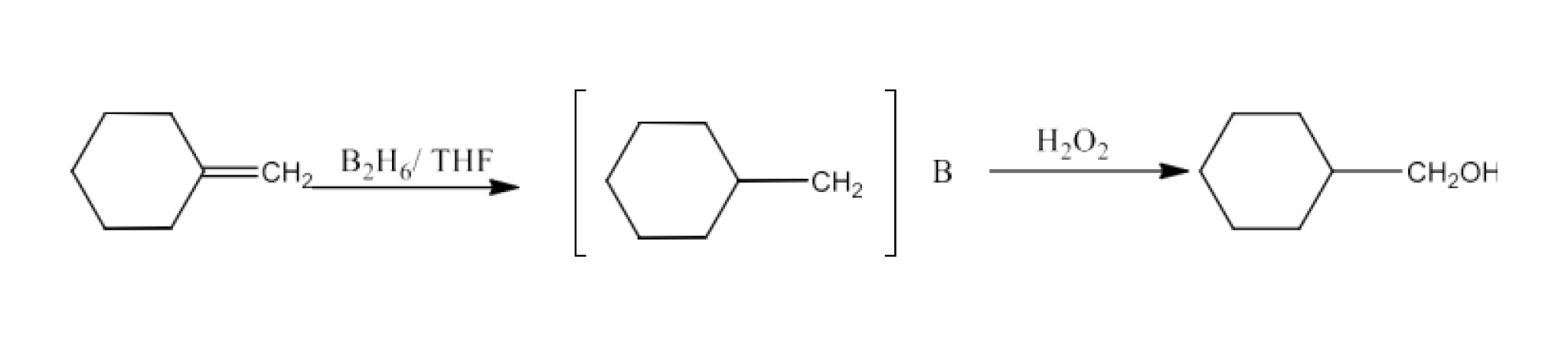
Ans: The complete reaction is given below:
Ans: The reactant for this reaction will be Cyclohexylidene Cyclohexanone. The reaction is given below:
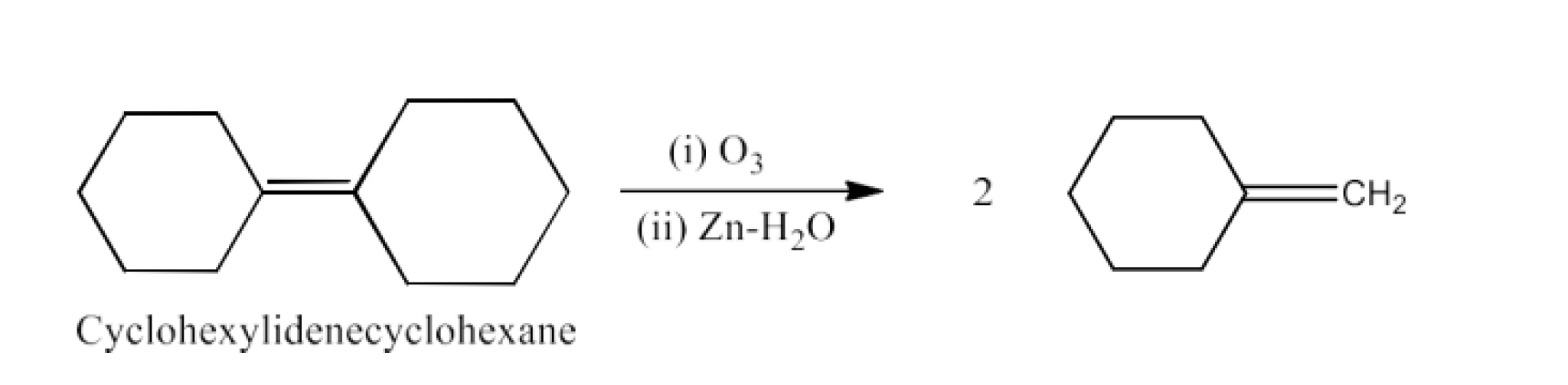
18. Give plausible explanation for each of the following:
Cyclohexanone forms cyanohydrin in good yield but 2, 2, 6- trimethylcyclohexanone does not.
Ans: Cyclohexanones form cyanohydrins according to the following equation.
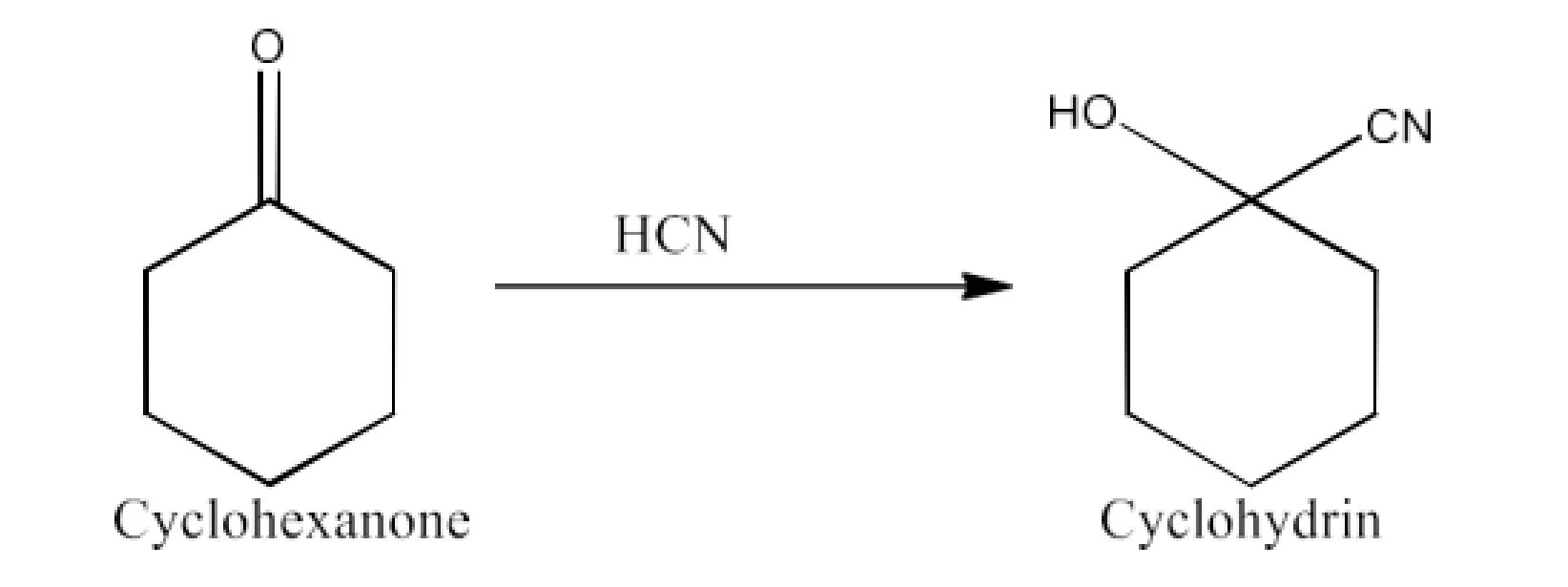
In this scenario, nucleophiles $\text{C}{{\text{N}}^{\text{-}}}$ can approach freely without steric obstruction. But for 2-2, 6-trimethylcyclohexanone, $\text{ }\!\!\alpha\!\!\text{ }$-positioned methyl groups present steric barriers and as a result $\text{C}{{\text{N}}^{\text{-}}}$ cannot successfully attack.

That’s why it cannot form cyanohydrins.
There are two $\text{-N}{{\text{H}}_{\text{2}}}$ groups in semicarbazide. However, only one is involved in the formation of semicarbazones.
Ans: Semicarbazide is resonant with just one of two groups $\text{-N}{{\text{H}}_{\text{2}}}$ which is immediately linked to the carbonyl carbon atom.
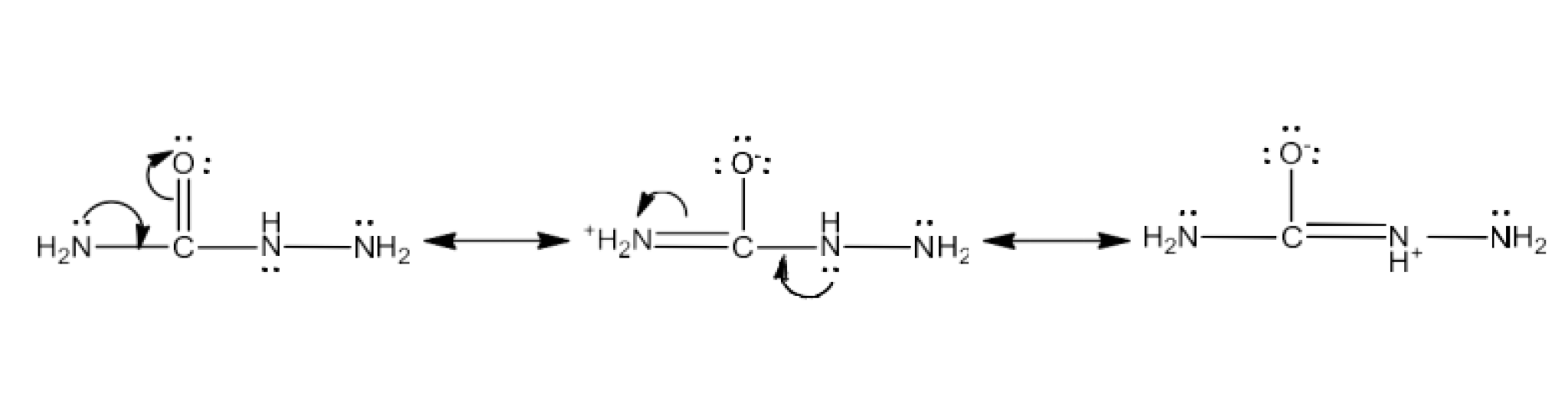
Consequently, the electron density at $\text{-N}{{\text{H}}_{\text{2}}}$ likewise decreases with the resonance. It cannot therefore operate as a nucleophile. Since the other group $\text{-N}{{\text{H}}_{\text{2}}}$ does not engage itself in resonance, it may function as a nucleophile and can attack aldehydrogen and ketone carbonic atoms to generate semicarbazones.
During the preparation of esters from a carboxylic acid and an alcohol in the presence of an acid catalyst, the water or the ester should be removed as soon as it is formed.
Ans: In presence of acid, ester along with water is reversibly made of carboxylic acid and alcohol.
\[\text{RCOOH+R }\!\!'\!\!\text{ OH}\xrightarrow{{{\text{H}}^{\text{+}}}}\text{RCOOR }\!\!'\!\!\text{ +}{{\text{H}}_{\text{2}}}\text{O}\]
If water or ester is not eliminated as it forms, then the reactants react to the reversibility of the reaction. Therefore, one of the two should be eliminated to push the balance forward in a more ester way.
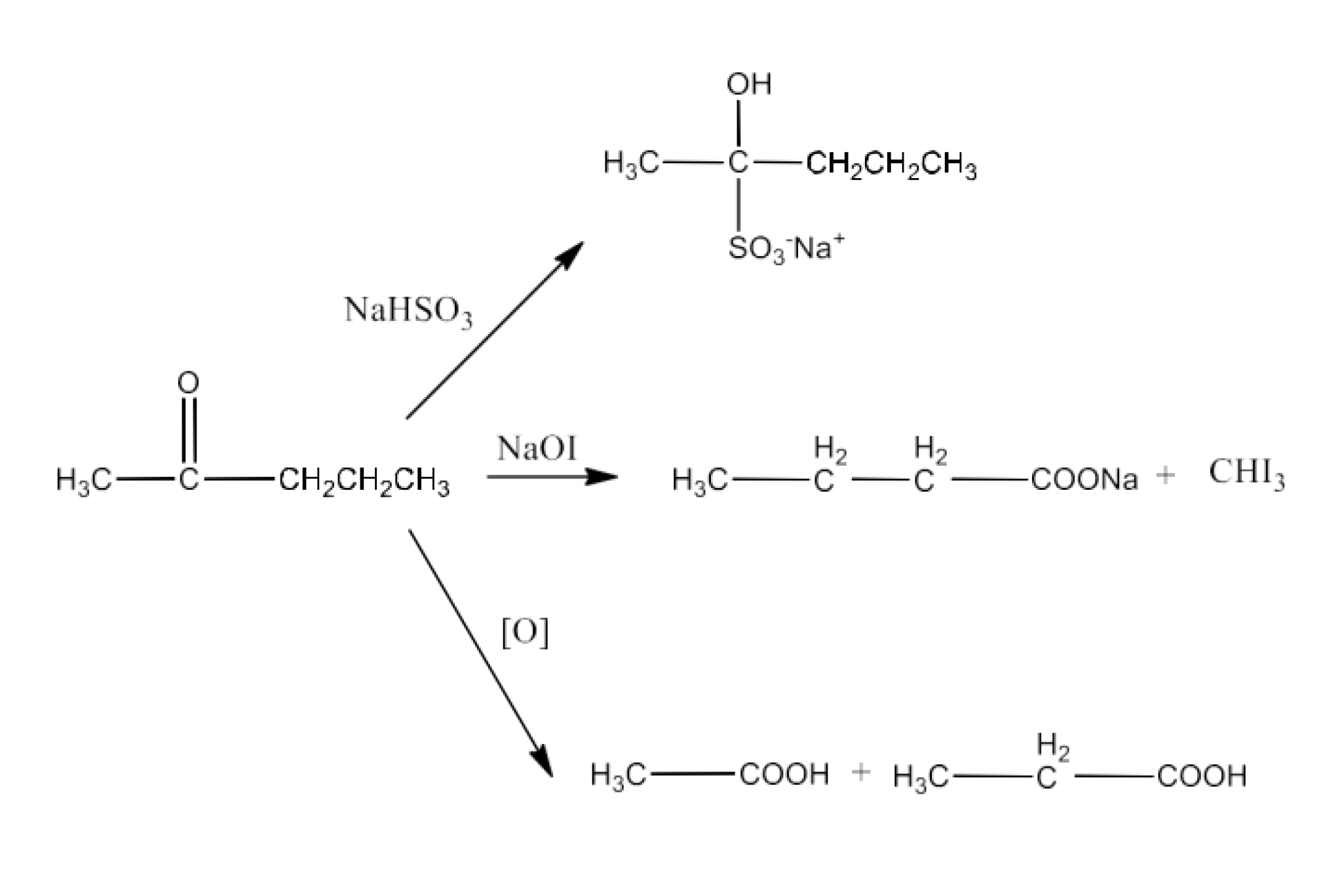
19. An organic compound contains 69.77% carbon, 11.63% hydrogen and rest oxygen. The molecular mass of the compound is 86. It does not reduce Tollens’ reagent but forms an additional compound with sodium hydrogen sulphite and gives a positive iodoform test. On vigorous oxidation it gives ethanoic and propanoic acid. Write the possible structure of the compound.
Ans: The percentage of carbon atom = 69.77%
The percentage of hydrogen atom = 11.63%
The percentage of oxygen atom = 100 – (69.77 + 11.63) = 18.6%
Thus, the ratio of the number of carbon, hydrogen, and oxygen atoms in the organic compound can be given as:
\[\text{C : H : O = }\frac{\text{69}\text{.77}}{\text{12}}\text{ : }\frac{\text{11}\text{.63}}{\text{1}}\text{ : }\frac{\text{18}\text{.6}}{\text{16}}\]
= 5.81 : 11.63 : 1.16
= 5 : 10 : 1
Hence the empirical formula of the compound is ${{\text{C}}_{\text{5}}}{{\text{H}}_{\text{10}}}\text{O}$. So, the mass of the compound is = 5 $\text{ }\!\!\times\!\!\text{ }$ 12 + 10 $\text{ }\!\!\times\!\!\text{ }$ 1 + 1 $\text{ }\!\!\times\!\!\text{ }$ 16 = 86
Since the given molecular mass is also 86. The molecular formula is also ${{\text{C}}_{\text{5}}}{{\text{H}}_{\text{10}}}\text{O}$.
As Tollen's reagent is not reduced by that molecule, it is not an aldehyde. The chemical once again provides a positive Iodoform test to form sodium hydrogen sulphate supplements. The molecule must be a methyl ketone, because it is not an aldehyde. A combination of ethanoic acid and propanoic acid also results in the given chemical. The chemical is thus pentan−2−ol.
The reactions in the question are given below:
20. Although phenoxide ion has more number of resonating structures than carboxylate ion, carboxylic acid is a stronger acid than phenol. Why?
Ans: Resonance structures of phenoxide ion are:
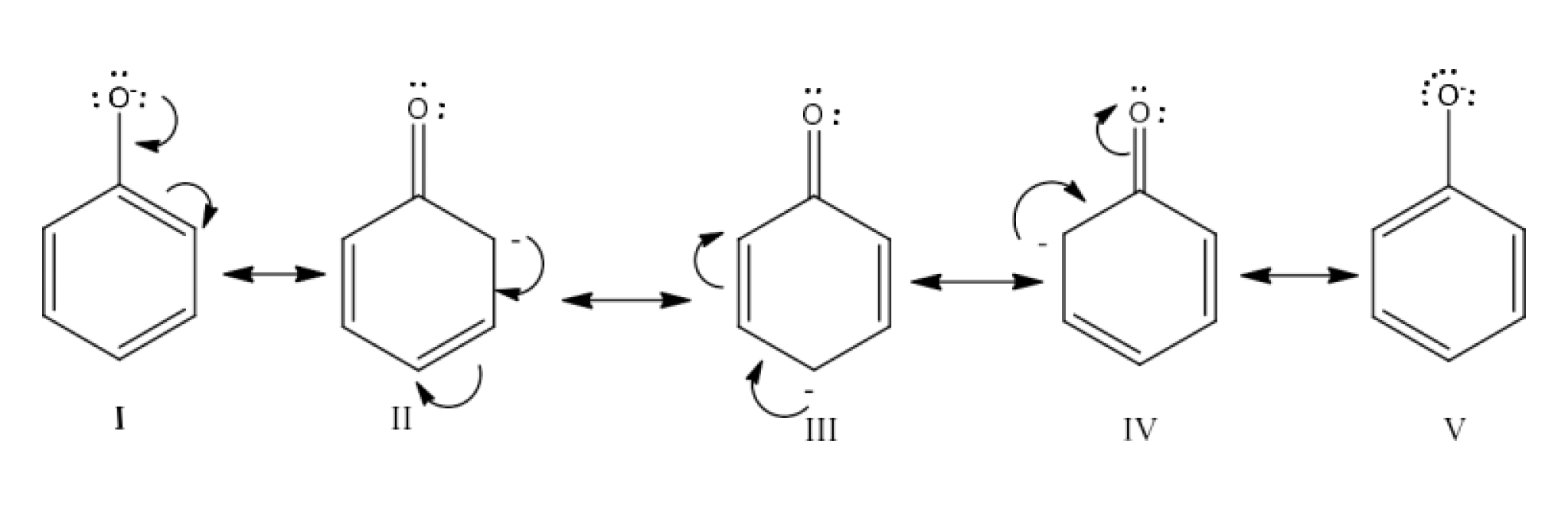
Resonance structures of phenoxide ion may be shown to have a negative charge for fewer electronegative carbon atoms in II, III and IV. Those three configurations therefore make a negligible contribution to the phenoxide ion's resonance stability. Such constructions can thus be removed. The most electronegative oxygen atom carries a negative load only in structures I and V.
The resonating structures of carboxylate ion are given below:
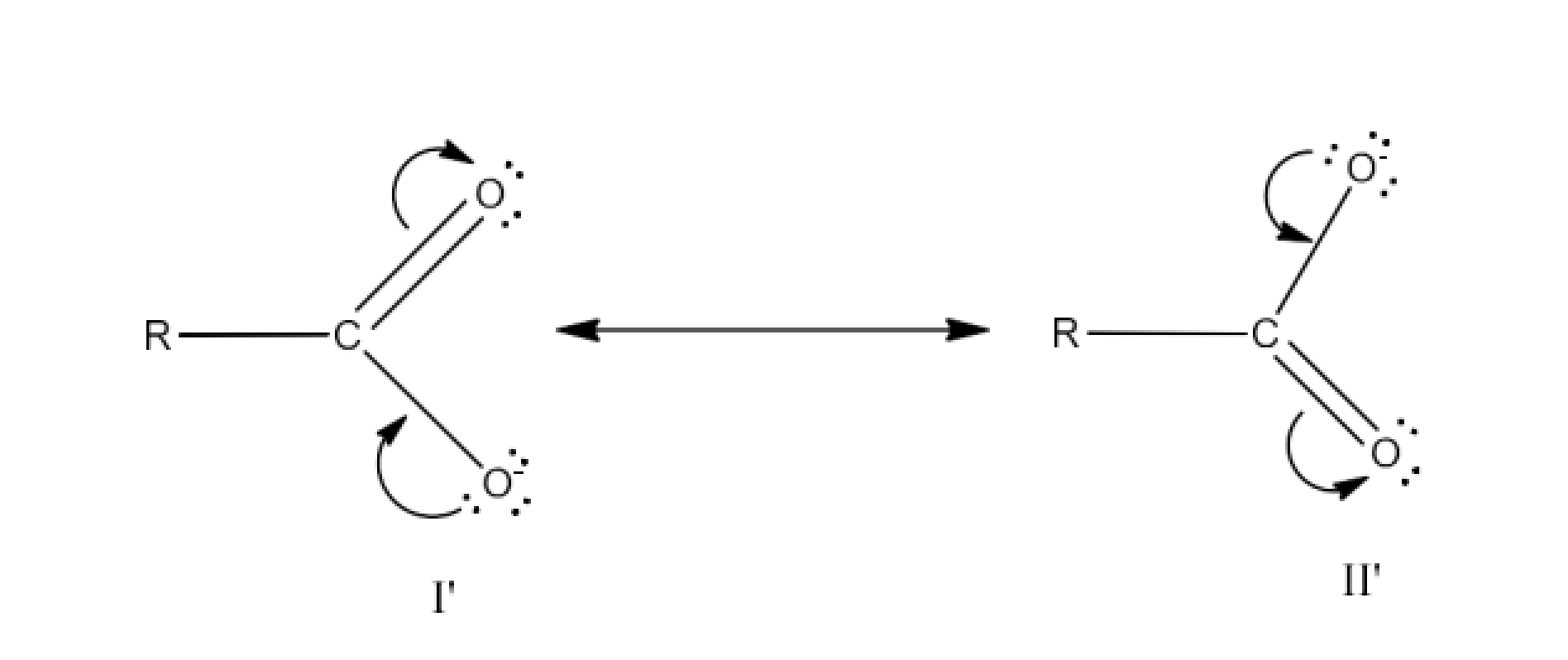
In the case of carboxylate ion, resonating structures I′ and II′ contain a charge carried by a more electronegative oxygen atom. Further, in resonating structures I′ and II′, the negative charge is delocalized over two oxygen atoms. But in resonating structures I and V of the phenoxide ion, the negative charge is localized on the same oxygen atom. Therefore, the resonating structures of carboxylate ion contribute more towards its stability than those of phenoxide ion. As a result, carboxylate ion is more resonance-stabilized than phenoxide ion. Hence, carboxylic acid is a stronger acid than phenol.
INTEXT SOLUTIONS
1. Write the structures of the following compounds.
α-Methoxypropionaldehyde
Ans: The structure of α-Methoxypropionaldehyde is given below:
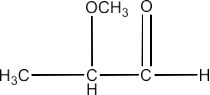
3-Hydroxybutanal
Ans: The structure of 3-Hydroxybutanal is given below:
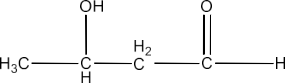
2-Hydroxycyclopentane carbaldehyde
Ans: The structure of 2-Hydroxycyclopentane carbaldehyde is given below:
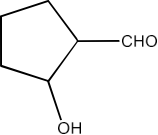
4-Oxopentanal
Ans: The structure of 4-Oxopentanal is given below:
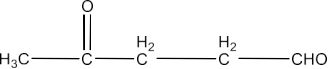
Di-sec-butyl ketone
Ans: The structure of Di-sec-butyl ketone is given below:

4-Fluoroacetophenone
Ans: The structure of 4-Fluoroacetophenone is given below:
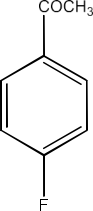
2. Write the structures of products of the following reactions;
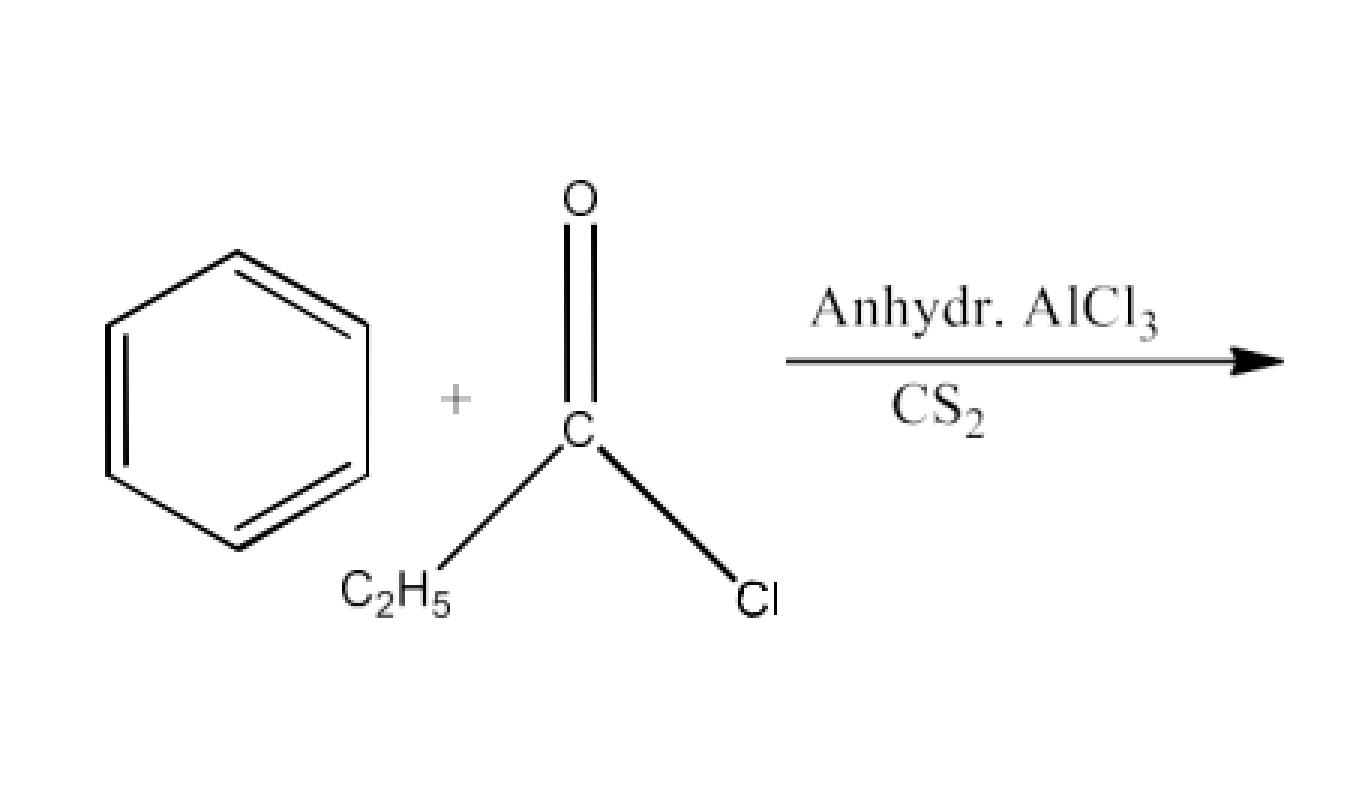
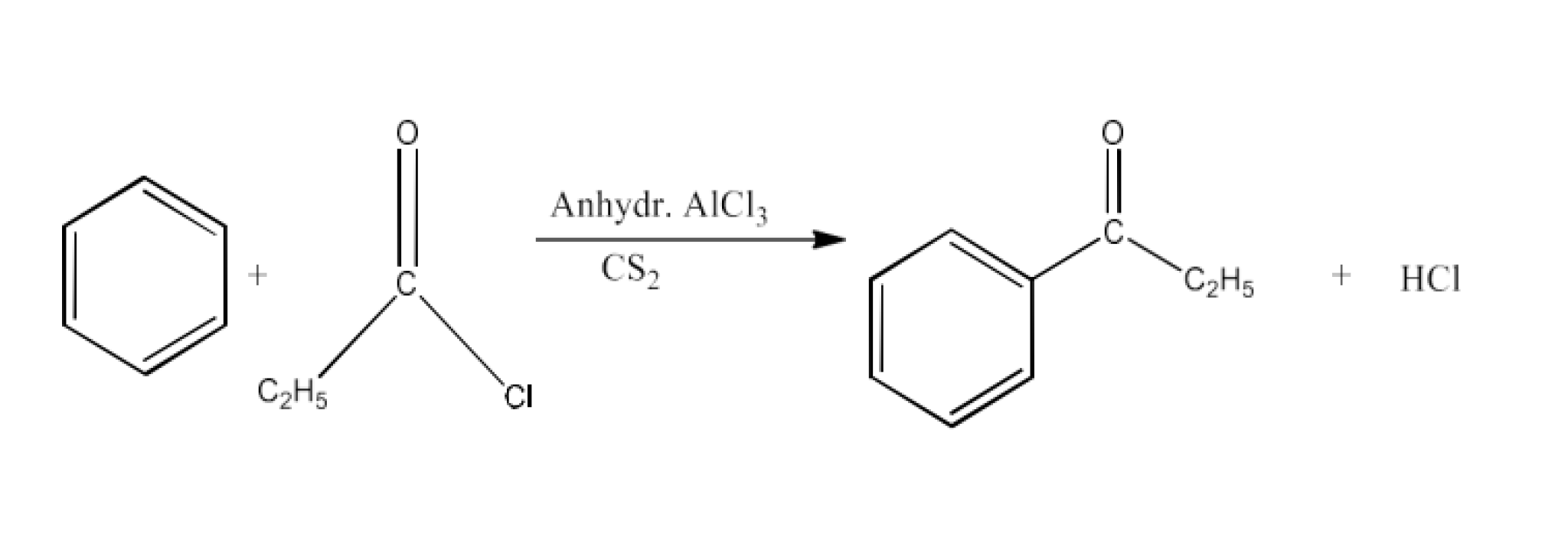
Ans: The product formed in this reaction is Propiophenone. The reaction is given below:
${{\text{(}{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}\text{C}{{\text{H}}_{\text{2}}}\text{)}}_{\text{2}}}\text{Cd + 2C}{{\text{H}}_{\text{3}}}\text{COCl }\to $
Ans: The product formed in this reaction is 1-Phenylpropan-2-one. The reaction is given below: \[{{\text{(}{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}\text{C}{{\text{H}}_{\text{2}}}\text{)}}_{\text{2}}}\text{Cd + 2C}{{\text{H}}_{\text{3}}}\text{COCl }\to \text{ 2C}{{\text{H}}_{\text{3}}}\text{COC}{{\text{H}}_{\text{2}}}{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}\text{ + CdC}{{\text{l}}_{\text{2}}}\]
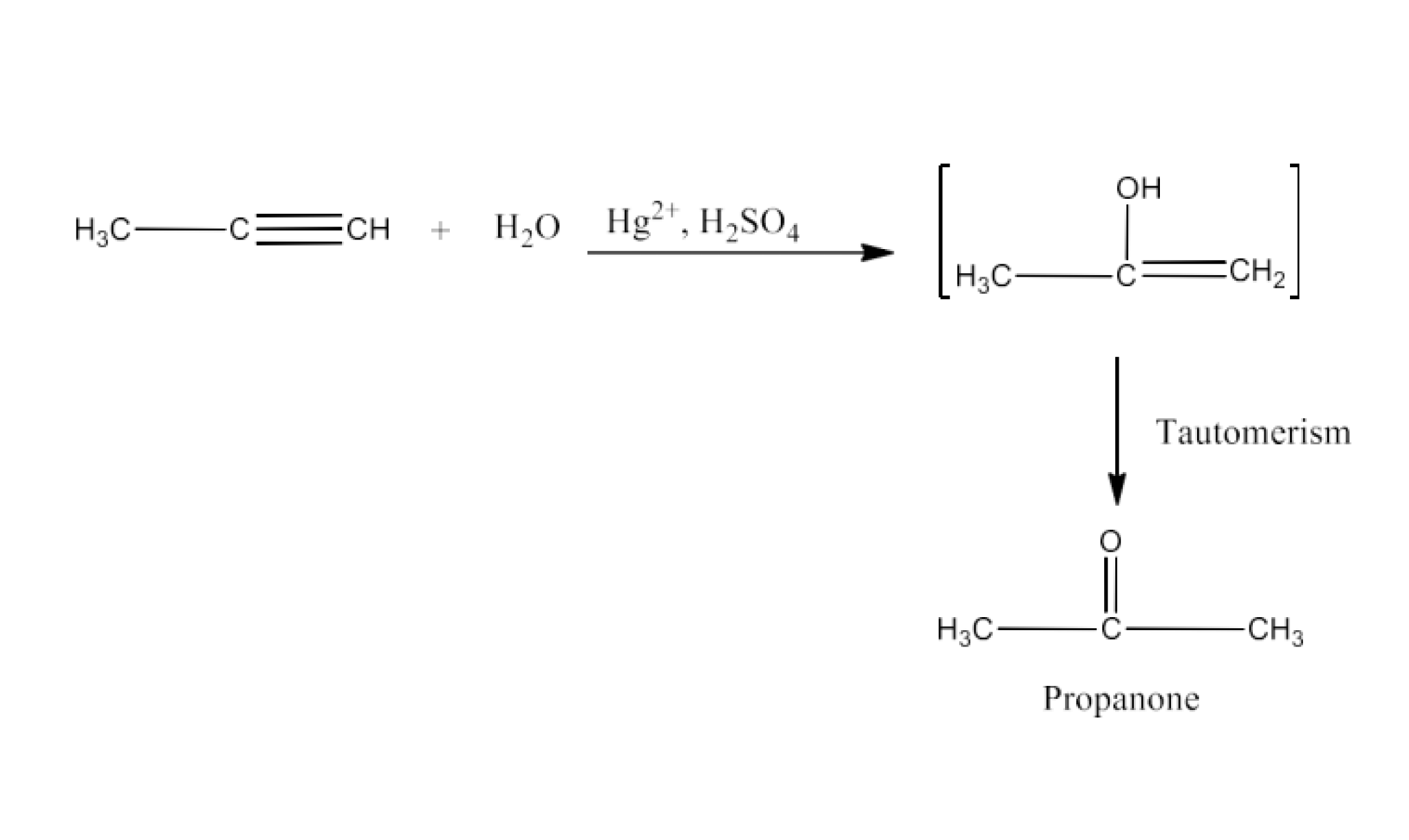
$\text{C}{{\text{H}}_{\text{3}}}\text{-C}\equiv \text{CH }\xrightarrow{\text{H}{{\text{g}}^{\text{2+}}}\text{,}{{\text{H}}_{\text{2}}}\text{S}{{\text{O}}_{\text{4}}}}$
Ans: The product formed in this reaction is Propanone. The reaction is given below:
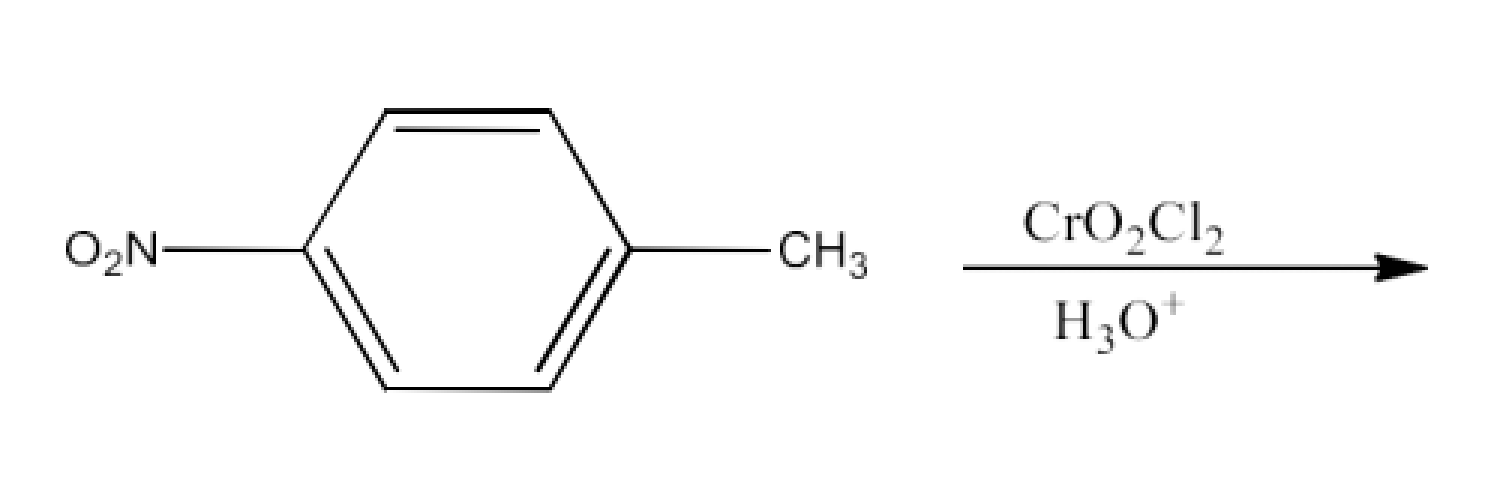
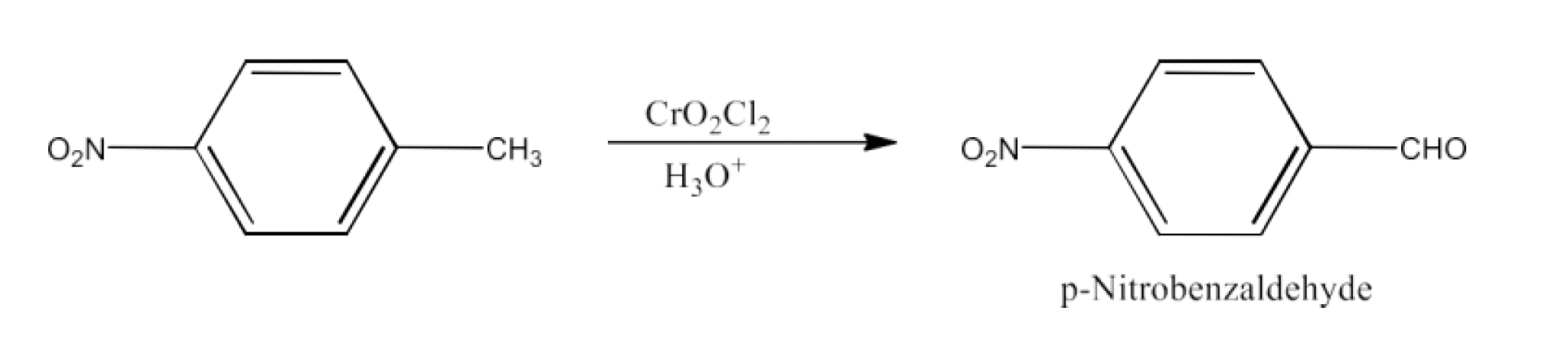
Ans: The product formed in this reaction is p-Nitrobenzaldehyde. The reaction is given below:
3. Arrange the following compounds in increasing order of their boiling points.
\[\text{C}{{\text{H}}_{\text{3}}}\text{CHO, C}{{\text{H}}_{\text{3}}}\text{C}{{\text{H}}_{\text{2}}}\text{OH, C}{{\text{H}}_{\text{3}}}\text{OC}{{\text{H}}_{\text{3}}}\text{, C}{{\text{H}}_{\text{3}}}\text{C}{{\text{H}}_{\text{2}}}\text{C}{{\text{H}}_{\text{3}}}\]
Ans: The molecular masses are in the range 44 to 46 of the listed compounds. $\text{C}{{\text{H}}_{\text{3}}}\text{C}{{\text{H}}_{\text{2}}}\text{OH}$ has the greatest boiling point is consequently subjected to substantial, intermolecular H-bonding, culminating in the combination between molecules.
$\text{C}{{\text{H}}_{\text{3}}}\text{CHO}$ is more polar than $\text{C}{{\text{H}}_{\text{3}}}\text{OC}{{\text{H}}_{\text{3}}}$, thus the dipole is greater than $\text{C}{{\text{H}}_{\text{3}}}\text{OC}{{\text{H}}_{\text{3}}}$, and $\text{C}{{\text{H}}_{\text{3}}}\text{C}{{\text{H}}_{\text{2}}}\text{C}{{\text{H}}_{\text{3}}}$ is weaker than the van der Waals.
The order will be:
\[\text{C}{{\text{H}}_{\text{3}}}\text{C}{{\text{H}}_{\text{2}}}\text{C}{{\text{H}}_{\text{3}}}\text{ C}{{\text{H}}_{\text{3}}}\text{OC}{{\text{H}}_{\text{3}}}\text{ C}{{\text{H}}_{\text{3}}}\text{CHO C}{{\text{H}}_{\text{3}}}\text{C}{{\text{H}}_{\text{2}}}\text{OH}\]
4. Arrange the following compounds in increasing order of their reactivity in Nucleophilic addition reactions.
Ethanal, Propanal, Propanone, Butanone.
Ans: The structures of all the compounds are given below:
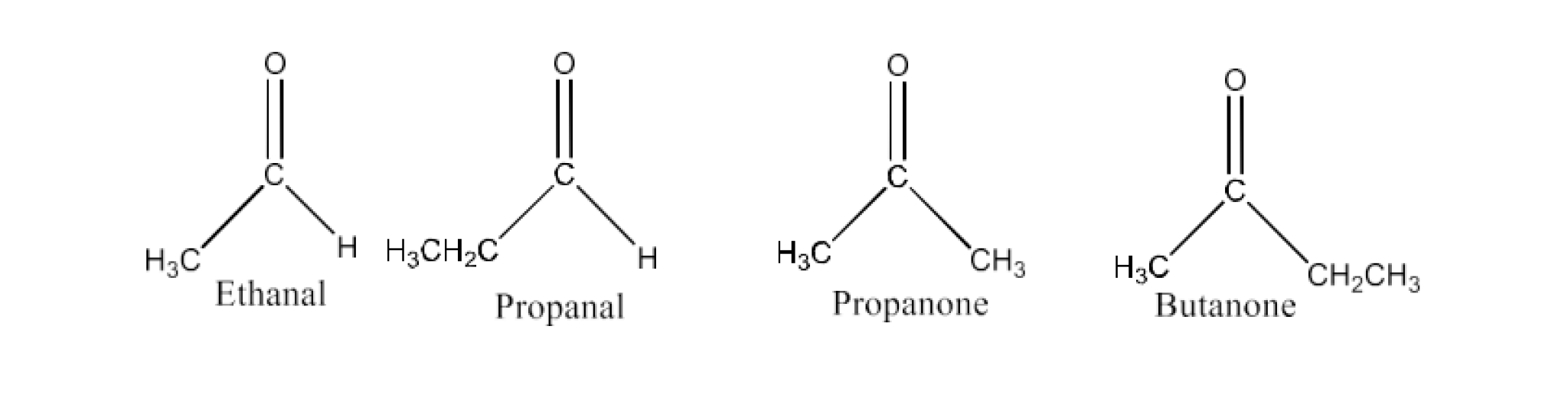
The alkyl group +I impact increases:
Ethanal < Propanal < Propanone < Butanone
With the +I effect increasing, the electron density of carbonyl carbon rises. As a consequence, the possibilities of a nucleophile attack are reduced. Thus, in nucleophilic addition reactions, the increasing order of reactivities of given carbonyl compounds is Butanone < Propanone < Propanal < Ethanal.
Benzaldehyde, p-Tolualdehyde, p-Nitrobenzaldehyde, Acetophenone
Ans: The structure of the following compounds is given below:
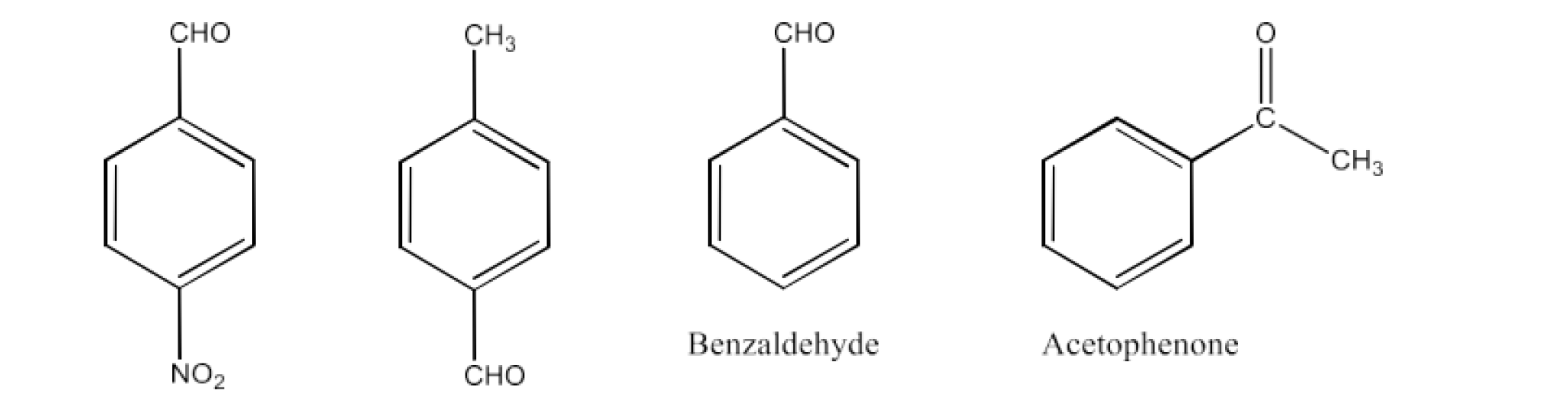
The impact of +I in ketone is higher than aldehyde. Acetophenone is therefore the least reactive in nuclear processes. The +I effect in p-tolualdehyde is greatest among aldehydes, due to the electron-donating group $\text{-C}{{\text{H}}_{\text{3}}}$ and the presence of the electron-donating group $\text{-N}{{\text{O}}_{\text{2}}}$ and the existence of the p-nitrobenzaldehyde.
Therefore, the increasing order of the compound reactivities is:
Benzaldehyde < p-Nitrobenzaldehyde < Acetophenone < p-tolualdehyde.
5. Predict the products of the following reactions:
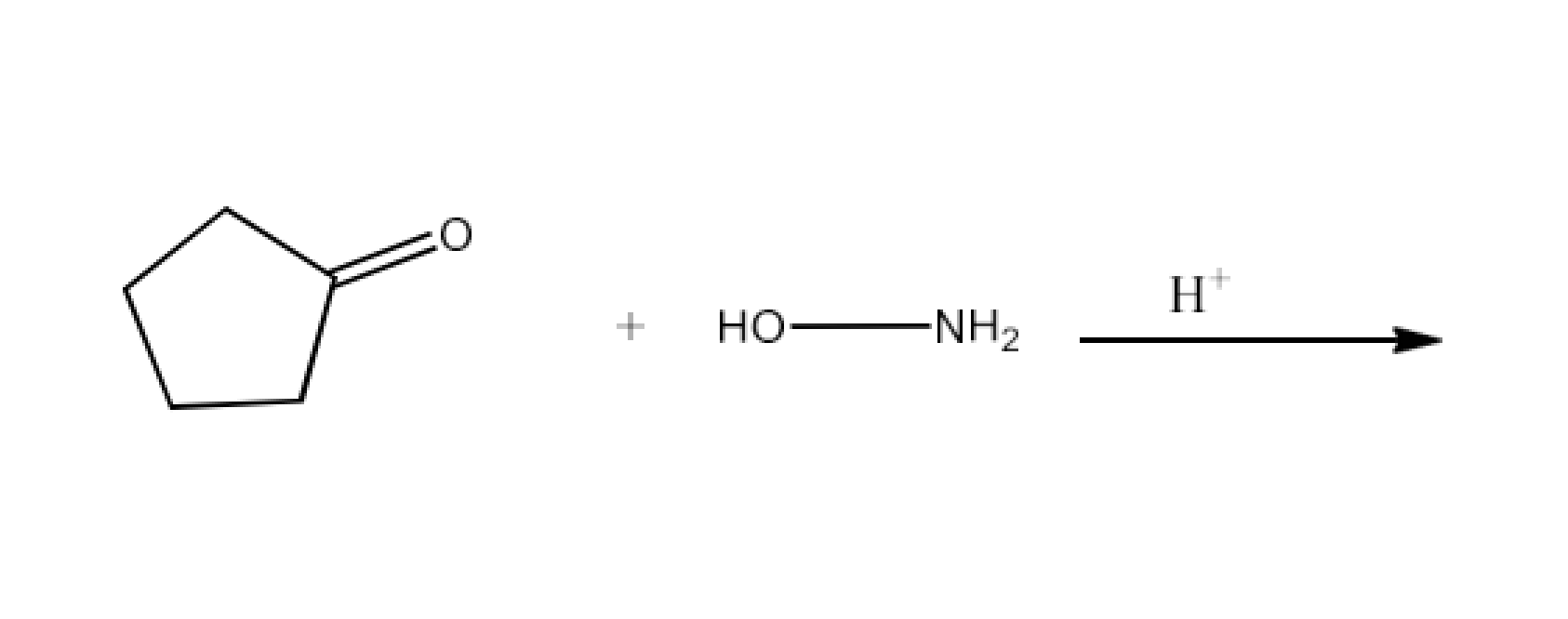
Ans: The complete reaction is given below:
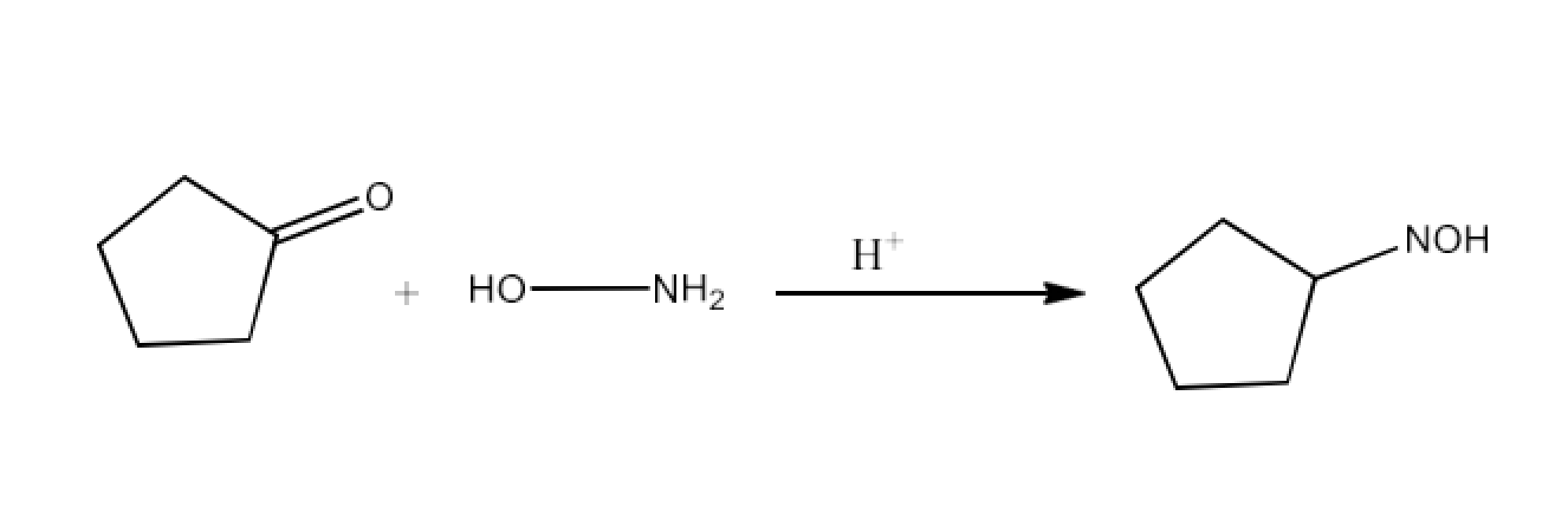
Ans: The complete reaction is given below:
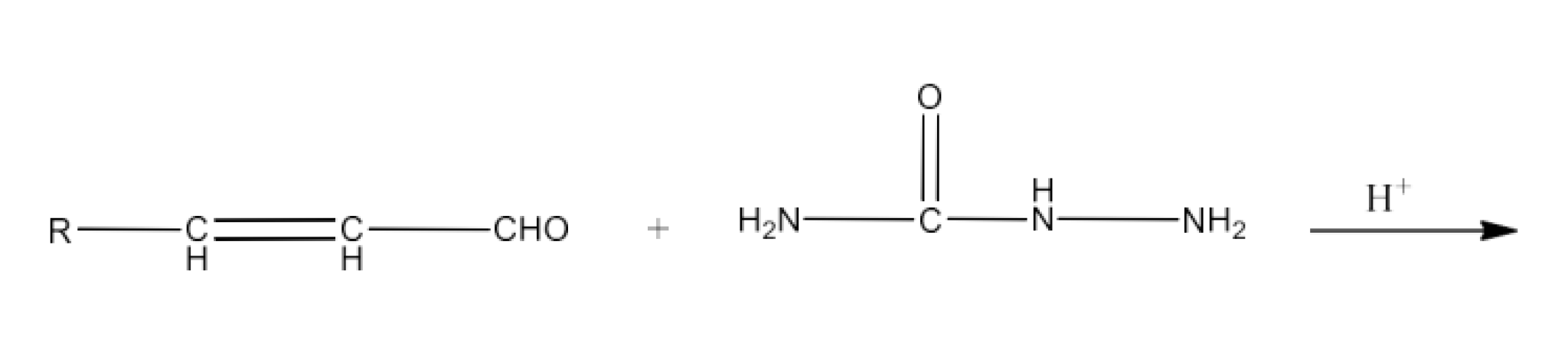
Ans: The complete reaction is given below:
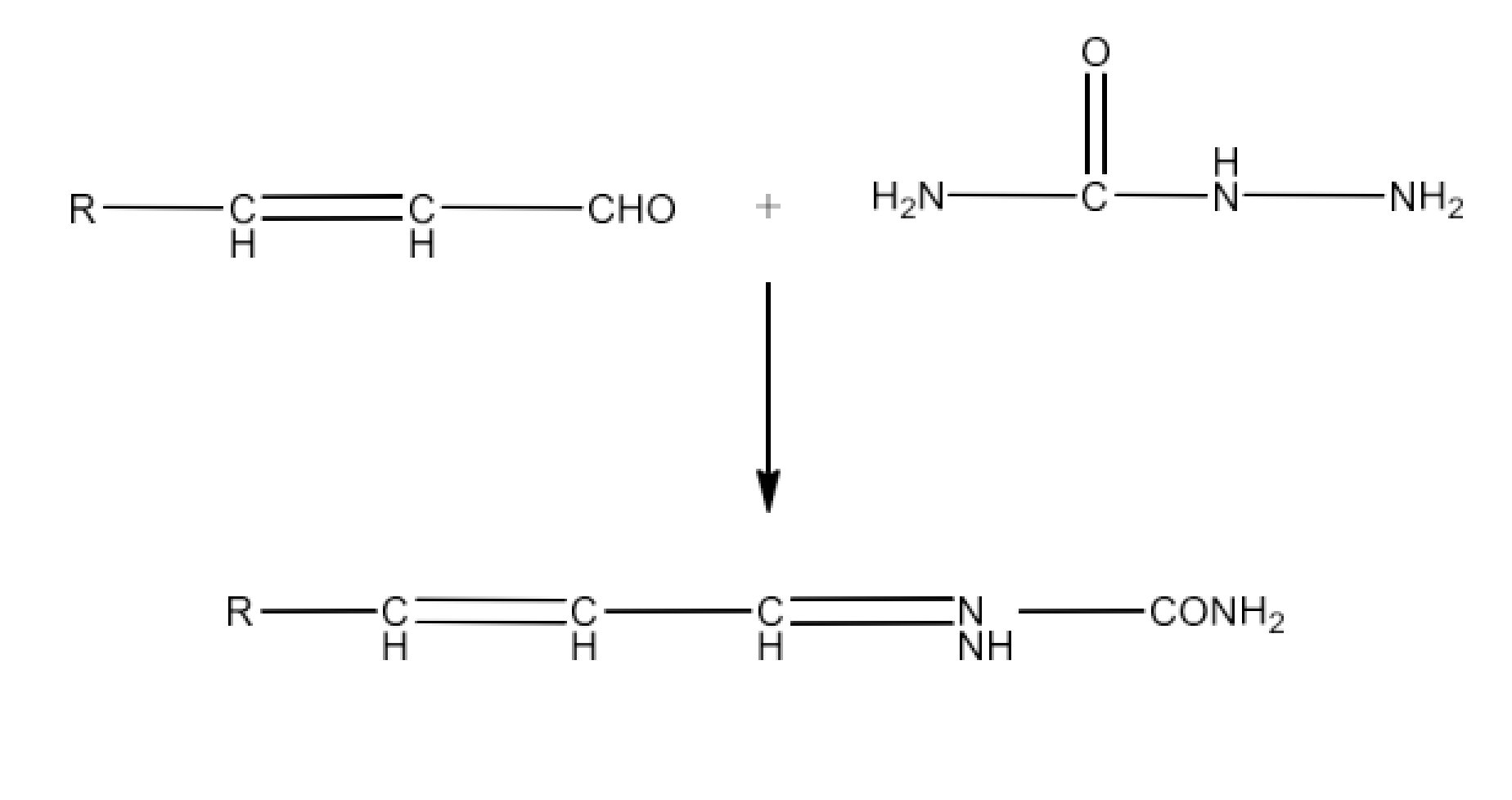
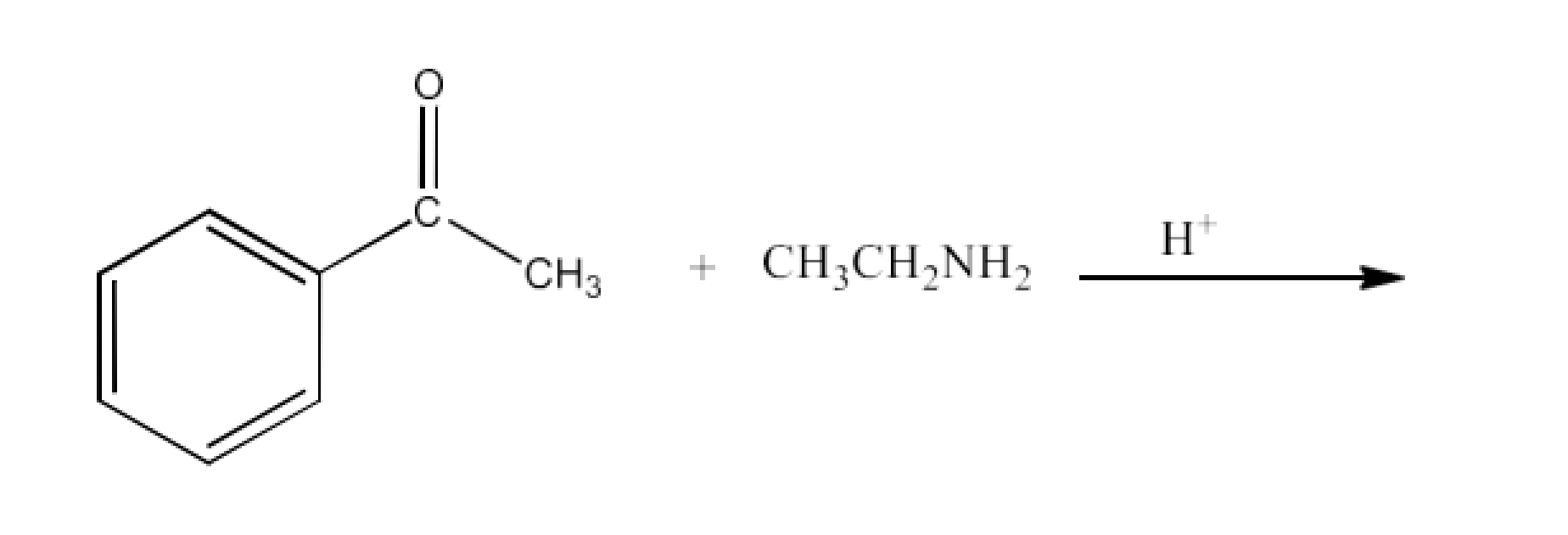
Ans: The complete reaction is given below:
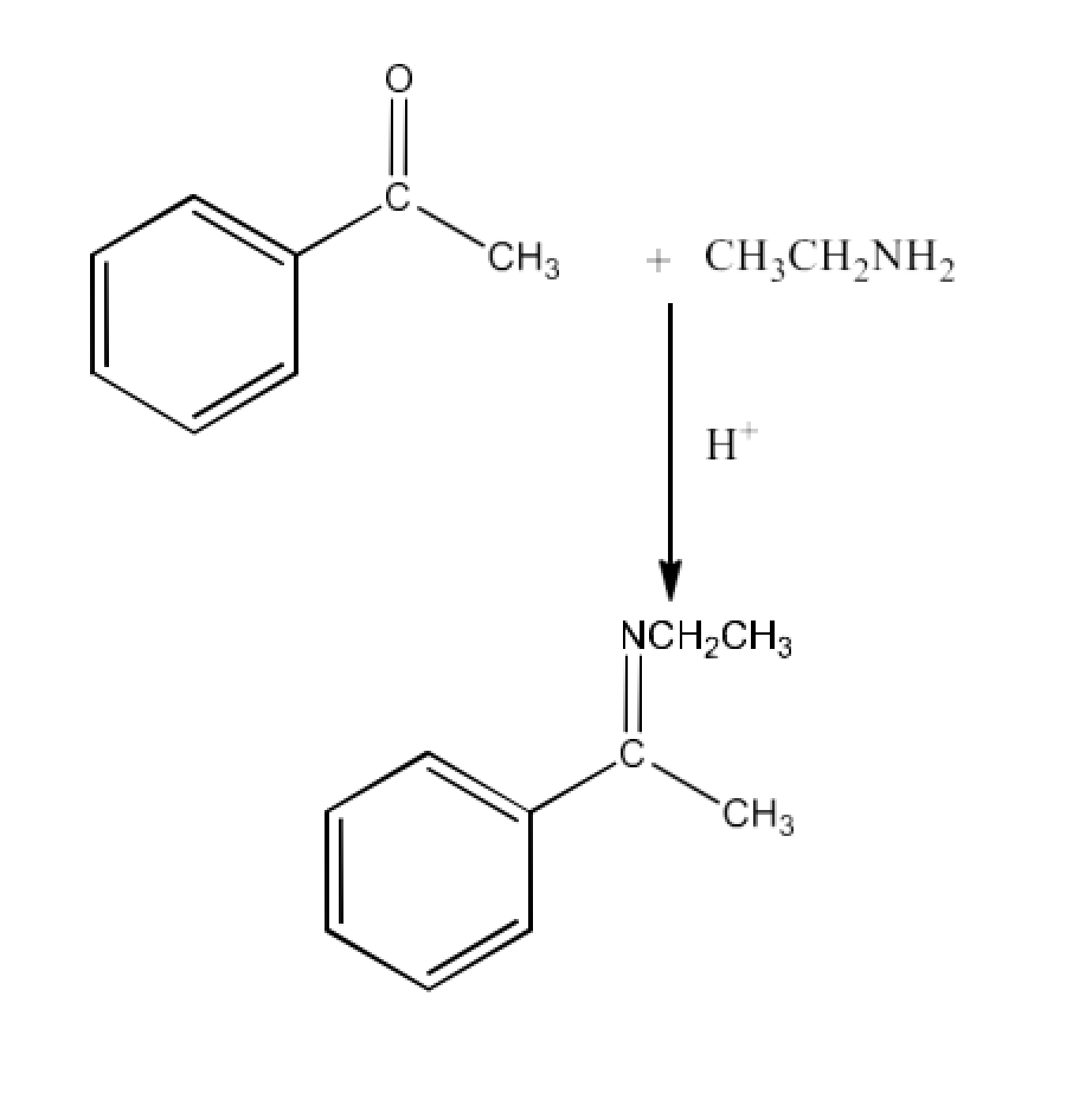
6. Give the IUPAC names of the following compounds:
$\text{PhC}{{\text{H}}_{\text{2}}}\text{C}{{\text{H}}_{\text{2}}}\text{COOH}$
Ans: The IUPAC name of the compound is 3-Phenylpropanoic acid.
${{\text{(C}{{\text{H}}_{\text{3}}}\text{)}}_{\text{2}}}\text{C=CHCOOH}$
Ans: The IUPAC name of the compound is 3-Methylbut-2-enoic acid.
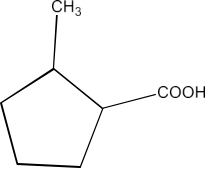
Ans: The IUPAC name of the compound is 2-Methylcyclopentanecarboxylic acid.
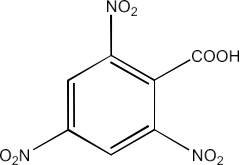
Ans: The IUPAC name of the compound is 2, 4, 6-Trinitrobenzoic acid.
7. Show how each of the following compounds can be converted to benzoic acid.
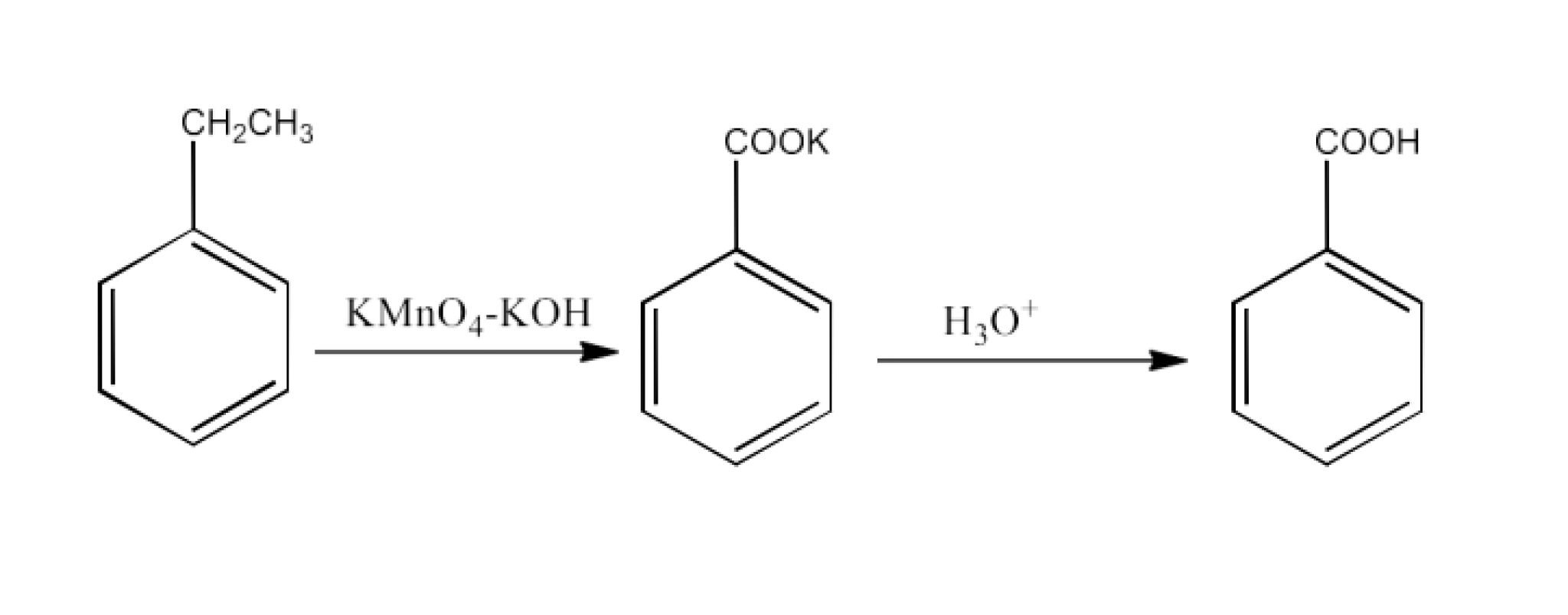
Ethylbenzene
Ans: Ethylbenzene will convert into potassium benzoate. Now, this potassium benzoate will be hydrolyzed to form benzoic acid. The reaction is given below:
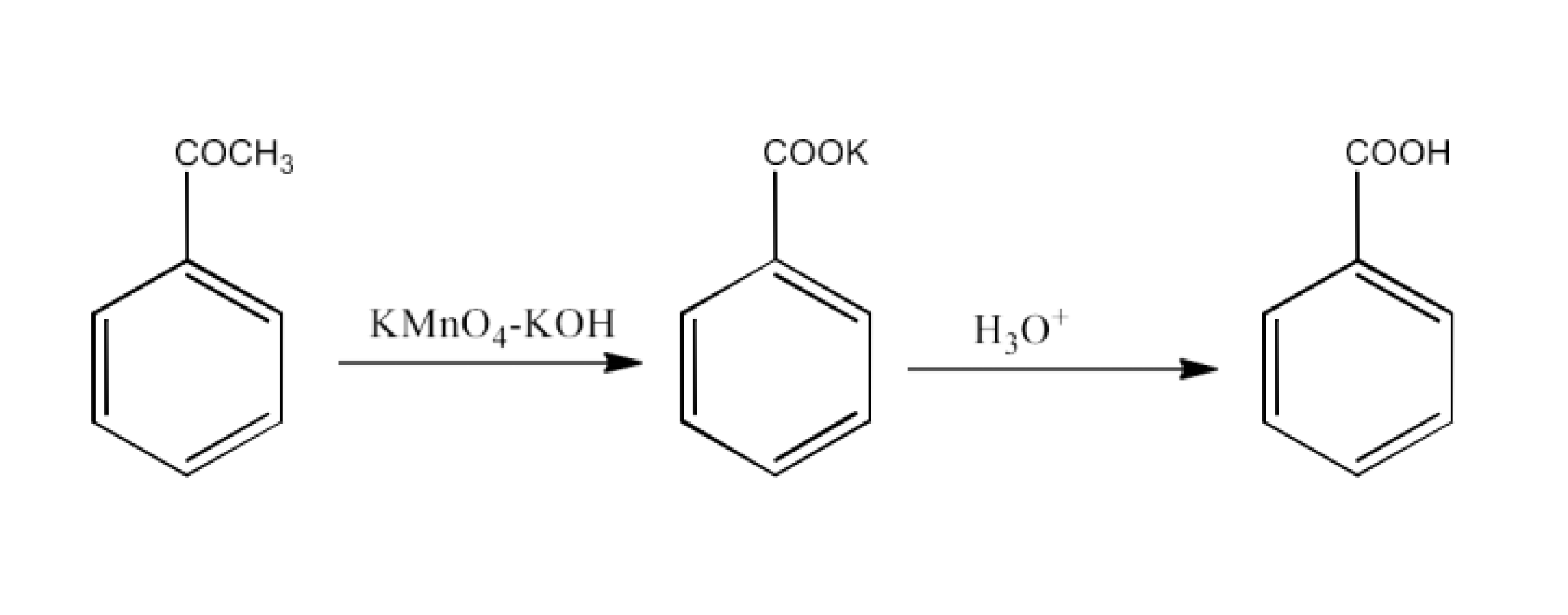
Acetophenone
Ans: Acetophenone will convert into potassium benzoate. Now, this potassium benzoate will be hydrolyzed to form benzoic acid. The reaction is given below:
Bromobenzene
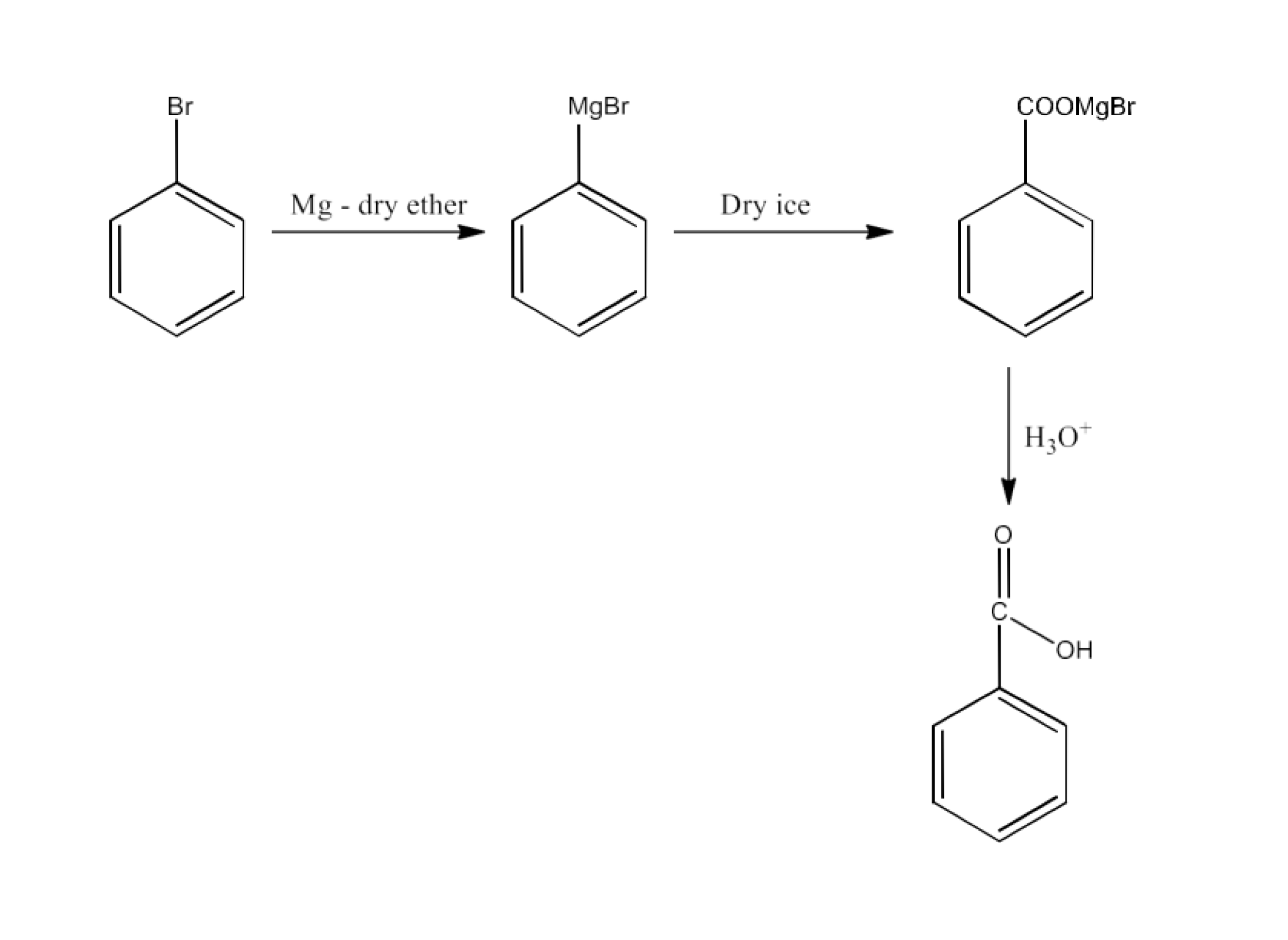
Ans: Bromobenzene will react with magnesium to form Phenyl magnesium bromide. This will be converted into benzoic acid. The reaction is given below:
Phenylethene (Styrene)
Ans: Phenylethene will convert into potassium benzoate. Now, this potassium benzoate will be hydrolyzed to form benzoic acid. The reaction is given below:
8. Which acid of each pair shown here would you expect to be stronger?
$\text{C}{{\text{H}}_{\text{3}}}\text{C}{{\text{O}}_{\text{2}}}\text{H o rC}{{\text{H}}_{\text{2}}}\text{FC}{{\text{O}}_{\text{2}}}\text{H}$
Ans: The structures of the compounds are given below:
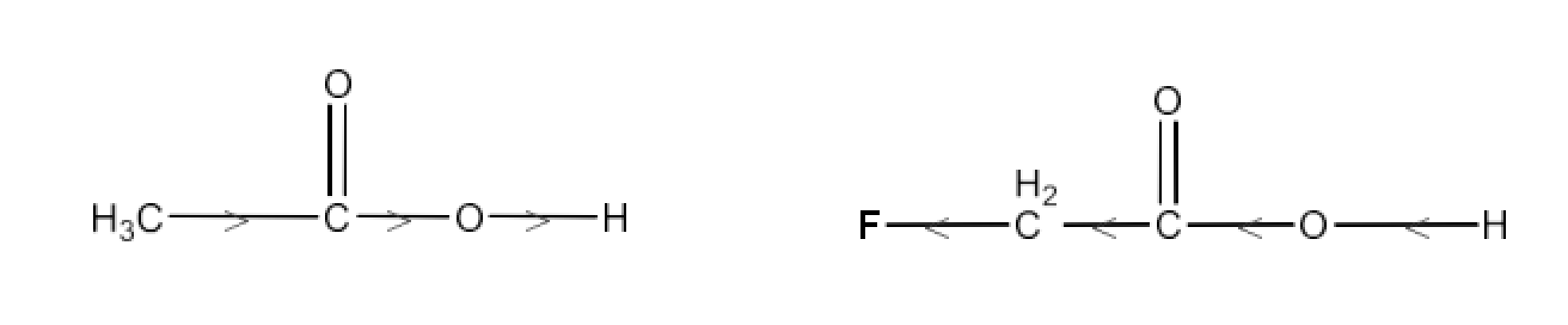
The $\text{-C}{{\text{H}}_{\text{3}}}$ Group's +I action increases the electron density on the O-H connection. Thus, it is tough to release the proton. The −I impact of F on the O-H bond, on the other hand, reduces the electron density. Proton may therefore readily be released. $\text{C}{{\text{H}}_{\text{2}}}\text{FC}{{\text{O}}_{\text{2}}}\text{H}$ is hence stronger than $\text{C}{{\text{H}}_{\text{3}}}\text{C}{{\text{O}}_{\text{2}}}\text{H}$.
$\text{C}{{\text{H}}_{\text{2}}}\text{FC}{{\text{O}}_{\text{2}}}\text{H or C}{{\text{H}}_{\text{2}}}\text{ClC}{{\text{O}}_{\text{2}}}\text{H}$
Ans: F has stronger −I effect than Cl. Therefore, $\text{C}{{\text{H}}_{\text{2}}}\text{FC}{{\text{O}}_{\text{2}}}\text{H}$ can release proton more easily than $\text{C}{{\text{H}}_{\text{2}}}\text{ClC}{{\text{O}}_{\text{2}}}\text{H}$. Hence, $\text{C}{{\text{H}}_{\text{2}}}\text{FC}{{\text{O}}_{\text{2}}}\text{H}$ is stronger acid than $\text{C}{{\text{H}}_{\text{2}}}\text{ClC}{{\text{O}}_{\text{2}}}\text{H}$.
$\text{C}{{\text{H}}_{\text{2}}}\text{FC}{{\text{H}}_{\text{2}}}\text{C}{{\text{H}}_{\text{2}}}\text{C}{{\text{O}}_{\text{2}}}\text{H or C}{{\text{H}}_{\text{3}}}\text{CHFC}{{\text{H}}_{\text{2}}}\text{C}{{\text{O}}_{\text{2}}}\text{H}$
Ans: The structures of the compounds are given below:
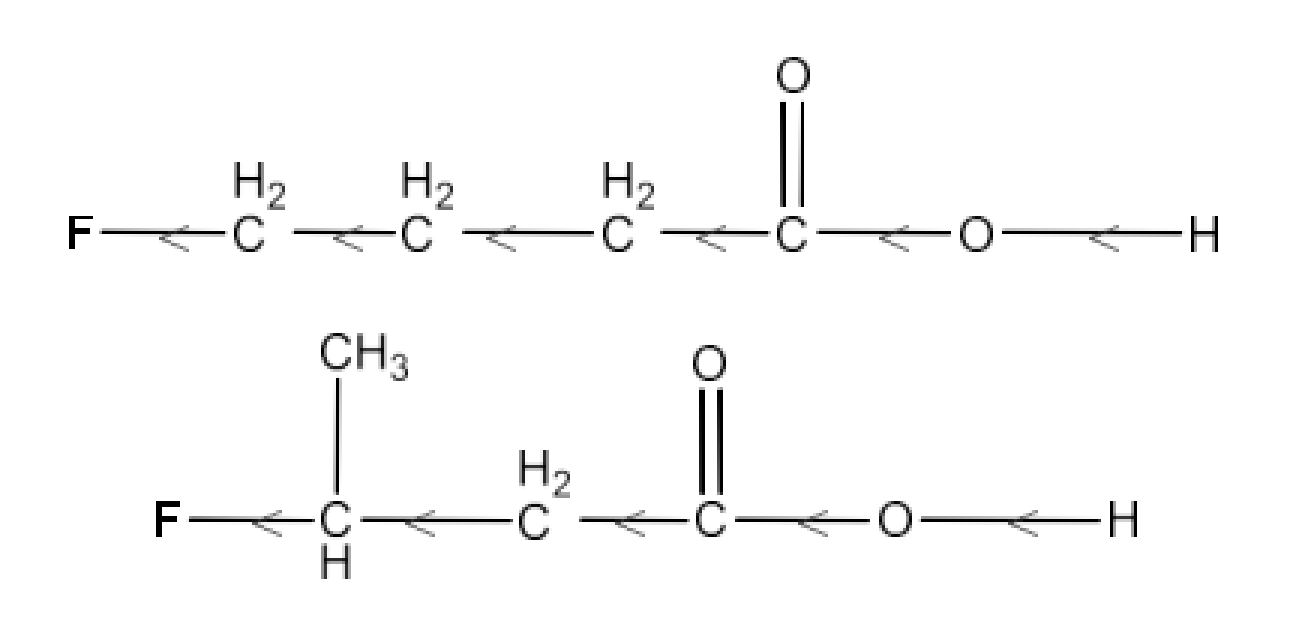
Inductive effect decreases with increase in distance. Hence, the +I effect of F in $\text{C}{{\text{H}}_{\text{3}}}\text{CHFC}{{\text{H}}_{\text{2}}}\text{C}{{\text{O}}_{\text{2}}}\text{H}$ is more than it is in $\text{C}{{\text{H}}_{\text{2}}}\text{FC}{{\text{H}}_{\text{2}}}\text{C}{{\text{H}}_{\text{2}}}\text{C}{{\text{O}}_{\text{2}}}\text{H}$. Hence, $\text{C}{{\text{H}}_{\text{3}}}\text{CHFC}{{\text{H}}_{\text{2}}}\text{C}{{\text{O}}_{\text{2}}}\text{H}$ is stronger acid than $\text{C}{{\text{H}}_{\text{2}}}\text{FC}{{\text{H}}_{\text{2}}}\text{C}{{\text{H}}_{\text{2}}}\text{C}{{\text{O}}_{\text{2}}}\text{H}$.
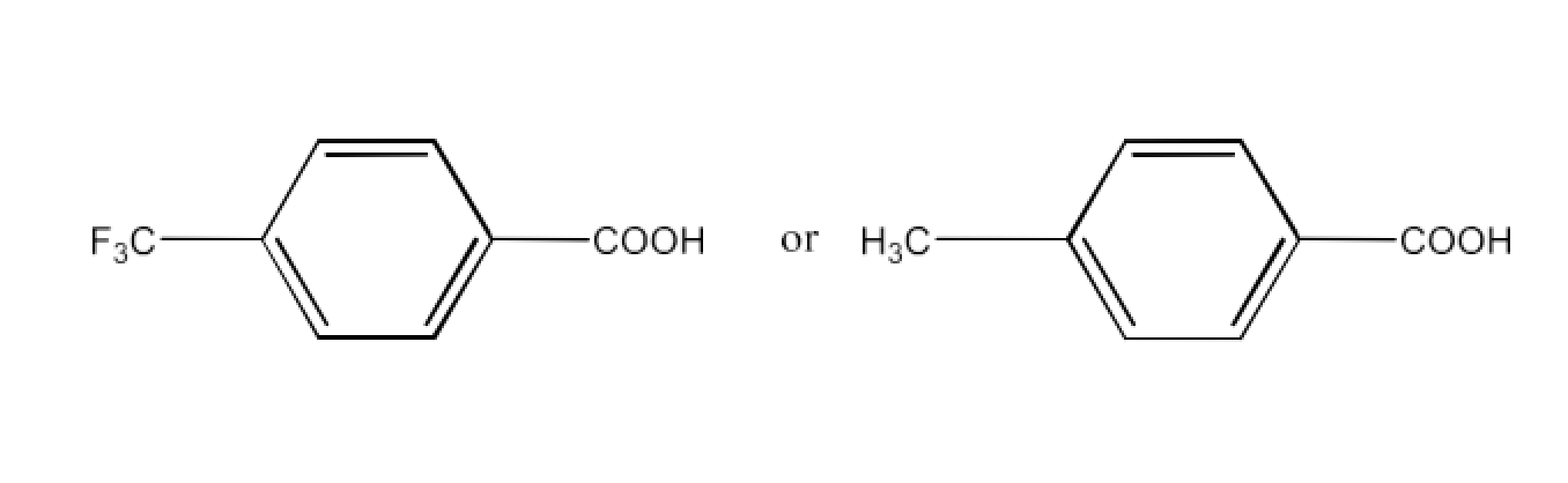
Ans: Due to the −I action of F, the release of proton for the chemical is simpler (A). However, proton release is problematic in the case of compound (B), because of the +I action of a group of $\text{-C}{{\text{H}}_{\text{3}}}$. (A) is hence stronger than acid (B).
Class 12 Chemistry Chapter 8 Quick Overview of Topics
Chemistry class 12 chapter 8 NCERT Solutions - Quick Overview of Detailed Structure of Topics and Subtopics Covered.
Topic | Subtopics |
Aldehyde | - Structure and classification |
- Methods of preparation | |
- Chemical properties and reactions | |
Ketone | - Structure and properties |
- Preparation methods | |
- Chemical reactions and applications | |
Carboxylic Acids | - Structure and nomenclature |
- Preparation techniques | |
- Acidic nature and reactions | |
- Derivatives: Esters, acid chlorides, and amides | |
Comparative Analysis | - Comparison of properties and reactivity among aldehyde, |
ketone, and carboxylic acids |
Some Important Highlights of Aldehydes, Ketones, and Carboxylic Acid NCERT Solutions
Class 12 NCERT solutions help the students to go through the formulas easily. Here find the Important topics of Chapter 8 - Aldehydes, Ketones, and Carboxylic Acid to crack your exams.
Nomenclature: Understanding the IUPAC nomenclature rules for aldehydes, ketones, and carboxylic acids is crucial.
Preparation Methods:
Aldehydes: From primary alcohols by mild oxidation (PCC, Tollens' reagent, Fehling's solution, etc.)
Ketones: From secondary alcohols by oxidation or from alkyl halides by Friedel-Crafts acylation.
Carboxylic Acids: From primary alcohols by strong oxidation from Grignard reagents, etc.
Reactions of Aldehydes and Ketones:
Nucleophilic Addition: Aldehydes and ketones undergo nucleophilic addition reactions with nucleophiles.
Oxidation: Aldehydes are oxidised to carboxylic acids, whereas ketones are not easily oxidised under mild conditions.
Reactions of Carboxylic Acids:
Esterification: Reaction with alcohols to form esters in the presence of an acid catalyst.
Decarboxylation: Carboxylic acids undergo decarboxylation to produce carbon dioxide and a lower alkane upon heating with soda lime
Acidity of Carboxylic Acids: Carboxylic acids are acidic due to the presence of the carboxyl group.
Benefits of Referring to Vedantu’s NCERT Solutions for Class 12 Chemistry Aldehydes, Ketones, and Carboxylic Acid
By referring to Vedantu’s NCERT Solutions for Class 12 Chemistry Chapter on Aldehydes, Ketones, and Carboxylic Acids, students can build a strong foundation, excel in their exams, and develop a deeper understanding of essential organic chemistry concepts. Here are some of the Benefits of Referring to Vedantu’s NCERT Solutions for Class 12 Chemistry Aldehydes, Ketones, and Carboxylic Acid:
Detailed explanations and step-by-step solutions for all topics in the chapter.
Includes preparation, properties, reactions, and uses of aldehydes, ketones, and carboxylic acids.
Solutions curated by experienced educators ensuring accuracy and clarity.
Covers important concepts like nucleophilic addition reactions, oxidation and reduction of carbonyl compounds, and methods of preparation of carboxylic acids.
Clear, concise explanations using precise chemical terminology.
Detailed analysis of the physical and chemical properties of aldehydes, ketones, and carboxylic acids.
Detailed explanation of important reactions such as the Aldol condensation, Cannizzaro reaction, and the Kolbe reaction.
Solutions to a variety of problems to strengthen analytical and problem-solving abilities.
Related Study Materials for Chemistry Class 12 Chapter 8 NCERT Solutions
Students can access extra study materials on Aldehydes, Ketones, and Carboxylic Acid. These resources are available for download, offering additional support for your studies.
S.No | Related Links for Class 12 Chemistry Chapter 8: Aldehydes, Ketones, and Carboxylic Acids |
1. | Aldehydes, Ketones, and Carboxylic Acids Important Questions |
2. | |
3. | Aldehydes, Ketones, and Carboxylic Acids NCERT Exemplar Solutions |
Conclusion
Chapter 8, "Aldehydes, Ketones, and Carboxylic Acids," provides a comprehensive understanding of these essential organic compounds. Through detailed study and analysis, students gain insights into their preparation, properties, reactions, and uses. This chapter highlights crucial concepts such as nucleophilic addition reactions, oxidation and reduction of carbonyl compounds, and the methods of preparation of carboxylic acids. Key reactions like Aldol condensation, Cannizzaro reaction, and the Kolbe reaction are explored thoroughly.
By mastering this chapter, students are well-equipped to tackle complex organic chemistry problems and can apply their knowledge to real-life scenarios. Understanding the behavior and reactions of aldehydes, ketones, and carboxylic acids is fundamental for further studies in organic chemistry and related fields. The thorough coverage provided by Vedantu’s NCERT Solutions ensures that students can approach their exams with confidence and achieve academic success.
NCERT Solutions For Class 12 Chemistry | Chapter-wise Links
S.No. | NCERT Solutions Class 12 Chemistry Chapter-wise List |
1 | |
2 | |
3 | |
4 | |
5 | |
6 | |
7 | |
8 | |
9 |
NCERT Solutions Class 12 Chemistry - Related Links
S.No | Important Resources Links for Class 12 Chemistry |
1 | |
2 | |
3 | |
4 | |
5 |
FAQs on NCERT Solutions For Class 12 Chemistry Chapter 8 Aldehydes Ketones And Carboxylic Acids - 2025-26
1. Where can I find complete and correct NCERT Solutions for Class 12 Chemistry Chapter 8?
You can find complete, step-by-step NCERT Solutions for Class 12 Chemistry Chapter 8 (Aldehydes, Ketones, and Carboxylic Acids) right here on Vedantu. Our solutions are prepared by expert teachers to be fully compliant with the CBSE 2025-26 syllabus, covering every in-text and exercise question accurately.
2. Do these NCERT Solutions explain the answers for both in-text and chapter-end exercises?
Yes, our solutions provide detailed explanations for all questions in Chapter 8. This includes every problem from the in-text questions as well as the comprehensive end-of-chapter exercises, ensuring you have a complete resource for your exam preparation.
3. What is the correct method to write the IUPAC name for a complex compound like CH₃CH(CH₃)CH₂C(CH₃)₂COCH₃ as shown in the NCERT exercises?
To solve this according to the NCERT solutions methodology, you should follow these steps:
- First, identify the principal functional group, which is the ketone (C=O).
- Next, find the longest carbon chain that includes the ketone group. This is a 6-carbon chain (hexane), making the parent name Hexan-2-one.
- Number the chain from the end that gives the ketone the lowest number (in this case, from the right).
- Finally, identify and number the substituent groups: two methyl groups at carbon 3 and one at carbon 5.
4. In the NCERT solutions, why does Benzaldehyde undergo the Cannizzaro reaction but Cyclohexanone undergoes Aldol condensation?
The critical difference, as explained by the principles in Chapter 8, is the presence of an alpha-hydrogen.
- Aldol Condensation requires at least one alpha-hydrogen (a hydrogen on the carbon next to the C=O group). Cyclohexanone has alpha-hydrogens, which a base can remove to initiate the reaction.
- Cannizzaro Reaction is specific to aldehydes that lack an alpha-hydrogen, such as Benzaldehyde. In the presence of a strong base, these aldehydes undergo self-oxidation and reduction.
5. How should I use the NCERT solutions to solve problem 8.19, which asks to identify a compound from its molecular formula and chemical reactions?
The NCERT solutions guide you to solve such problems systematically. For a compound with formula C₅H₁₀O, the approach is:
- Analyse the Tests: "Does not reduce Tollens’ reagent" means it is a ketone. "Gives a positive iodoform test" indicates a methyl ketone (CH₃CO–) structure.
- Deduce the Structure: A five-carbon methyl ketone must be Pentan-2-one.
- Verify the Structure: The final clue, that vigorous oxidation gives ethanoic and propanoic acid, confirms the cleavage of Pentan-2-one at the carbonyl group, validating the answer.
6. When converting ethanal to but-2-enoic acid (NCERT Exercise 8.8), what is the specific role of Tollen's reagent in the final step?
In this multi-step conversion, Tollen's reagent functions as a mild oxidising agent. After the initial aldol condensation forms but-2-enal, Tollen's reagent is used to selectively oxidise the aldehyde group (–CHO) into a carboxylate ion. This is a crucial step because it achieves the oxidation without affecting the carbon-carbon double bond present in the molecule.
7. Why is the Sodium Bicarbonate test, mentioned in the NCERT solutions, effective for distinguishing between Benzoic acid and Ethyl benzoate?
The Sodium Bicarbonate test works by identifying the acidic carboxyl group (–COOH). Benzoic acid is acidic enough to react with the weak base sodium bicarbonate, producing brisk effervescence from the release of carbon dioxide gas. In contrast, Ethyl benzoate is an ester and lacks this acidic hydrogen, so it does not react. This clear, observable difference makes it an excellent distinguishing test.
8. What is the chemical principle that explains why Di-tert-butyl ketone is less reactive towards HCN than Acetaldehyde, as ordered in the NCERT solutions?
The lower reactivity of Di-tert-butyl ketone is due to two main factors:
- Steric Hindrance: The two large, bulky tert-butyl groups physically block the nucleophile (CN⁻) from attacking the carbonyl carbon. Acetaldehyde has only a small methyl group and a hydrogen atom, offering very little hindrance.
- Electronic Effects: The alkyl groups in ketones have a positive inductive effect (+I), which reduces the partial positive charge on the carbonyl carbon, making it less attractive to nucleophiles. The two tert-butyl groups create a much stronger +I effect than the single methyl group in acetaldehyde.
9. Are the NCERT Solutions for Chapter 8 updated for the CBSE 2025-26 board exams?
Yes, all NCERT solutions provided by Vedantu for Class 12 Chemistry Chapter 8 are fully updated and aligned with the latest CBSE 2025-26 syllabus and the current NCERT textbook. The methods and answers are designed to help you score effectively in your board examinations.


























