
How Can Coordination Chemistry Class 12 NCERT Solutions Help With Chapter 5 Questions And Answers For Exam Preparation
Coordination chemistry class 12 questions and answers help students understand complex compounds and their properties. This chapter covers metal ions bonding with ligands to form coordination compounds. Class 12 chemistry chapter 5 NCERT solutions explain each concept step by step.
 Table of Content
Table of ContentThese solutions help students solve problems quickly and build confidence. Every answer includes detailed explanations and important formulas. Download the NCERT Solutions PDF for free and master coordination chemistry today.
How Can Coordination Chemistry Class 12 NCERT Solutions Help With Chapter 5 Questions And Answers For Exam Preparation
Intext Exercise
1. Write the formulas for the following coordination compounds:
(i) Tetraamminediaquacobalt (III) chloride
Ans: The formula of Tetraamminediaquacobalt (III) chloride is $\text{ }\!\![\!\!\text{ Co(}{{\text{H}}_{\text{2}}}\text{O)(N}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{4}}}\text{ }\!\!]\!\!\text{ C}{{\text{l}}_{\text{3}}}$.
(ii) Potassium tetracyanonickelate(II)
Ans: The formula of Potassium tetracyanonickelate(II) is ${{\text{K}}_{\text{2}}}\text{ }\!\![\!\!\text{ Ni(CN}{{\text{)}}_{\text{4}}}\text{ }\!\!]\!\!\text{ }$.
(iii) Tris(ethane-1,2-diamine) chromium(III) chloride
Ans: The formula of Tris(ethane-1,2-diamine) chromium(III) chloride is $\text{ }\!\![\!\!\text{ Cr(en}{{\text{)}}_{\text{3}}}\text{ }\!\!]\!\!\text{ C}{{\text{l}}_{\text{3}}}$
(iv) Amminebromidochloridonitrito-N-platinate(II)
Ans: The formula of Amminebromidochloridenitrito-N-platinate(II) is ${{\text{ }\!\![\!\!\text{ Pt(N}{{\text{H}}_{\text{3}}}\text{)BrCl(N}{{\text{O}}_{\text{2}}}\text{) }\!\!]\!\!\text{ }}^{\text{-}}}$
(v) Dichloridobis(ethane-1,2-diamine)platinum(IV) nitrate
Ans: The formula of Dichloridobis(ethane-1,2-diamine)platinum(IV) nitrate is $\text{ }\!\![\!\!\text{ PtC}{{\text{l}}_{\text{2}}}{{\text{(en)}}_{\text{2}}}\text{ }\!\!]\!\!\text{ (N}{{\text{O}}_{\text{3}}}{{\text{)}}_{\text{2}}}$
(vi) Iron(III) hexacyanoferrate(II)
Ans: The formula of Iron(III) hexacyanoferrate(II) is $\text{F}{{\text{e}}_{\text{4}}}{{\text{ }\!\![\!\!\text{ Fe(CN}{{\text{)}}_{\text{6}}}\text{ }\!\!]\!\!\text{ }}_{\text{3}}}$
2. Write the IUPAC names of the following coordination compounds:
(i) $\text{ }\!\![\!\!\text{ Co(N}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{6}}}\text{ }\!\!]\!\!\text{ C}{{\text{l}}_{\text{3}}}$
Ans: The IUPAC name of the compound is Hexaamminecobalt(III) chloride.
(ii) $\text{ }\!\![\!\!\text{ Co(N}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{6}}}\text{Cl }\!\!]\!\!\text{ C}{{\text{l}}_{\text{2}}}$
Ans: The IUPAC name of the compound is Pentaamminechloridocobalt(III) chloride.
(iii) ${{\text{K}}_{\text{3}}}\text{ }\!\![\!\!\text{ Fe(CN}{{\text{)}}_{\text{6}}}\text{ }\!\!]\!\!\text{ }$
Ans: The IUPAC name of the compound is Potassium hexacyanoferrate (III).
(iv) ${{\text{K}}_{\text{3}}}\text{ }\!\![\!\!\text{ Fe(}{{\text{C}}_{\text{2}}}{{\text{O}}_{\text{4}}}{{\text{)}}_{\text{3}}}\text{ }\!\!]\!\!\text{ }$
Ans: The IUPAC name of the compound is Potassium trioxalatoferrate(III).
(v) ${{\text{K}}_{\text{2}}}\text{ }\!\![\!\!\text{ PdC}{{\text{l}}_{\text{4}}}\text{ }\!\!]\!\!\text{ }$
Ans: The IUPAC name of the compound is Potassium tetrachloridopalladate(II).
(vi) $\text{ }\!\![\!\!\text{ Pt(N}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{2}}}\text{Cl(N}{{\text{H}}_{\text{2}}}\text{C}{{\text{H}}_{\text{3}}}\text{) }\!\!]\!\!\text{ Cl}$
Ans: The IUPAC name of the compound is Diamminechloride (methylamine) platinum(II) chloride.
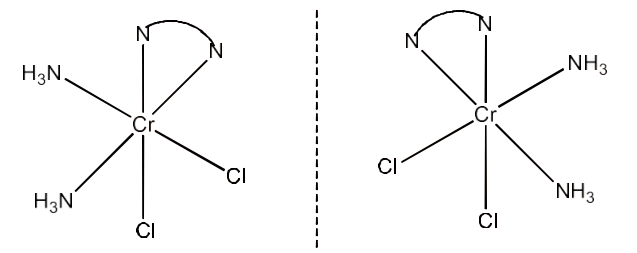
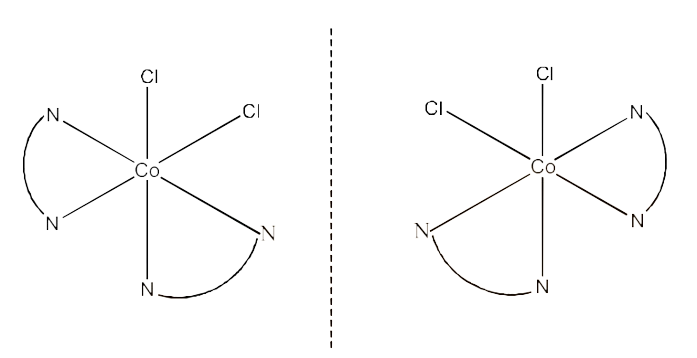
3. Indicate the types of isomerism exhibited by the following complexes and draw the structures for these isomers:
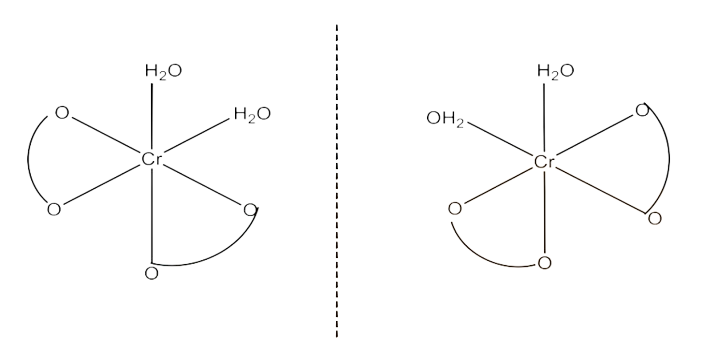
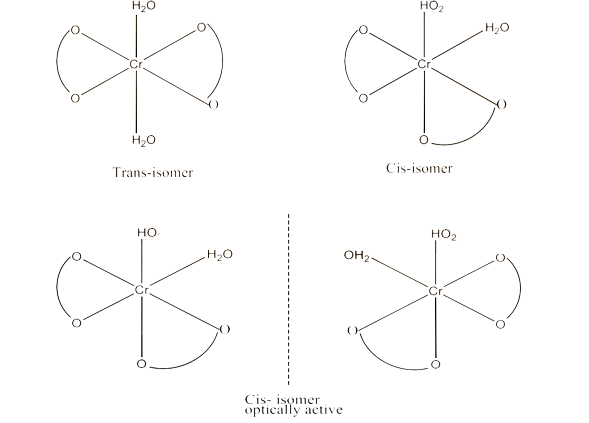
(i) $\text{K }\!\![\!\!\text{ Cr(}{{\text{H}}_{\text{2}}}\text{O}{{\text{)}}_{\text{2}}}{{\text{(}{{\text{C}}_{\text{2}}}{{\text{O}}_{\text{4}}}\text{)}}_{\text{2}}}\text{ }\!\!]\!\!\text{ }$
Ans: Both geometrical isomers (cis and trans) are possible for this compound. These are given below:
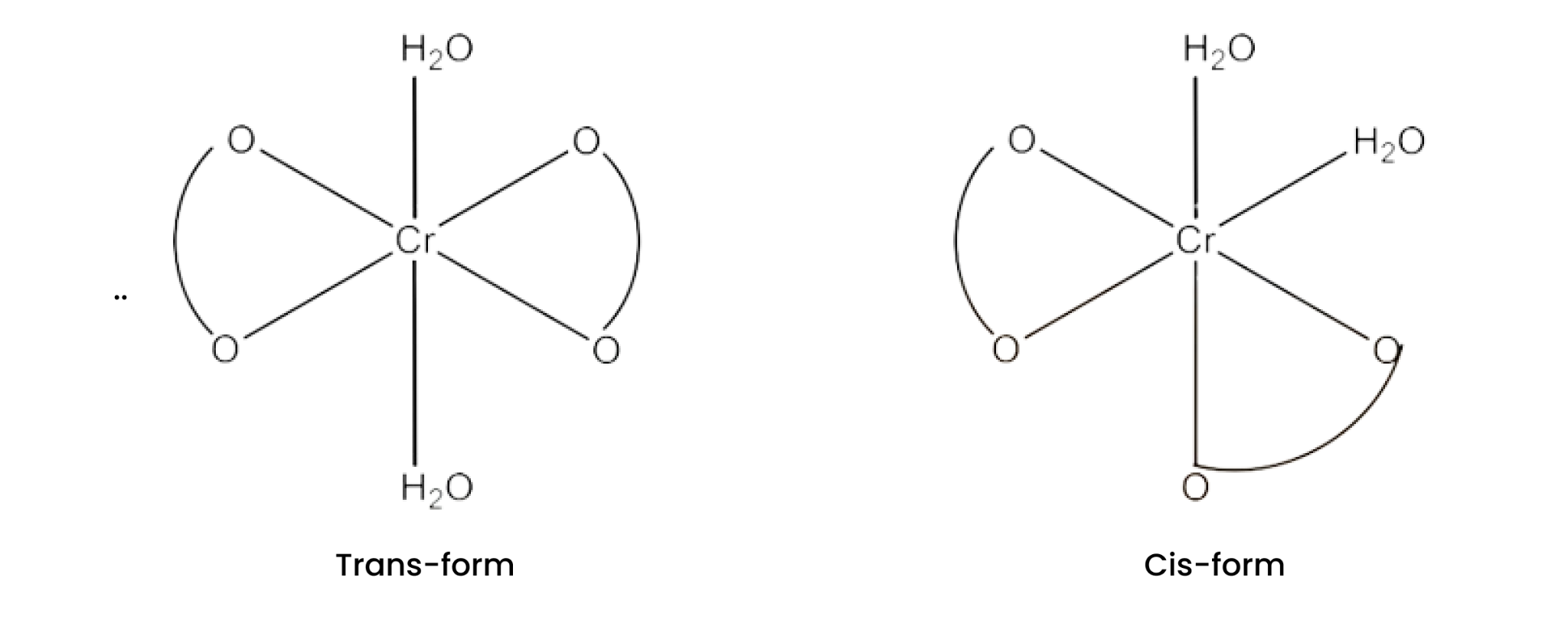
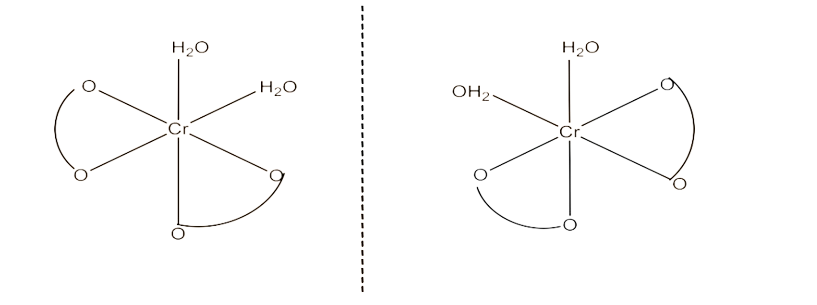
The trans-isomers of this compound is optically inactive but the cis-isomer is optically active. Therefore, it can also show optical isomerism. This is given below:
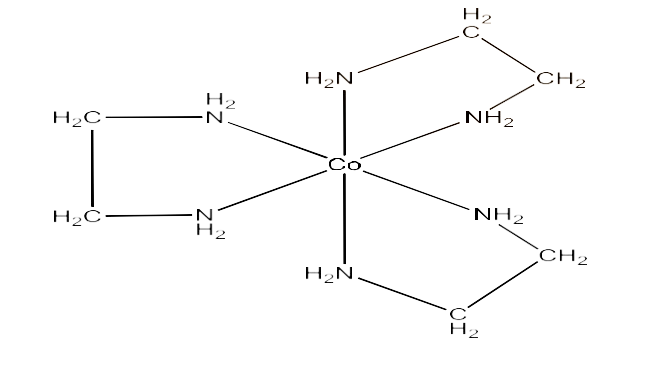
(ii) $\text{ }\!\![\!\!\text{ Co(en}{{\text{)}}_{\text{3}}}\text{ }\!\!]\!\!\text{ C}{{\text{l}}_{\text{3}}}$
Ans: This compound can show optical isomerism. The structure is given below:
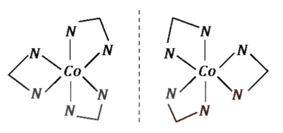
Its optical isomers are given below:
(iii) $\text{ }\!\![\!\!\text{ Co(N}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{5}}}\text{(N}{{\text{O}}_{\text{2}}}\text{) }\!\!]\!\!\text{ (N}{{\text{O}}_{\text{3}}}{{\text{)}}_{\text{2}}}$
Ans: It can show three types of isomerism.
A pair of optical isomer, these are given below:
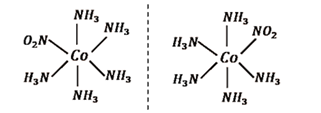
It can show linkage isomerism. These are given below:
$\text{ }\!\![\!\!\text{ Co(N}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{5}}}\text{(N}{{\text{O}}_{\text{2}}}\text{) }\!\!]\!\!\text{ (N}{{\text{O}}_{\text{3}}}{{\text{)}}_{\text{2}}}\text{ and }\!\![\!\!\text{ Co(N}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{5}}}\text{(ONO) }\!\!]\!\!\text{ (N}{{\text{O}}_{\text{3}}}{{\text{)}}_{\text{2}}}$
It can also show ionization isomerism. These are given below:
$\text{ }\!\![\!\!\text{ Co(N}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{5}}}\text{(N}{{\text{O}}_{\text{2}}}\text{) }\!\!]\!\!\text{ (N}{{\text{O}}_{\text{3}}}{{\text{)}}_{\text{2}}}\text{ and }\!\![\!\!\text{ Co(N}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{5}}}\text{(N}{{\text{O}}_{\text{3}}}\text{) }\!\!]\!\!\text{ (N}{{\text{O}}_{\text{3}}}\text{)(N}{{\text{O}}_{\text{2}}}\text{)}$
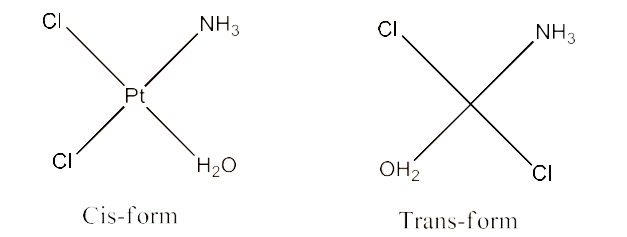
(iv) $\text{ }\!\![\!\!\text{ Pt(N}{{\text{H}}_{\text{3}}}\text{)(}{{\text{H}}_{\text{2}}}\text{O)C}{{\text{l}}_{\text{2}}}\text{ }\!\!]\!\!\text{ }$
Ans: Geometrical isomerism of this compound is possible. Both cis-form and trans-form are given below:
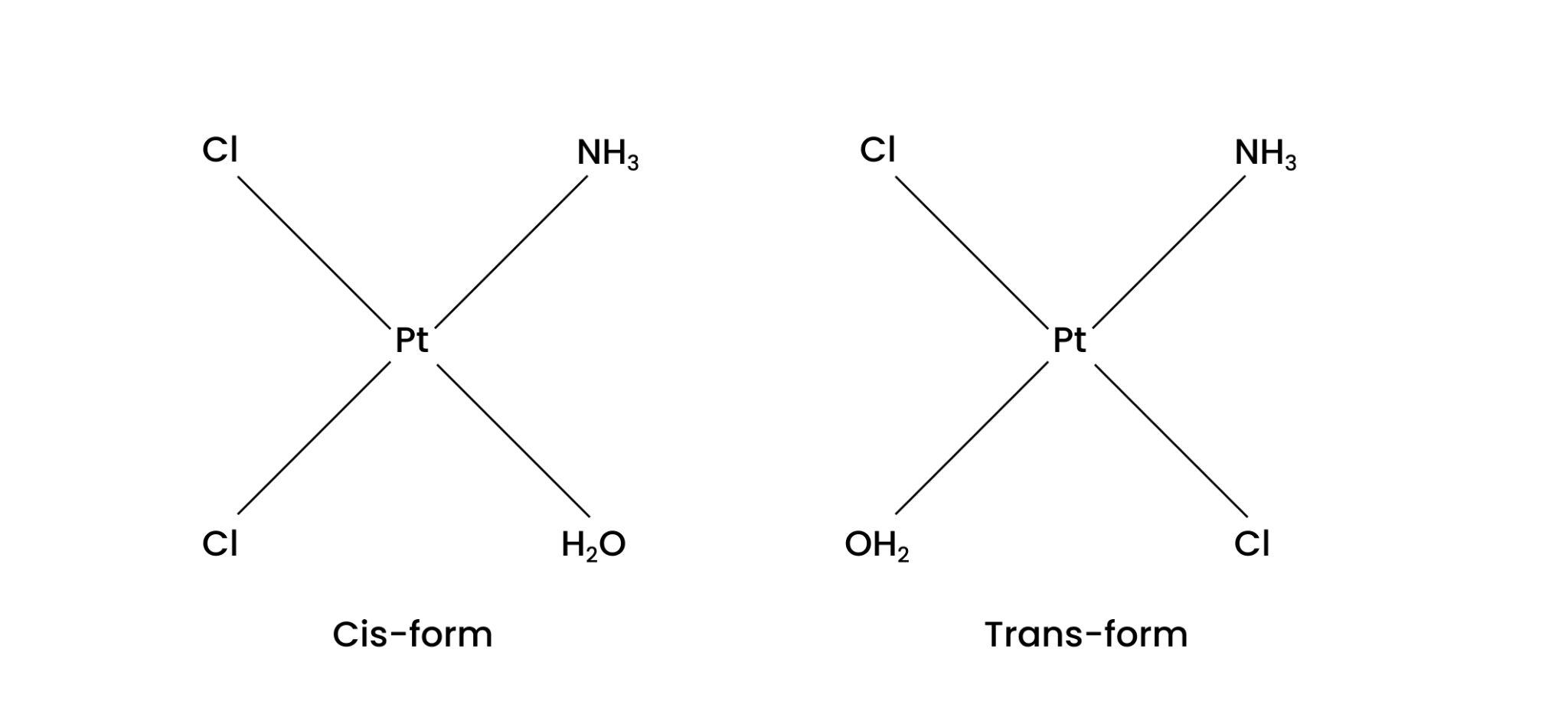
4. Give evidence that $\text{ }\!\![\!\!\text{ Co(N}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{5}}}\text{Cl }\!\!]\!\!\text{ S}{{\text{O}}_{\text{4}}}$ and $\text{ }\!\![\!\!\text{ Co(N}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{5}}}\text{S}{{\text{O}}_{\text{4}}}\text{ }\!\!]\!\!\text{ Cl}$ are ionization isomers.
Ans: If isomers of ionization are ionized into water, various ionization isomers are ionized. These ions react differently to various products with different reagents. The compounds given in the questions are tested with $\text{AgN}{{\text{O}}_{\text{3}}}$ solution and $\text{BaC}{{\text{l}}_{\text{2}}}$ solution. The reactions are given below:\[\text{ }\!\![\!\!\text{ Co(N}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{5}}}\text{Cl }\!\!]\!\!\text{ S}{{\text{O}}_{\text{4}}}\text{(aq) + BaC}{{\text{l}}_{\text{2}}}\text{(aq) }\to \text{ }\underset{\text{White precipitate}}{\mathop{\text{BaS}{{\text{O}}_{\text{4}}}\downarrow }}\,\]
\[\text{ }\!\![\!\!\text{ Co(N}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{5}}}\text{Cl }\!\!]\!\!\text{ S}{{\text{O}}_{\text{4}}}\text{(aq) + AgN}{{\text{O}}_{\text{3}}}\text{(aq) }\to \text{ No reaction}\] \[\text{ }\!\![\!\!\text{ Co(N}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{5}}}\text{S}{{\text{O}}_{\text{4}}}\text{ }\!\!]\!\!\text{ Cl(aq) + BaC}{{\text{l}}_{\text{2}}}\text{(aq) }\to \text{ No reaction}\]
\[\text{ }\!\![\!\!\text{ Co(N}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{5}}}\text{S}{{\text{O}}_{\text{4}}}\text{ }\!\!]\!\!\text{ Cl(aq) + AgN}{{\text{O}}_{\text{3}}}\text{(aq) }\to \text{ }\underset{\text{White precipitate}}{\mathop{\text{AgCl}\downarrow }}\,\]
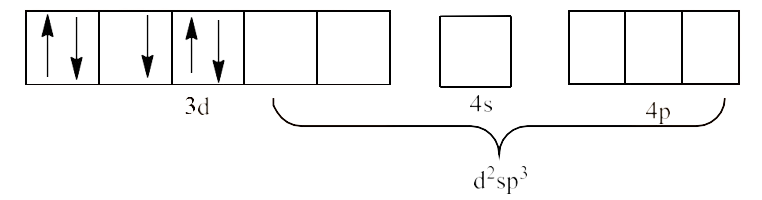
5. Explain on the basis of valence bond theory that ${{\text{ }\!\![\!\!\text{ Ni(CN}{{\text{)}}_{\text{4}}}\text{ }\!\!]\!\!\text{ }}^{\text{2-}}}$ ion with square planer structure is diamagnetic and the ${{\text{ }\!\![\!\!\text{ NiC}{{\text{l}}_{\text{4}}}\text{ }\!\!]\!\!\text{ }}^{\text{2-}}}$ ion with tetrahedral geometry is paramagnetic.
Ans: In ${{\text{d}}^{\text{8}}}$ configuration, Ni is in +2 oxidation state. This is shown below:

As we can see that in the question, there are 4 ligands in the compound which means it is a square planar complex.
Since, $\text{C}{{\text{N}}^{\text{-}}}$ is a strong field ligand and it will cause the pairing of the 3d electrons. This will lead to $\text{ds}{{\text{p}}^{\text{2}}}$ hybridization. It is given below:
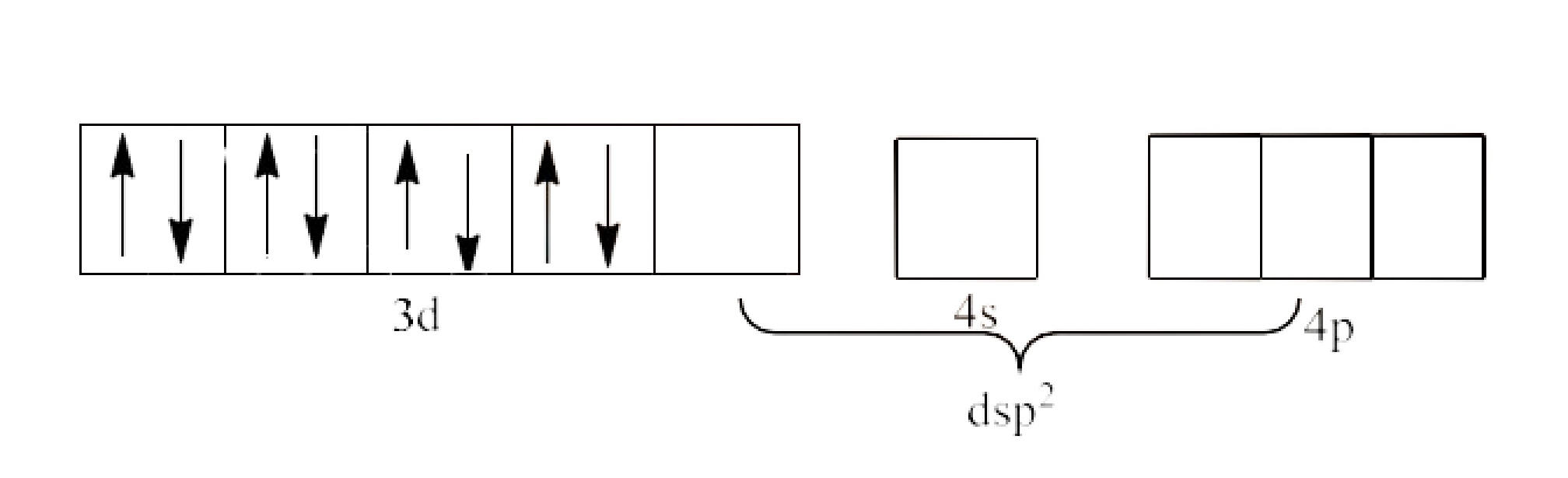
Since, all the electrons are paired, ${{\text{ }\!\![\!\!\text{ Ni(CN}{{\text{)}}_{4}}\text{ }\!\!]\!\!\text{ }}^{\text{2-}}}$ is diamagnetic complex.
Since, $\text{C}{{\text{l}}^{\text{-}}}$ is a weak field ligand and it will not cause the pairing of the 3d electrons. This will lead to $\text{s}{{\text{p}}^{3}}$ hybridization. It is given below:
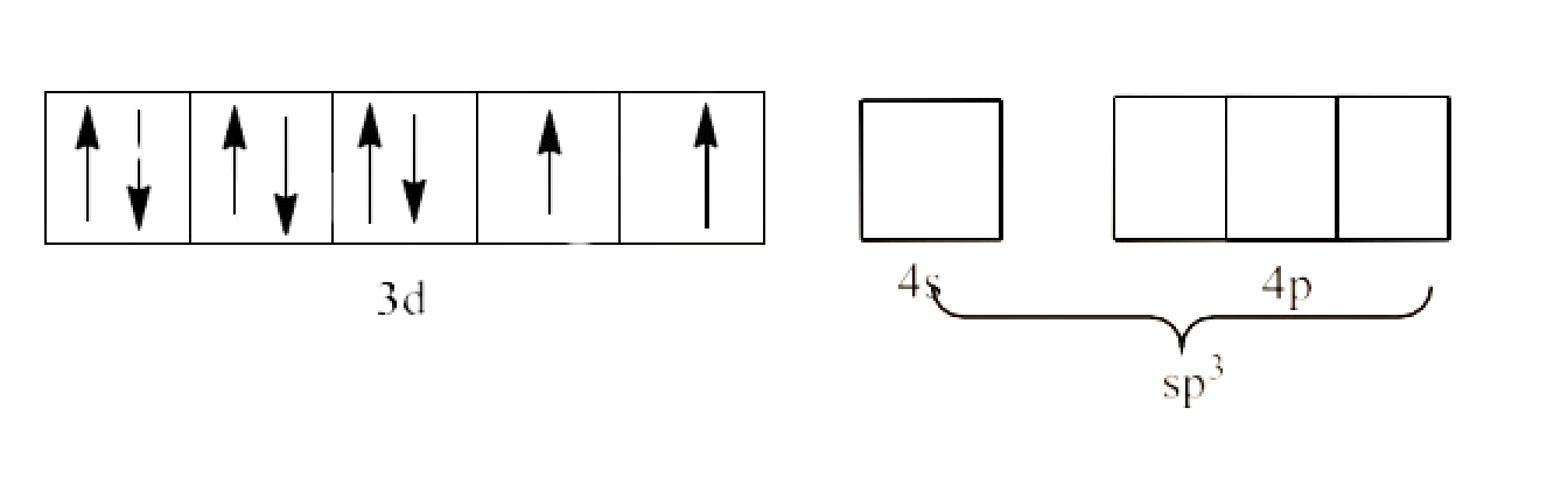
Since, two electrons are unpaired, ${{\text{ }\!\![\!\!\text{ NiC}{{\text{l}}_{4}}\text{ }\!\!]\!\!\text{ }}^{\text{2-}}}$ is paramagnetic complex.
6. ${{\text{ }\!\![\!\!\text{ NiC}{{\text{l}}_{\text{4}}}\text{ }\!\!]\!\!\text{ }}^{\text{2-}}}$ is paramagnetic while $\text{ }\!\![\!\!\text{ Ni(CO}{{\text{)}}_{\text{4}}}\text{ }\!\!]\!\!\text{ }$ is diamagnetic though both are tetrahedral. Why?
Ans: As we can see that in the question, there are 4 ligands in the compound which means it is a tetrahedral complex.
Since, $\text{C}{{\text{l}}^{\text{-}}}$ is a strong field ligand and it will not cause the pairing of the 3d electrons. This will lead to $\text{s}{{\text{p}}^{3}}$ hybridization. It is given below:
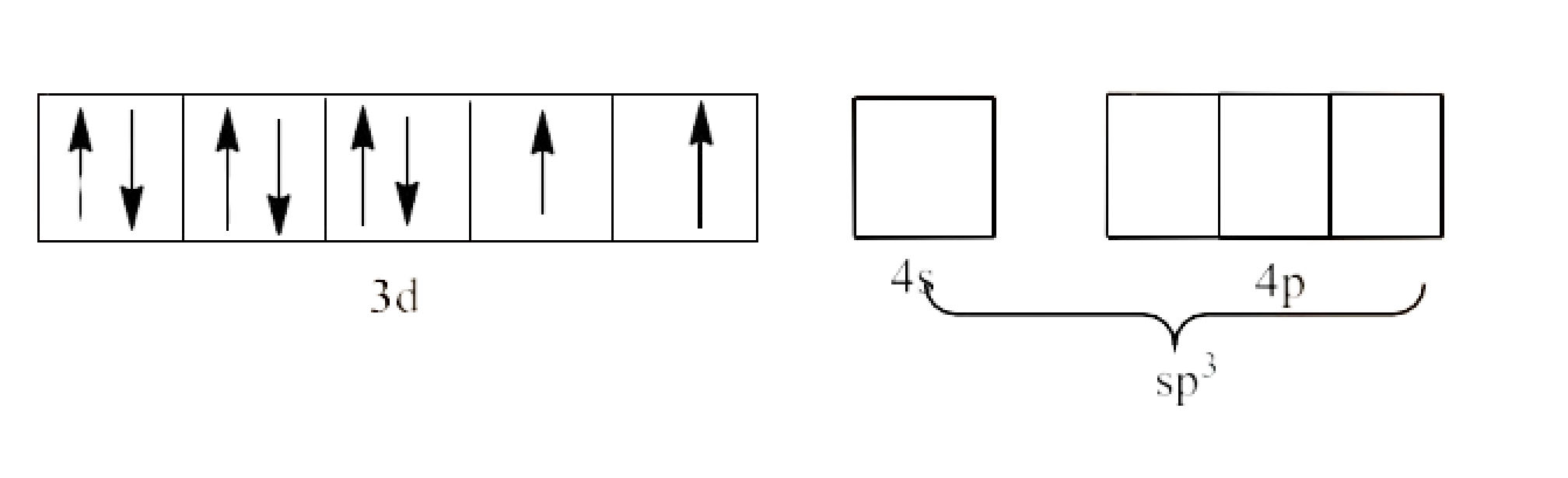
Since, two electrons are unpaired, ${{\text{ }\!\![\!\!\text{ NiC}{{\text{l}}_{4}}\text{ }\!\!]\!\!\text{ }}^{\text{2-}}}$ is paramagnetic complex.
In $\text{ }\!\![\!\!\text{ Ni(CO}{{\text{)}}_{\text{4}}}\text{ }\!\!]\!\!\text{ }$, Ni is in the zero oxidation state i.e., it has a configuration of $\text{3}{{\text{d}}^{\text{8}}}\text{ 4}{{\text{s}}^{\text{2}}}$.
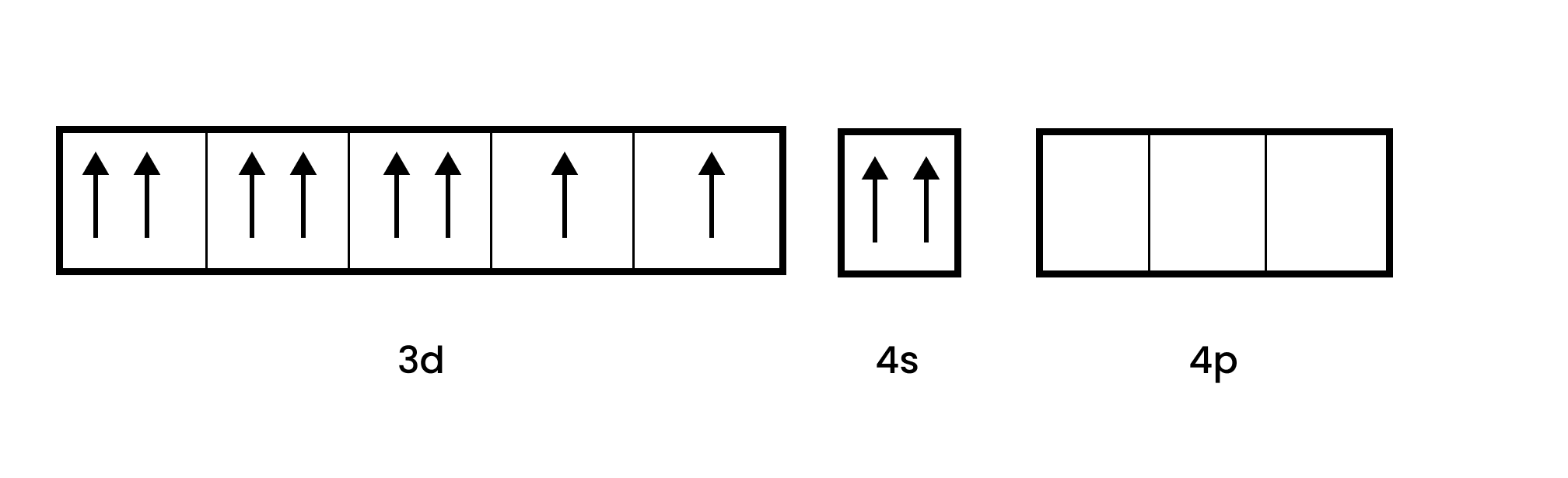
But CO is a strong field ligand. This leads unpaired 3d electrons to be coupled with one another. It also leads the 4s to go to the 3d orbital, which results in a hybridization of $\text{s}{{\text{p}}^{\text{3}}}$. Since in this situation, $\text{ }\!\![\!\!\text{ Ni(CO}{{\text{)}}_{\text{4}}}\text{ }\!\!]\!\!\text{ }$ is diamagnetic as no unpaired electrons are present.
7. ${{\text{ }\!\![\!\!\text{ Fe(}{{\text{H}}_{\text{2}}}\text{O}{{\text{)}}_{\text{6}}}\text{ }\!\!]\!\!\text{ }}^{\text{3+}}}$ is strongly paramagnetic whereas ${{\text{ }\!\![\!\!\text{ Fe(CN}{{\text{)}}_{\text{6}}}\text{ }\!\!]\!\!\text{ }}^{\text{3-}}}$ is weakly paramagnetic. Explain.
Ans: In the given complexes, Fe is the central metal ion and in both the cases the oxidation state of Fe is +3. Therefore, it will be in ${{\text{d}}^{\text{5}}}$ configuration.
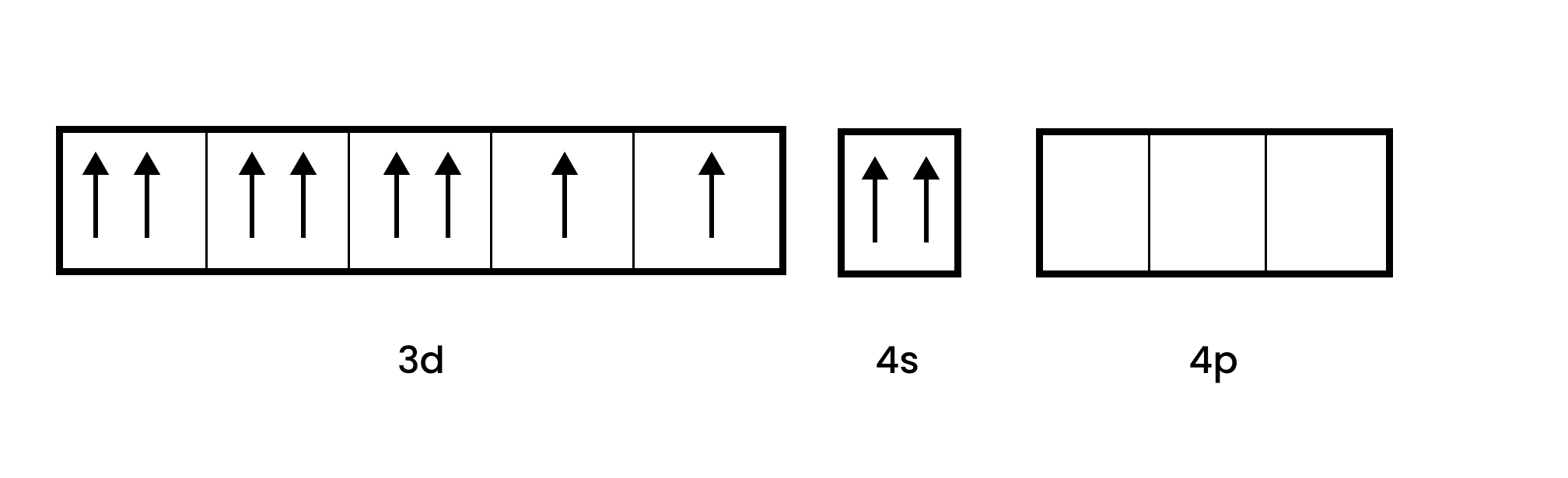
Since $\text{C}{{\text{N}}^{\text{-}}}$ is a strong field ligand, it causes unpaired electrons to be paired. There is just one unpaired electron in the d-orbital thus remaining.
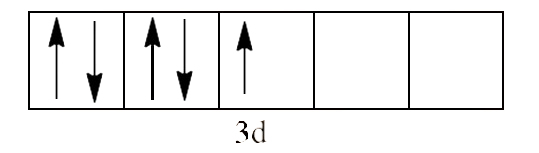
So, we can calculate the magnetic moment as:
\[\text{ }\!\!\mu\!\!\text{ = }\sqrt{\text{n(n+2)}}\]
\[\text{ }\!\!\mu\!\!\text{ = }\sqrt{\text{1(1+2)}}\]
\[\text{ }\!\!\mu\!\!\text{ = }\sqrt{3}\]
\[\text{ }\!\!\mu\!\!\text{ =1}\text{.732 BM}\]
Since ${{\text{H}}_{\text{2}}}\text{O}$ is a weak field ligand, it does not cause unpaired electrons to be paired. There are five unpaired electrons in the d-orbital.
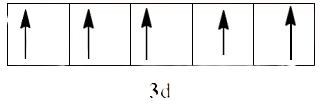
So, we can calculate the magnetic moment as:
\[\text{ }\!\!\mu\!\!\text{ = }\sqrt{\text{n(n+2)}}\]
\[\text{ }\!\!\mu\!\!\text{ = }\sqrt{\text{5(5+2)}}\]
\[\text{ }\!\!\mu\!\!\text{ = }\sqrt{35}\]
\[\text{ }\!\!\mu\!\!\text{ =5}\text{.91 BM}\]
Therefore, ${{\text{ }\!\![\!\!\text{ Fe(}{{\text{H}}_{\text{2}}}\text{O}{{\text{)}}_{\text{6}}}\text{ }\!\!]\!\!\text{ }}^{\text{3+}}}$ is strongly paramagnetic while ${{\text{ }\!\![\!\!\text{ Fe(CN}{{\text{)}}_{\text{6}}}\text{ }\!\!]\!\!\text{ }}^{\text{3+}}}$ is weakly paramagnetic complex.
8. Explain ${{\text{ }\!\![\!\!\text{ Co(N}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{6}}}\text{ }\!\!]\!\!\text{ }}^{\text{3+}}}$ is an inner orbital complex whereas ${{\text{ }\!\![\!\!\text{ Ni(N}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{6}}}\text{ }\!\!]\!\!\text{ }}^{\text{2+}}}$ is an outer orbital complex.
Ans: In ${{\text{ }\!\![\!\!\text{ Co(N}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{6}}}\text{ }\!\!]\!\!\text{ }}^{\text{3+}}}$ the oxidation state of Co is in +3 oxidation state, which will cause the configuration of Co as ${{\text{d}}^{\text{6}}}$.
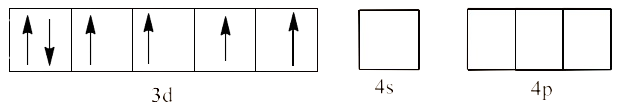
Since $\text{N}{{\text{H}}_{\text{3}}}$ is a strong field ligand, it causes unpaired electrons to be paired. This will lead to ${{\text{d}}^{\text{2}}}\text{s}{{\text{p}}^{\text{3}}}$ hybridization. This is shown below:
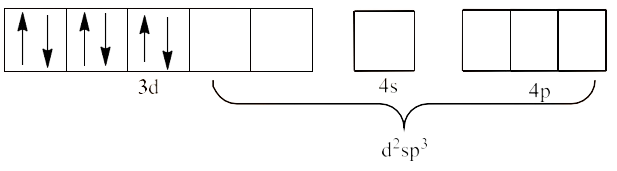
Therefore, it is an inner orbital complex.
In ${{\text{ }\!\![\!\!\text{ Ni(N}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{6}}}\text{ }\!\!]\!\!\text{ }}^{\text{2+}}}$ the oxidation state of Ni is in +2 oxidation state, which will cause the configuration of Ni as ${{\text{d}}^{8}}$.
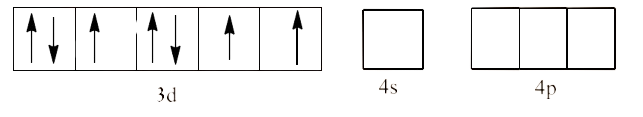
Since $\text{N}{{\text{H}}_{\text{3}}}$ is a strong field ligand, it causes unpaired electrons to be paired. There is only one d-orbital left and it cannot form the ${{\text{d}}^{\text{2}}}\text{s}{{\text{p}}^{\text{3}}}$. Therefore, it will act as a weak field ligand. This will lead to $\text{s}{{\text{p}}^{\text{3}}}{{\text{d}}^{\text{2}}}$ hybridization. This is shown below:
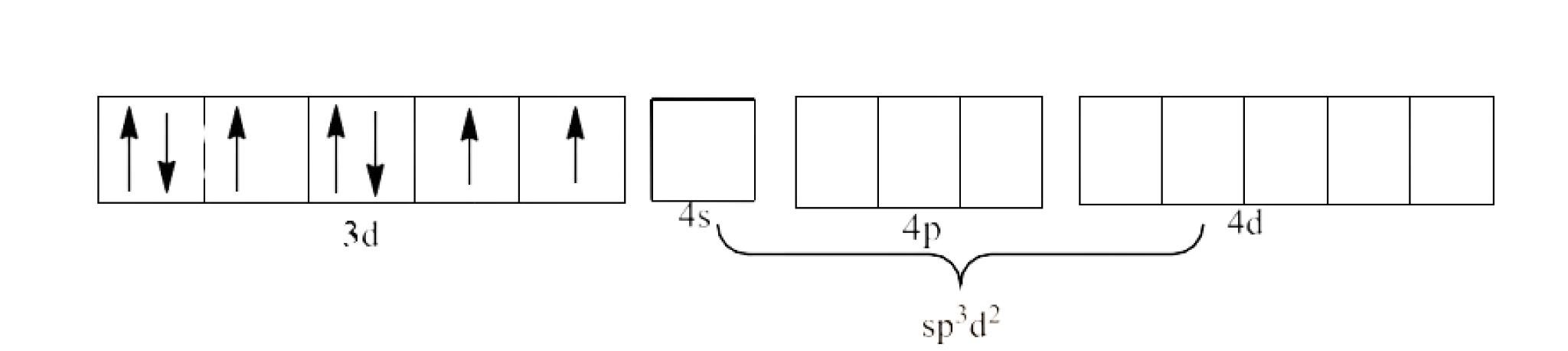
Therefore, it is an outer orbital complex.
9. Predict the number of unpaired electrons in the square planar ${{\text{ }\!\![\!\!\text{ Pt(CN}{{\text{)}}_{\text{4}}}\text{ }\!\!]\!\!\text{ }}^{\text{2-}}}$ ion.
Ans: The given complex is ${{\text{ }\!\![\!\!\text{ Pt(CN}{{\text{)}}_{\text{4}}}\text{ }\!\!]\!\!\text{ }}^{\text{2-}}}$. In this complex the central metal ion is platinum and its oxidation state is +2. It forms a square planar structure. This means that it will have the hybridization of $\text{ds}{{\text{p}}^{\text{2}}}$. So, the electronic configuration Pt (+2) of is $\text{5}{{\text{d}}^{\text{8}}}$.
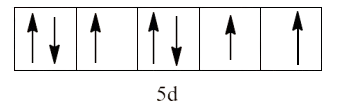
In the complex, $\text{C}{{\text{N}}^{\text{-}}}$ is a strong field ligand which will cause the pairing of all the unpaired electrons. Therefore, there will be no unpaired electrons.
10. The hexaquomanganese (II) ion contains five unpaired electrons, while the hexacyanoion contains only one unpaired electron. Explain using Crystal Field Theory.
Ans: In ${{\text{ }\!\![\!\!\text{ Mn(}{{\text{H}}_{\text{2}}}\text{O}{{\text{)}}_{\text{6}}}\text{ }\!\!]\!\!\text{ }}^{\text{2+}}}$, the Mn is in +2 oxidation state and its electronic configuration is ${{\text{d}}^{\text{5}}}$. The crystal field is octahedral. Water is a weak field ligand. Therefore, the arrangement of the electrons in ${{\text{ }\!\![\!\!\text{ Mn(}{{\text{H}}_{\text{2}}}\text{O}{{\text{)}}_{\text{6}}}\text{ }\!\!]\!\!\text{ }}^{\text{2+}}}$ will be ${{\text{t}}_{\text{2}}}{{\text{g}}^{\text{3}}}\text{ e}{{\text{g}}^{\text{2}}}$.
In ${{\text{ }\!\![\!\!\text{ Mn(CN}{{\text{)}}_{\text{6}}}\text{ }\!\!]\!\!\text{ }}^{\text{2-}}}$, the Mn is in +2 oxidation state and its electronic configuration is ${{\text{d}}^{\text{5}}}$. The crystal field is octahedral. Cyanide is a strong field ligand. Therefore, the arrangement of the electrons in ${{\text{ }\!\![\!\!\text{ Mn(CN}{{\text{)}}_{\text{6}}}\text{ }\!\!]\!\!\text{ }}^{\text{2-}}}$ will be ${{\text{t}}_{\text{2}}}{{\text{g}}^{5}}\text{ e}{{\text{g}}^{0}}$.
11. Calculate the overall complex dissociation equilibrium constant for the $\text{Cu(N}{{\text{H}}_{\text{3}}}\text{)}_{\text{4}}^{\text{2+}}$ ion, given that ${{\text{ }\!\!\beta\!\!\text{ }}_{\text{4}}}$ for this complex is $\text{2}\text{.1 }\!\!\times\!\!\text{ 1}{{\text{0}}^{\text{13}}}$.
Ans: We are given the overall stability constant (${{\text{ }\!\!\beta\!\!\text{ }}_{\text{4}}}$) = $\text{2}\text{.1 }\!\!\times\!\!\text{ 1}{{\text{0}}^{\text{13}}}$.
The overall complex dissociation equilibrium constant is the reciprocal of the overall stability constant. This is given below:\[\text{Overall dissociation constant = }\frac{\text{1}}{{{\text{ }\!\!\beta\!\!\text{ }}_{\text{4}}}}\text{ = }\frac{\text{1}}{\text{2}\text{.1 }\!\!\times\!\!\text{ 1}{{\text{0}}^{\text{13}}}}\text{ = 4}\text{.7 }\!\!\times\!\!\text{ 1}{{\text{0}}^{\text{-14}}}\]
NCERT Exercise
1. Explain the bonding in coordination compounds in terms of Werner's postulates.
Ans: Werner's theory is the first theory to explain the nature of bonding in coordination compounds.
The main postulates of this theory are:
(i) Two types of valencies, primary and secondary valencies, are present in coordinated compound metals.
(ii) Negative ions are primary valencies, which are ionizable. The dotted line is depicted
(iii) The primary valence is equivalent to the metal ion oxidation number.
Secondary valencies are non-ionizing and neutral ions are both fulfilled. It is shown by a solid line.
(vi) The secondary valence refers to the metal ion coordination number.
(v) Those valencies project in the space allocated to a given geometry of the coordination compound in a specified direction.
2. $\text{FeS}{{\text{O}}_{\text{4}}}$ solution mixed with ${{\text{(N}{{\text{H}}_{\text{4}}}\text{)}}_{\text{2}}}\text{S}{{\text{O}}_{\text{4}}}$ solution in 1:1 molar ratio gives the test of $\text{F}{{\text{e}}^{\text{2+}}}$ ion but $\text{CuS}{{\text{O}}_{\text{4}}}$ solution mixed with aqueous ammonia in 1:4 molar ratio does not give the test of $\text{C}{{\text{u}}^{\text{2+}}}$ ion. Explain why?
Ans: Let us see the reactions happening in both the cases.
\[{{\text{(N}{{\text{H}}_{\text{4}}}\text{)}}_{\text{2}}}\text{S}{{\text{O}}_{\text{4}}}\text{ + FeS}{{\text{O}}_{\text{4}}}\text{ + 6}{{\text{H}}_{\text{2}}}\text{O }\to \text{ FeS}{{\text{O}}_{\text{4}}}\text{.(N}{{\text{H}}_{\text{4}}}{{\text{)}}_{\text{2}}}\text{S}{{\text{O}}_{\text{4}}}\text{.6}{{\text{H}}_{\text{2}}}\text{O}\]
\[\text{4N}{{\text{H}}_{3}}\text{ + CuS}{{\text{O}}_{\text{4}}}\text{ + 5}{{\text{H}}_{\text{2}}}\text{O }\to \text{ }\!\![\!\!\text{ Cu(N}{{\text{H}}_{3}}{{\text{)}}_{4}}\text{ }\!\!]\!\!\text{ S}{{\text{O}}_{\text{4}}}\text{.5}{{\text{H}}_{\text{2}}}\text{O}\]
The compound \[\text{FeS}{{\text{O}}_{\text{4}}}\text{.(N}{{\text{H}}_{\text{4}}}{{\text{)}}_{\text{2}}}\text{S}{{\text{O}}_{\text{4}}}\text{.6}{{\text{H}}_{\text{2}}}\text{O}\] is Mohr’s salt while $\text{ }\!\![\!\!\text{ Cu(N}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{4}}}\text{ }\!\!]\!\!\text{ S}{{\text{O}}_{\text{4}}}\text{.5}{{\text{H}}_{\text{2}}}\text{O}$ is Tetraamminocopper(II) sulphate.
Both \[\text{FeS}{{\text{O}}_{\text{4}}}\text{.(N}{{\text{H}}_{\text{4}}}{{\text{)}}_{\text{2}}}\text{S}{{\text{O}}_{\text{4}}}\text{.6}{{\text{H}}_{\text{2}}}\text{O}\] and $\text{ }\!\![\!\!\text{ Cu(N}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{4}}}\text{ }\!\!]\!\!\text{ S}{{\text{O}}_{\text{4}}}\text{.5}{{\text{H}}_{\text{2}}}\text{O}$ are classified as compounds, with one main distinction, i.e., the former is an example of a double salt, while the latter is a compound for co-ordination.
\[\text{FeS}{{\text{O}}_{\text{4}}}\text{.(N}{{\text{H}}_{\text{4}}}{{\text{)}}_{\text{2}}}\text{S}{{\text{O}}_{\text{4}}}\text{.6}{{\text{H}}_{\text{2}}}\text{O}\] is a double salt and it contains the $\text{F}{{\text{e}}^{\text{2+}}}$ ions that are in ionizable form. Therefore, it will give the $\text{F}{{\text{e}}^{\text{2+}}}$ test.
$\text{ }\!\![\!\!\text{ Cu(N}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{4}}}\text{ }\!\!]\!\!\text{ S}{{\text{O}}_{\text{4}}}\text{.5}{{\text{H}}_{\text{2}}}\text{O}$ is a coordination compound and it contains the $\text{C}{{\text{u}}^{\text{+}}}$ ion and it is not in an ionizable form. Therefore, it doesn’t give the $\text{C}{{\text{u}}^{\text{+}}}$ ion test.
3. Explain with two examples each of the following: coordination entity, ligand, coordination number, coordination polyhedron, homoleptic and heteroleptic.
Ans: All of them are explained below:
(i) Coordination entity
A central metal atom or an ions connected to a set number of ions or molecules known as ligands comprises a coordination entity.
For example:
${{\text{ }\!\![\!\!\text{ Ni(N}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{6}}}\text{ }\!\!]\!\!\text{ }}^{\text{2+}}}\text{, }\!\![\!\!\text{ Fe(CN}{{\text{)}}_{\text{6}}}{{\text{ }\!\!]\!\!\text{ }}^{\text{4+}}}$ are cationic complex.
${{\text{ }\!\![\!\!\text{ PtC}{{\text{l}}_{\text{4}}}\text{ }\!\!]\!\!\text{ }}^{\text{2-}}}\text{, }\!\![\!\!\text{ Ag(CN}{{\text{)}}_{\text{2}}}{{\text{ }\!\!]\!\!\text{ }}^{\text{-}}}$ are anionic complex.
$\text{ }\!\![\!\!\text{ Ni(CO}{{\text{)}}_{\text{4}}}\text{ }\!\!]\!\!\text{ , }\!\![\!\!\text{ Co(N}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{4}}}\text{C}{{\text{l}}_{\text{2}}}\text{ }\!\!]\!\!\text{ }$ are neutral complex.
(ii) Ligand
Ligands are regarded as the neutral molecules or negated ions which surround the metal atom in a coordinating entity. Ligands are generally polar and have a pair of valence electrons at least one unshared. For example, ${{\text{H}}_{\text{2}}}\text{O, C}{{\text{N}}^{\text{-}}}\text{, N}{{\text{H}}_{\text{3}}}\text{, CO}$ etc are some ligands.
(iii) Coordination number
A coordination number of central-metal atom is called the total number of ligands (whether neutral or negative ion) that are connected to the central-metal atom in the coordinating sphere. It is also known for its ligancy.
For example, ${{\text{K}}_{\text{2}}}\text{ }\!\![\!\!\text{ PtC}{{\text{l}}_{\text{6}}}\text{ }\!\!]\!\!\text{ }$ contains platinum as the central metal ion and chloride ions as ligands. Since, there are 6 ligands, therefore the coordination number is 6.
(iv) Coordination polyhedron
The coordination polyhedrons on the core atom may be described as the spatial arrangement of the ligands directly associated in the coordinating field to the central metal ion.
For example, square planar is a coordination polyhedron.
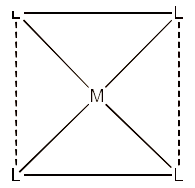
(v) Homoleptic complex
These are the complexes where the metal ion is linked to just one type of donor group. For example, ${{\text{ }\!\![\!\!\text{ PtC}{{\text{l}}_{\text{6}}}\text{ }\!\!]\!\!\text{ }}^{\text{2-}}}$ is a homoleptic complex.
(vi) Heteroleptic complex.
These are the complexes where the metal ion is linked to more than one type of donor group. For example, ${{\text{ }\!\![\!\!\text{ Co(N}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{4}}}\text{C}{{\text{l}}_{\text{2}}}\text{ }\!\!]\!\!\text{ }}^{\text{+}}}$ is a heteroleptic complex.
4. What is meant by unidentate, bidentate and ambidentate ligands? Give two examples for each.
Ans: These are explained below:
(i) Unidentate ligand
Ligands with only one donor sites are called unidentate ligands. For example, $\text{C}{{\text{l}}^{\text{-}}}$ and $\text{N}{{\text{H}}_{\text{3}}}$ are unidentate ligand.
(ii) Didentate ligand.
Ligands that have two donor sites are called didentate ligands. For example, ethane-1,2-diamine and oxalate ion. These are given below:
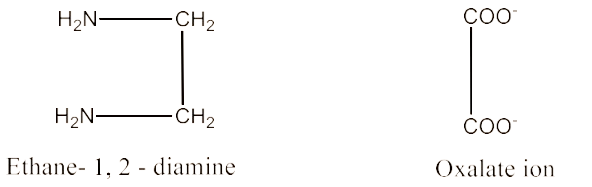
(iii) Ambidentate ligand
Ligands that can attach themselves to the central metal atom through two different atoms are called ambidentate ligands. For example, $\text{N}{{\text{O}}_{\text{2}}}$ group and $\text{SCN}$ group.
$\text{N}{{\text{O}}_{\text{2}}}$ group can attack from the nitrogen atom and oxygen atom. SCN group can attack from nitrogen atom and sulfur atom.
5. Specify the oxidation numbers of the metals in the following coordination entities:
(i) ${{\text{ }\!\![\!\!\text{ Co(}{{\text{H}}_{\text{2}}}\text{O)(CN)(en}{{\text{)}}_{\text{2}}}\text{ }\!\!]\!\!\text{ }}^{\text{2+}}}$
Ans: Let us assume that the coordination number of Co is x.
Therefore, we can write:
\[\text{x + 0 + (-1) + 2 (0) = +2}\]
\[\text{x - 1 = +2}\]
x = +3
So, the coordination number of cobalt is +3.
(ii) ${{\text{ }\!\![\!\!\text{ PtC}{{\text{l}}_{\text{4}}}\text{ }\!\!]\!\!\text{ }}^{\text{2-}}}$
Ans: Let us assume that the coordination number of Pt is x.
Therefore, we can write:
\[\text{x + 4 (-1) = -2}\]
x = +2
So, the coordination number of platinum is +2
(iii) $\text{ }\!\![\!\!\text{ Cr(N}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{3}}}\text{C}{{\text{l}}_{\text{3}}}\text{ }\!\!]\!\!\text{ }$
Ans: Let us assume that the coordination number of Cr is x.
Therefore, we can write:
\[\text{x + 0 + 3 (-1) = 0}\]
\[\text{x - 3 = 0}\]
x = +3
So, the oxidation number of chromium is +3
(iv) ${{\text{ }\!\![\!\!\text{ CoB}{{\text{r}}_{\text{2}}}{{\text{(en)}}_{\text{2}}}\text{ }\!\!]\!\!\text{ }}^{\text{+}}}$
Ans: Let us assume that the coordination number of Co is x.
Therefore, we can write:
\[\text{x + 2 (-1) + 0 = +1}\]
\[\text{x - 2 = +1}\]
x = +3
So, the oxidation number of cobalt is +3
(v) ${{\text{K}}_{\text{3}}}\text{ }\!\![\!\!\text{ Fe(CN}{{\text{)}}_{\text{6}}}\text{ }\!\!]\!\!\text{ }$
Ans: This can be written as ${{\text{ }\!\![\!\!\text{ Fe(CN}{{\text{)}}_{\text{6}}}\text{ }\!\!]\!\!\text{ }}^{\text{3-}}}$
Let us assume that the coordination number of Fe is x.
Therefore, we can write:
\[\text{x + 6 (-1) = -3}\]
\[\text{x - 6 = -3}\]
x = +3
So, the oxidation number of iron is +3
6. Using IUPAC norms write the formulas for the following:
(i) Tetrahydroxozincate(II)
Ans: The formula of Tetrahydroxozincate(II) is ${{\text{ }\!\![\!\!\text{ Zn(OH}{{\text{)}}_{\text{4}}}\text{ }\!\!]\!\!\text{ }}^{\text{2-}}}$
(ii) Potassium tetrachloridopalladate(II)
Ans: The formuls of Potassium tetrachloridopalladate(II) is ${{\text{K}}_{\text{2}}}\text{ }\!\![\!\!\text{ PdC}{{\text{l}}_{\text{4}}}\text{ }\!\!]\!\!\text{ }$
(iii) Diamminedichloridoplatinum(II)
Ans: The formula of Diamminedichloridoplatinum(II) is $\text{ }\!\![\!\!\text{ Pt(N}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{2}}}\text{C}{{\text{l}}_{\text{2}}}\text{ }\!\!]\!\!\text{ }$.
(iv) Potassium tetracyanonickelate(II)
Ans: The formula of Potassium tetracyanonickelate(II) is ${{\text{K}}_{\text{2}}}\text{ }\!\![\!\!\text{ Ni(CN}{{\text{)}}_{\text{4}}}\text{ }\!\!]\!\!\text{ }$
(v) Pentaamminenitrito-O-cobalt(III)
Ans: The formula of Pentaamminenitrito-O-cobalt(III) is ${{\text{ }\!\![\!\!\text{ Co(N}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{5}}}\text{(ONO) }\!\!]\!\!\text{ }}^{\text{2+}}}$
(vi) Hexaamminecobalt(III)sulphate
Ans: The formula of Hexaamminecobalt(III)sulphate is ${{\text{ }\!\![\!\!\text{ Co(N}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{6}}}\text{ }\!\!]\!\!\text{ }}_{\text{2}}}{{\text{(S}{{\text{O}}_{\text{4}}}\text{)}}_{\text{3}}}$
(vii) Potassium tri(oxalato)chromate(III)
Ans: The formula of Potassium tri(oxalato)chromate(III) is ${{\text{K}}_{\text{3}}}\text{ }\!\![\!\!\text{ Cr(}{{\text{C}}_{\text{2}}}{{\text{O}}_{\text{4}}}{{\text{)}}_{\text{3}}}\text{ }\!\!]\!\!\text{ }$
(viii) Hexaammineplatinum(IV)
Ans: The formula of Hexaammineplatinum(IV) is ${{\text{ }\!\![\!\!\text{ Pt(N}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{6}}}\text{ }\!\!]\!\!\text{ }}^{\text{4+}}}$
(ix) Tetrabromidocuprate(II)
Ans: The formula of Tetrabromidocuprate(II) is ${{\text{ }\!\![\!\!\text{ CuB}{{\text{r}}_{\text{4}}}\text{ }\!\!]\!\!\text{ }}^{\text{2-}}}$
(x) Pentaamminenitrito-N-cobalt(III)
Ans: The formula of Pentaamminenitrito-N-cobalt(III) is ${{\text{ }\!\![\!\!\text{ Co(N}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{5}}}\text{(N}{{\text{O}}_{\text{2}}}\text{) }\!\!]\!\!\text{ }}^{\text{2+}}}$
8. Using IUPAC norms write the systematic names of the following:
(i) $\text{ }\!\![\!\!\text{ Co(N}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{6}}}\text{ }\!\!]\!\!\text{ C}{{\text{l}}_{\text{3}}}$
Ans: The IUPAC name of the compound is Hexaamminecobalt(III) chloride.
(ii) $\text{ }\!\![\!\!\text{ Pt(N}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{2}}}\text{(N}{{\text{H}}_{\text{2}}}\text{C}{{\text{H}}_{\text{3}}}\text{) }\!\!]\!\!\text{ Cl}$
Ans: The IUPAC name of the compound is Diamminechloride (methyl amine) platinum(II) chloride.
(iii) ${{\text{ }\!\![\!\!\text{ Ti(}{{\text{H}}_{\text{2}}}\text{O}{{\text{)}}_{\text{6}}}\text{ }\!\!]\!\!\text{ }}^{\text{3+}}}$
Ans: The IUPAC name of the compound is Hexaaquatitanium(III) ion.
(iv) $\text{ }\!\![\!\!\text{ Co(N}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{4}}}\text{Cl(N}{{\text{O}}_{\text{2}}}\text{) }\!\!]\!\!\text{ Cl}$
Ans: The IUPAC name of the compound is Tetraamminechloridonitrito-N-cobalt(III) chloride.
(v) ${{\text{ }\!\![\!\!\text{ Mn(}{{\text{H}}_{\text{2}}}\text{O}{{\text{)}}_{\text{6}}}\text{ }\!\!]\!\!\text{ }}^{\text{2+}}}$
Ans: The IUPAC name of the compound is Hexaaquamanganese(II) ion.
(iv) ${{\text{ }\!\![\!\!\text{ NiC}{{\text{l}}_{\text{4}}}\text{ }\!\!]\!\!\text{ }}^{\text{2-}}}$
Ans: The IUPAC name of the compound is Tetrachloridenickelate(II) ion.
(vii) $\text{ }\!\![\!\!\text{ Ni(N}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{6}}}\text{ }\!\!]\!\!\text{ C}{{\text{l}}_{\text{2}}}$
Ans: The IUPAC name of the compound is Hexaamminenickel(II) chloride.
(viii) ${{\text{ }\!\![\!\!\text{ Co(en}{{\text{)}}_{\text{3}}}\text{ }\!\!]\!\!\text{ }}^{\text{3+}}}$
Ans: The IUPAC name of the compound is Tris (ethane-1, 2-diamine) cobalt(III) ion.
(ix) $\text{ }\!\![\!\!\text{ Ni(CO}{{\text{)}}_{\text{4}}}\text{ }\!\!]\!\!\text{ }$
Ans: The IUPAC name of the compound is Tetracarbonyl nickel(0).
8. List various types of isomerism possible for coordination compounds, giving an example of each.
Ans: Let us see the types of isomerism. These are discussed below with examples.
Geometrical isomerism:
In heteroleptic complexes this isomerism is prevalent. The various geometric arrangements of the ligands are responsible for it. For example:
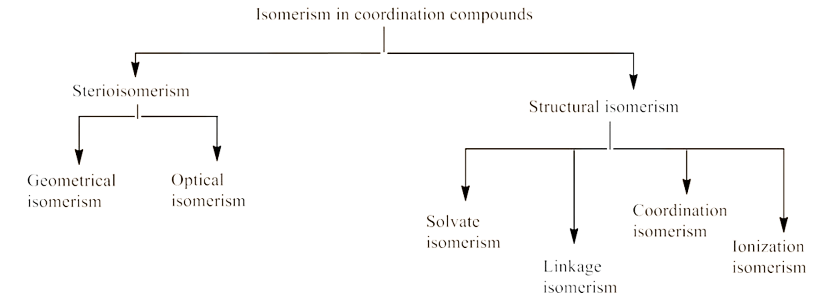
Optical isomerism:
The isomerism of this sort occurs in chiral compounds. Isomers represent each other's mirror reflections and are not superimposable. For example:
Linkage isomerism:
When the coordination sphere has ambidentate ligand, this isomerism occurs. For example, $\text{ }\!\![\!\!\text{ Co(N}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{5}}}\text{(N}{{\text{O}}_{\text{2}}}\text{) }\!\!]\!\!\text{ (N}{{\text{O}}_{\text{3}}}{{\text{)}}_{\text{2}}}\text{ and }\!\![\!\!\text{ Co(N}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{5}}}\text{(ONO) }\!\!]\!\!\text{ (N}{{\text{O}}_{\text{3}}}{{\text{)}}_{\text{2}}}$
Coordination isomerism:
This form of isomerism occurs when ligands of various metal ions in the complex are exchanged among cationic and anionic entities. For example, $\text{ }\!\![\!\!\text{ Co(N}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{6}}}\text{ }\!\!]\!\!\text{ }\!\![\!\!\text{ Cr(CN}{{\text{)}}_{\text{6}}}\text{ }\!\!]\!\!\text{ and }\!\![\!\!\text{ Cr(N}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{6}}}\text{ }\!\!]\!\!\text{ }\!\![\!\!\text{ Co(CN}{{\text{)}}_{\text{6}}}\text{ }\!\!]\!\!\text{ }$
Ionization isomerism:
The counter-ion replaces the ligand in the coordinating sphere, this is the kind of isomerism. Thus, ionisation isomers are termed complexes which have the same composition but which provide distinct ions when dissolved in water. For example, $\text{ }\!\![\!\!\text{ Co(N}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{5}}}\text{(N}{{\text{O}}_{\text{2}}}\text{) }\!\!]\!\!\text{ (N}{{\text{O}}_{\text{3}}}{{\text{)}}_{\text{2}}}\text{ and }\!\![\!\!\text{ Co(N}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{5}}}\text{(N}{{\text{O}}_{\text{3}}}\text{) }\!\!]\!\!\text{ (N}{{\text{O}}_{\text{3}}}\text{)(N}{{\text{O}}_{\text{2}}}\text{)}$
Solvate isomerism:
Solvent isomers differ in that the solvent molecule is directly connected to the metal ion or is simply in the crystal grid as a free solvent molecule. For example, $\text{ }\!\![\!\!\text{ Cr(}{{\text{H}}_{\text{2}}}\text{O}{{\text{)}}_{\text{6}}}\text{ }\!\!]\!\!\text{ C}{{\text{l}}_{\text{3}}}\text{ and }\!\![\!\!\text{ Cr(}{{\text{H}}_{\text{2}}}\text{O}{{\text{)}}_{\text{5}}}\text{Cl }\!\!]\!\!\text{ (}{{\text{H}}_{\text{2}}}\text{O)C}{{\text{l}}_{\text{2}}}$
9. How many geometrical isomers are possible in the following coordination entities?
(i) ${{\text{ }\!\![\!\!\text{ Cr(}{{\text{C}}_{\text{2}}}{{\text{O}}_{\text{4}}}{{\text{)}}_{\text{3}}}\text{ }\!\!]\!\!\text{ }}^{\text{3-}}}$
Ans: For this complex no geometrical isomerism is possible there is only one type of ligand and it is a bidentate ligand.
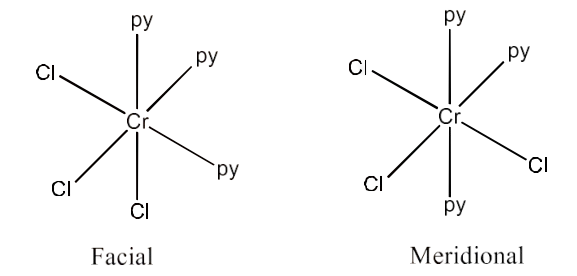
(ii) $\text{ }\!\![\!\!\text{ Co(N}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{3}}}\text{C}{{\text{l}}_{\text{3}}}\text{ }\!\!]\!\!\text{ }$
Ans: For this compound there are two geometrical isomers possible, i.e., Facial-form and Meridional form. These are given below:
![structures of optical isomers of Cr(C2O4)3 ] 3-](https://www.vedantu.com/seo/content-images/b8771478-acb9-45ee-8782-588c408d8d2c_26..png)
10. Draw the structures of optical isomers of:
(i) ${{\text{ }\!\![\!\!\text{ Cr(}{{\text{C}}_{\text{2}}}{{\text{O}}_{\text{4}}}{{\text{)}}_{\text{3}}}\text{ }\!\!]\!\!\text{ }}^{\text{3-}}}$
Ans: The structure is given below:
![structures of optical isomers of [ PtCl2(en)2 ] 2+](https://www.vedantu.com/seo/content-images/93fdc989-5c9f-4713-992b-99d9fb1eb180_27..png)
(ii) ${{\text{ }\!\![\!\!\text{ PtC}{{\text{l}}_{\text{2}}}{{\text{(en)}}_{\text{2}}}\text{ }\!\!]\!\!\text{ }}^{\text{2+}}}$
Ans: The structure is given below:
![structures of optical isomers of [ Cr(NH3)2Cl2(en) ] +](https://www.vedantu.com/seo/content-images/6831eed0-e821-4a39-9283-fe7af49ce8ef_28..png)
(iii) ${{\text{ }\!\![\!\!\text{ Cr(N}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{2}}}\text{C}{{\text{l}}_{\text{2}}}\text{(en) }\!\!]\!\!\text{ }}^{\text{+}}}$
Ans: The structures are given below:
This is of the cis-form
![isomers of CoCl2(en)2 ] 2+](https://www.vedantu.com/seo/content-images/88993bfe-e43c-4ad2-bb26-e1e9ed417e65_30..png)
This is of the trans-form
11. Draw all the isomers (geometrical and optical) of:
(i) ${{\text{ }\!\![\!\!\text{ CoC}{{\text{l}}_{\text{2}}}{{\text{(en)}}_{\text{2}}}\text{ }\!\!]\!\!\text{ }}^{\text{2+}}}$
Ans: The structures of optical isomers are given below:
![isomers of [ Co(NH3)Cl(en)2 ] 2+](https://www.vedantu.com/seo/content-images/fd186519-956d-434e-a055-0a35e1fed0d7_31..png)
In total, three isomers are possible.
(ii) ${{\text{ }\!\![\!\!\text{ Co(N}{{\text{H}}_{\text{3}}}\text{)Cl(en}{{\text{)}}_{\text{2}}}\text{ }\!\!]\!\!\text{ }}^{\text{2+}}}$
Ans: The structures of isomers are given below:
Trans-isomer is optically inactive
![isomers of [ Co(NH3)2Cl2(en) ] +](https://www.vedantu.com/seo/content-images/79bc099f-ccf3-47c1-bc6f-5683cd91e2e7_33..png)
(iii) ${{\text{ }\!\![\!\!\text{ Co(N}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{2}}}\text{C}{{\text{l}}_{\text{2}}}\text{(en) }\!\!]\!\!\text{ }}^{\text{+}}}$
Ans: The structures of all the isomers are given below:
![isomers of [ Co(NH3)2Cl2(en) ] +](https://www.vedantu.com/seo/content-images/05b56e4d-5694-494e-a28c-17fecf699809_34..png)
12. Write all the geometrical isomers of $\text{ }\!\![\!\!\text{ Pt(N}{{\text{H}}_{\text{3}}}\text{)(Br)(Cl)(py) }\!\!]\!\!\text{ }$ and how many of these will exhibit optical isomers?
Ans: There are three isomers possible:
![Geometrical isomers of [Pt(NH3)(Br)(Cl)(py)]](https://www.vedantu.com/seo/content-images/17f23eff-43c4-41ed-8b1e-a3c03b21d7c7_35..png)
No one will display optical isomers from the given isomers. Optical isomerization of tetrahedral compounds is uncommon. You only accomplish this in the presence of unsymmetric chelating substances.
13. Aqueous copper sulphate solution (blue in colour) gives:
Ans: Aqueous $\text{CuS}{{\text{O}}_{\text{4}}}$ solution exists as $\text{ }\!\![\!\!\text{ Cu(}{{\text{H}}_{\text{2}}}\text{O}{{\text{)}}_{\text{4}}}\text{ }\!\!]\!\!\text{ S}{{\text{O}}_{\text{4}}}$ which has blue color due to ${{\text{ }\!\![\!\!\text{ Cu(}{{\text{H}}_{\text{2}}}\text{O}{{\text{)}}_{\text{4}}}\text{ }\!\!]\!\!\text{ }}^{\text{2+}}}$ ions.
(i) a green precipitate with aqueous potassium fluoride, and
When KF is added, the weak ${{\text{H}}_{\text{2}}}\text{O}$ ligand is replaced by ${{\text{F}}^{\text{-}}}$ ligands forming ${{\text{ }\!\![\!\!\text{ Cu}{{\text{F}}_{\text{4}}}\text{ }\!\!]\!\!\text{ }}^{\text{2-}}}$ ions which is a green precipitate. The reaction is given below:
\[{{\text{ }\!\![\!\!\text{ Cu(}{{\text{H}}_{\text{2}}}\text{O}{{\text{)}}_{\text{4}}}\text{ }\!\!]\!\!\text{ }}^{\text{2+}}}\text{ + 4}{{\text{F}}^{\text{-}}}\text{ }\to \text{ }\!\![\!\!\text{ Cu(F}{{\text{)}}_{\text{4}}}{{\text{ }\!\!]\!\!\text{ }}^{\text{2-}}}\text{ + 4}{{\text{H}}_{\text{2}}}\text{O}\]
(ii) a bright green solution with aqueous potassium chloride
Explain these experimental results.
When KCl is added, $\text{C}{{\text{l}}^{\text{-}}}$ ligand replace the weak ${{\text{H}}_{\text{2}}}\text{O}$ ligands forming ${{\text{ }\!\![\!\!\text{ CuC}{{\text{l}}_{\text{4}}}\text{ }\!\!]\!\!\text{ }}^{\text{2-}}}$ ion which has bright green color. The reaction is given below:\[{{\text{ }\!\![\!\!\text{ Cu(}{{\text{H}}_{\text{2}}}\text{O}{{\text{)}}_{\text{4}}}\text{ }\!\!]\!\!\text{ }}^{\text{2+}}}\text{ + 4C}{{\text{l}}^{\text{-}}}\text{ }\to \text{ }\!\![\!\!\text{ Cu(Cl}{{\text{)}}_{\text{4}}}{{\text{ }\!\!]\!\!\text{ }}^{\text{2-}}}\text{ + 4}{{\text{H}}_{\text{2}}}\text{O}\]
14. What is the coordination entity formed when excess of aqueous KCN is added to an aqueous solution of copper sulphate? Why is it that no precipitate of copper sulphide is obtained when ${{\text{H}}_{\text{2}}}\text{S}$(g) is passed through this solution?
Ans: The reactions are given below:\[\text{CuS}{{\text{O}}_{\text{4}}}\text{(aq) + 4KCN(aq) }\to \text{ }{{\text{K}}_{\text{2}}}\text{ }\!\![\!\!\text{ Cu(CN}{{\text{)}}_{\text{4}}}\text{ }\!\!]\!\!\text{ (aq) + }{{\text{K}}_{\text{2}}}\text{S}{{\text{O}}_{\text{4}}}\text{(aq)}\]
i.e., ${{\text{ }\!\![\!\!\text{ Cu(}{{\text{H}}_{\text{2}}}\text{O}{{\text{)}}_{\text{4}}}\text{ }\!\!]\!\!\text{ }}^{\text{2+}}}\text{ + 4C}{{\text{N}}^{\text{-}}}\text{ }\to \text{ }\!\![\!\!\text{ Cu(CN}{{\text{)}}_{\text{4}}}{{\text{ }\!\!]\!\!\text{ }}^{\text{2-}}}\text{ + 4}{{\text{H}}_{\text{2}}}\text{O}$
Thus, the process-formed coordination entity is ${{\text{K}}_{\text{2}}}\text{ }\!\![\!\!\text{ Cu(CN}{{\text{)}}_{\text{4}}}\text{ }\!\!]\!\!\text{ }$, which is highly stable, which does not ionise to add $\text{C}{{\text{u}}^{\text{2+}}}$ ions to water. Therefore when the ${{\text{H}}_{\text{2}}}\text{S}$ (g) passes through the solution, $\text{C}{{\text{u}}^{\text{2+}}}$ ions are not precipitated.
15. Discuss the nature of bonding in the following coordination entities on the basis of valence bond theory:
(i) ${{\text{ }\!\![\!\!\text{ Fe(CN}{{\text{)}}_{\text{6}}}\text{ }\!\!]\!\!\text{ }}^{\text{4-}}}$
Ans: In ${{\text{ }\!\![\!\!\text{ Fe(CN}{{\text{)}}_{\text{6}}}\text{ }\!\!]\!\!\text{ }}^{4-}}$ the oxidation state of Fe is in +2 oxidation state, which will cause the configuration of Fe as ${{\text{d}}^{\text{6}}}$.
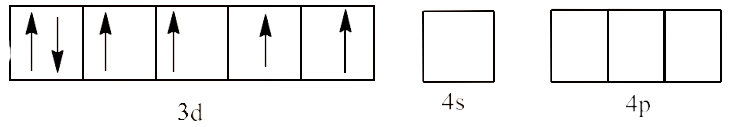
Since $\text{C}{{\text{N}}^{\text{-}}}$ is a strong field ligand, it causes unpaired electrons to be paired. This will lead to ${{\text{d}}^{\text{2}}}\text{s}{{\text{p}}^{\text{3}}}$ hybridization. This is shown below:
Therefore, the geometry of the complex is octahedral and it is a diamagnetic complex.
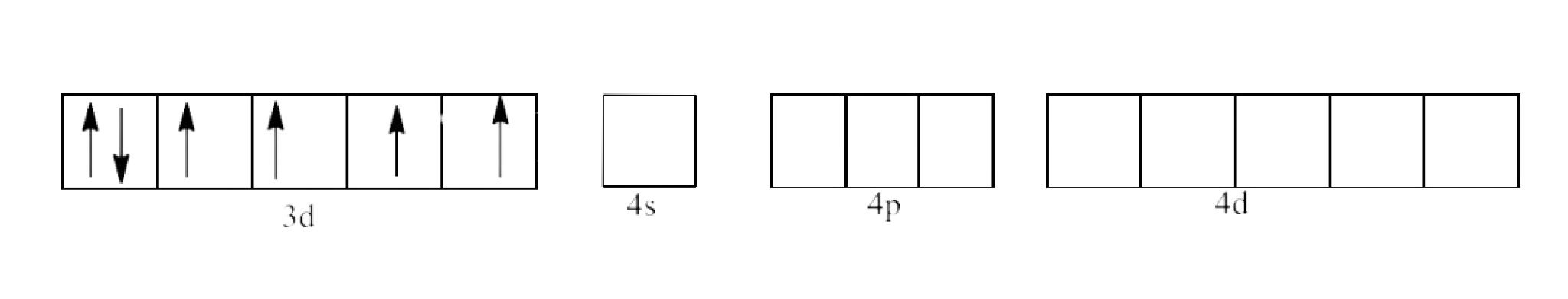
(ii) ${{\text{ }\!\![\!\!\text{ Fe}{{\text{F}}_{\text{6}}}\text{ }\!\!]\!\!\text{ }}^{\text{3-}}}$
Ans: In ${{\text{ }\!\![\!\!\text{ Fe(F}{{\text{)}}_{\text{6}}}\text{ }\!\!]\!\!\text{ }}^{\text{3-}}}$ the oxidation state of Fe is in +3 oxidation state, which will cause the configuration of Fe as ${{\text{d}}^{5}}$.
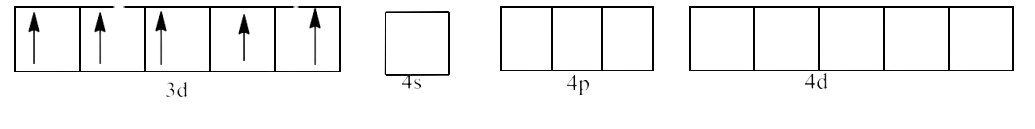
Since ${{\text{F}}^{\text{-}}}$ is a weak field ligand, it does not cause pairing of electrons. This will lead to $\text{s}{{\text{p}}^{\text{3}}}{{\text{d}}^{\text{2}}}$ hybridization. This is shown below:
The geometry of the complex is octahedral and it is paramagnetic.
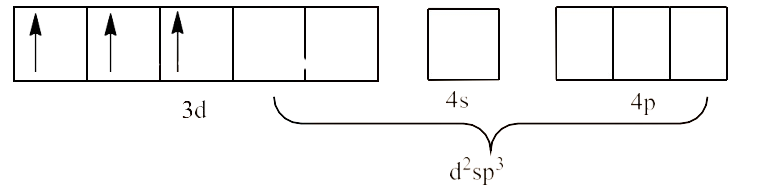
(iii) ${{\text{ }\!\![\!\!\text{ Co(}{{\text{C}}_{\text{2}}}{{\text{O}}_{\text{4}}}{{\text{)}}_{\text{3}}}\text{ }\!\!]\!\!\text{ }}^{\text{3-}}}$
Ans: In ${{\text{ }\!\![\!\!\text{ Co(}{{\text{C}}_{2}}{{\text{O}}_{4}}{{\text{)}}_{3}}\text{ }\!\!]\!\!\text{ }}^{3-}}$ the oxidation state of Co is in +3 oxidation state, which will cause the configuration of Co as ${{\text{d}}^{\text{6}}}$.
Since oxalate is a strong field ligand, it causes unpaired electrons to be paired. This will lead to ${{\text{d}}^{\text{2}}}\text{s}{{\text{p}}^{\text{3}}}$ hybridization. This is shown below:
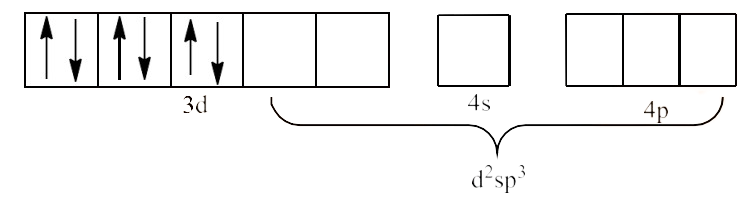
Therefore, the geometry of the complex is octahedral and it is a diamagnetic complex.
(iv) ${{\text{ }\!\![\!\!\text{ Co}{{\text{F}}_{\text{6}}}\text{ }\!\!]\!\!\text{ }}^{\text{3-}}}$
Ans: In ${{\text{ }\!\![\!\!\text{ Co}{{\text{F}}_{\text{6}}}\text{ }\!\!]\!\!\text{ }}^{\text{3-}}}$ the oxidation state of Co is in +3 oxidation state, which will cause the configuration of Co as ${{\text{d}}^{6}}$.
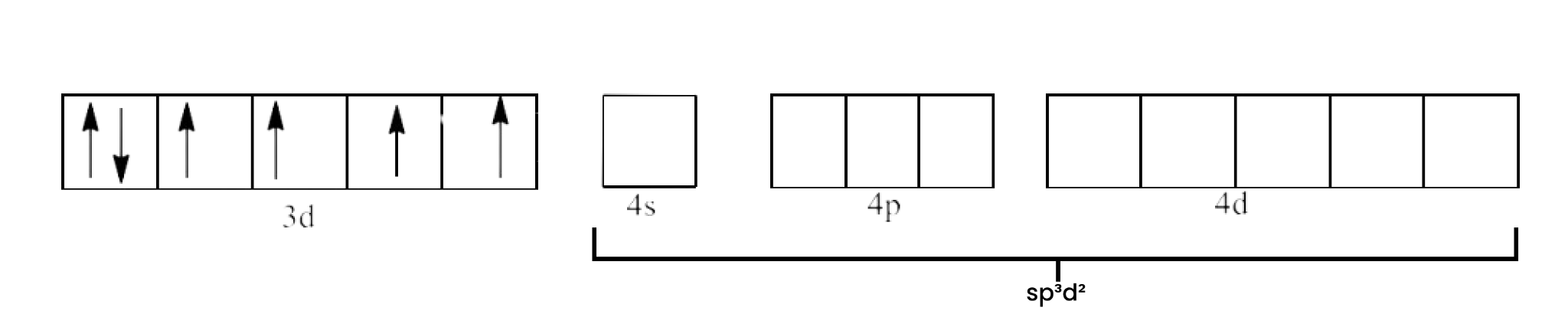
Since ${{\text{F}}^{\text{-}}}$ is a weak field ligand, it does not cause pairing of electrons. This will lead to $\text{s}{{\text{p}}^{\text{3}}}{{\text{d}}^{\text{2}}}$ hybridization.
The geometry of the complex is octahedral and it is paramagnetic.
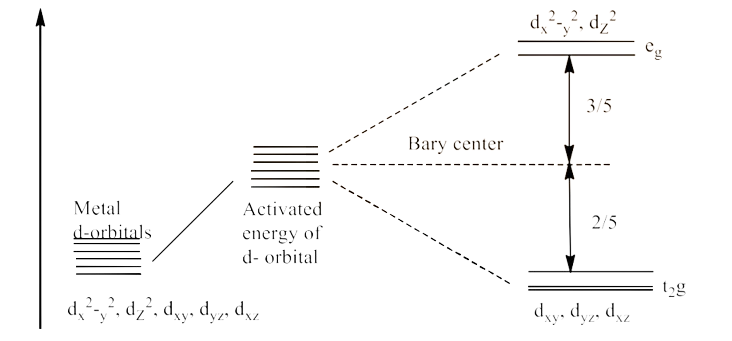
16. Draw figure to show the splitting of d orbitals in an octahedral crystal field.
Ans: We know that there are five subshells of d-orbital, i.e., ${{\text{d}}_{\text{xy}}}\text{, }{{\text{d}}_{\text{yz}}}\text{, }{{\text{d}}_{\text{zx}}}\text{, }{{\text{d}}_{{{\text{x}}^{\text{2}}}\text{-}{{\text{y}}^{\text{2}}}}}\text{ and }{{\text{d}}_{{{\text{z}}^{\text{2}}}}}$. When the energy is provided, the splitting occurs in such a way that ${{\text{d}}_{{{\text{x}}^{\text{2}}}\text{-}{{\text{y}}^{\text{2}}}}}\text{ and }{{\text{d}}_{{{\text{z}}^{\text{2}}}}}$ experience the rise in energy so, they form ${{\text{e}}_{\text{g}}}$ level, while ${{\text{d}}_{\text{xy}}}\text{, }{{\text{d}}_{\text{yz}}}\text{ and }{{\text{d}}_{\text{zx}}}$ experience the low energy level so, they form ${{\text{t}}_{\text{2}}}\text{g}$ level. This is shown below:
17. What is spectrochemical series? Explain the difference between a weak field ligand and a strong field ligand.
Ans: A spectrochemical set of ligands is the organisation of their crystal field splitting energy (CFSE) values in the ascending order. The ligands on the R.H.S are strong ligands of fields whereas L.H.S is weak ligands of field. In d'orbitals, strong field ligands are more divided than weak field ligands. The series is given below:
${{\text{I}}^{\text{-}}}$ < $\text{B}{{\text{r}}^{\text{-}}}$ < ${{\text{S}}^{\text{2-}}}$ < $\text{SC}{{\text{N}}^{\text{-}}}$ < $\text{C}{{\text{l}}^{\text{-}}}$ < ${{\text{F}}^{\text{-}}}$ < $\text{O}{{\text{H}}^{\text{-}}}$ < ${{\text{C}}_{\text{2}}}\text{O}_{\text{4}}^{\text{2-}}$ < ${{\text{O}}^{\text{2-}}}$ < ${{\text{H}}_{\text{2}}}\text{O}$ < $\text{NC}{{\text{S}}^{\text{-}}}$ < $\text{N}{{\text{H}}_{\text{3}}}$ < en < $\text{NO}_{\text{2}}^{\text{-}}$ < $\text{C}{{\text{N}}^{\text{-}}}$ < CO
18. What is crystal field splitting energy? How does the magnitude of ${{\text{ }\!\!\Delta\!\!\text{ }}_{\text{O}}}$ decide the actual configuration of d orbitals in a coordination entity?
Ans: D-orbitals degenerate into two levels (${{\text{e}}_{\text{g}}}$ and ${{\text{t}}_{\text{2}}}\text{g}$) in the presence of ligands in the spherical field surroundings. The separation of degenerated levels by the presence of ligands is called the splitting of the crystal field, and the energy difference between two levels (${{\text{e}}_{\text{g}}}$ and ${{\text{t}}_{\text{2}}}\text{g}$) is called the splitting energy of the crystal field. The name is ${{\text{ }\!\!\Delta\!\!\text{ }}_{\text{0}}}$.
The electrons are filled once the orbitals have divided. The filling of the fourth electron is done in two different methods after 1 electron (each) has been filled in 3 ${{\text{t}}_{\text{2}}}\text{g}$ orbitals.
The electron can be either in the ${{\text{e}}_{\text{g}}}$ orbital (giving birth to ${{\text{t}}_{\text{2}}}{{\text{g}}^{\text{3}}}\text{, }{{\text{e}}_{\text{g}}}^{\text{1}}$ similar electrical configuration) or in the ${{\text{t}}_{\text{2}}}\text{g}$ orbital (giving rise to ${{\text{t}}_{\text{2}}}{{\text{g}}^{4}}\text{, }{{\text{e}}_{\text{g}}}^{0}$ like electronic configuration). The electrons enter the ${{\text{e}}_{\text{g}}}$ orbital when the ${{\text{ }\!\!\Delta\!\!\text{ }}_{\text{0}}}$ value of the ligand is lower than pairing energy (p).
On the other hand, if a ligand's ${{\text{ }\!\!\Delta\!\!\text{ }}_{\text{0}}}$ value exceeds the pairing energy (P), the electrons then enter ${{\text{t}}_{\text{2}}}\text{g}$ orbital.
19. ${{\text{ }\!\![\!\!\text{ Cr(N}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{6}}}\text{ }\!\!]\!\!\text{ }}^{\text{3+}}}$ is paramagnetic while ${{\text{ }\!\![\!\!\text{ Ni(CN}{{\text{)}}_{\text{4}}}\text{ }\!\!]\!\!\text{ }}^{\text{2-}}}$ is diamagnetic. Explain why?
Ans: In ${{\text{ }\!\![\!\!\text{ Cr(N}{{\text{H}}_{3}}{{\text{)}}_{\text{6}}}\text{ }\!\!]\!\!\text{ }}^{\text{3+}}}$ the oxidation state of Cr is in +3 oxidation state, which will cause the configuration of Cr as ${{\text{d}}^{3}}$.
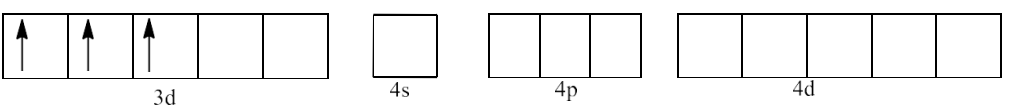
Since $\text{N}{{\text{H}}_{\text{3}}}$ is a weak field ligand, it does not cause pairing of electrons. This will lead to ${{\text{d}}^{\text{2}}}\text{s}{{\text{p}}^{\text{3}}}$ hybridization.

The geometry of the complex is octahedral and it is paramagnetic.
In ${{\text{ }\!\![\!\!\text{ Ni(CN}{{\text{)}}_{4}}\text{ }\!\!]\!\!\text{ }}^{2-}}$ the oxidation state of Ni is in +2 oxidation state, which will cause the configuration of Ni as ${{\text{d}}^{8}}$.
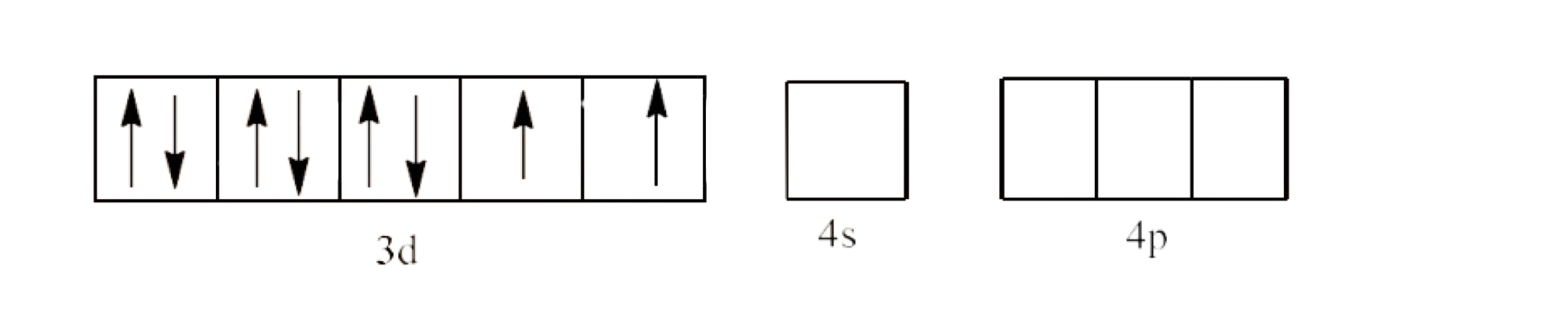
Since $\text{C}{{\text{N}}^{\text{-}}}$ is a strong field ligand, it causes unpaired electrons to be paired. This will lead to $\text{ds}{{\text{p}}^{2}}$ hybridization. This is shown below:
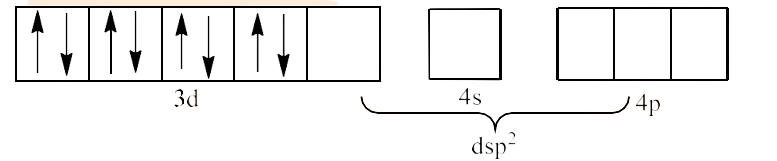
Therefore, the geometry of the structure is square planar and it is diamagnetic.
20. A solution of ${{\text{ }\!\![\!\!\text{ Ni(}{{\text{H}}_{\text{2}}}\text{O}{{\text{)}}_{\text{6}}}\text{ }\!\!]\!\!\text{ }}^{\text{2+}}}$ is green but a solution of ${{\text{ }\!\![\!\!\text{ Ni(CN}{{\text{)}}_{\text{4}}}\text{ }\!\!]\!\!\text{ }}^{\text{2-}}}$ is colorless. Explain.
Ans: ${{\text{ }\!\![\!\!\text{ Ni(}{{\text{H}}_{\text{2}}}\text{O}{{\text{)}}_{\text{6}}}\text{ }\!\!]\!\!\text{ }}^{\text{2+}}}$, the field ligand ${{\text{H}}_{\text{2}}}\text{O}$ is weak. Unpaired electrons are therefore available in $\text{N}{{\text{i}}^{\text{2+}}}$. In this complex d electrons can be stimulated to the higher energy level from the lower energy level, i.e. there is a potential to change d to d-orbital. Therefore, it has color. The ${{\text{ }\!\![\!\!\text{ Ni(CN}{{\text{)}}_{\text{4}}}\text{ }\!\!]\!\!\text{ }}^{\text{2-}}}$ have a strong ligand in the form of $\text{C}{{\text{N}}^{\text{-}}}$. As a result, the transition to d-d in ${{\text{ }\!\![\!\!\text{ Ni(CN}{{\text{)}}_{\text{4}}}\text{ }\!\!]\!\!\text{ }}^{\text{2-}}}$ is not feasible. It's colorless, therefore.
21. ${{\text{ }\!\![\!\!\text{ Fe(CN}{{\text{)}}_{\text{6}}}\text{ }\!\!]\!\!\text{ }}^{\text{4-}}}$ and ${{\text{ }\!\![\!\!\text{ Fe(}{{\text{H}}_{\text{2}}}\text{O}{{\text{)}}_{\text{6}}}\text{ }\!\!]\!\!\text{ }}^{\text{2+}}}$ are of different colors in dilute solution. Why?
Ans: The color of a certain coordinating chemical relies on the amount of the energy dividing the crystal field, ${{\text{ }\!\!\Delta\!\!\text{ }}_{\text{0}}}$. In turn, this CFSE depends on the ligand nature. The colors of ${{\text{ }\!\![\!\!\text{ Fe(CN}{{\text{)}}_{\text{6}}}\text{ }\!\!]\!\!\text{ }}^{\text{4-}}}$ and ${{\text{ }\!\![\!\!\text{ Fe(}{{\text{H}}_{\text{2}}}\text{O}{{\text{)}}_{\text{6}}}\text{ }\!\!]\!\!\text{ }}^{\text{2+}}}$ vary because the CFSE is different. Now, CN– is a powerful field ligand with greater CFSE than the CFSE water value. This means that intra-d-d transition absorption of energy also differs. The color also differs, however.
22. Discuss the nature of bonding in metal carbonyls.
Ans: The carbon metal linkages in metal carbonyls are characterized by both s and p. $\text{M-C }\!\!\sigma\!\!\text{ }$ bond consists of a donation into an empty metal orbital of a lone pair of electrons on the carbonyl carbon.
$\text{M-C }\!\!\pi\!\!\text{ }$ bond is the donation to the empty carbon dioxide vacant anti-bonding ${{\text{ }\!\!\pi\!\!\text{ }}^{\text{*}}}$ orbital of a pair of electrons from the d-filled metal orbital. The reverse bonding of the carbonyl group is also known. The ligand-metal interaction provides a synergistic effect that enhances the relationship between CO and metal. The synergistic action reinforces the connection between CO and metal. The diagram is given below:
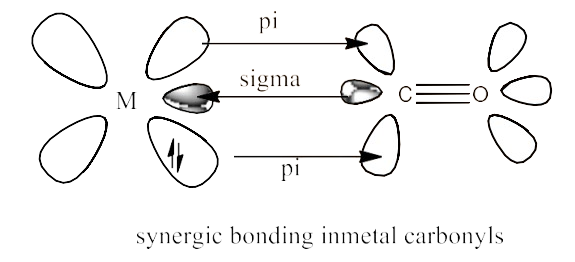
23. Give the oxidation state, d-orbital occupation and coordination number of the central metal ion in the following complexes:
(i) ${{\text{K}}_{\text{3}}}\text{ }\!\![\!\!\text{ Co(}{{\text{C}}_{\text{2}}}{{\text{O}}_{\text{4}}}{{\text{)}}_{\text{3}}}\text{ }\!\!]\!\!\text{ }$
Ans: The complex will be ${{\text{ }\!\![\!\!\text{ Co(}{{\text{C}}_{\text{2}}}{{\text{O}}_{\text{4}}}{{\text{)}}_{\text{3}}}\text{ }\!\!]\!\!\text{ }}^{\text{3-}}}$.
Oxidation state of cobalt will be = x – 6 = - 3
The oxidation state is = +3
Coordination number = 6
In +3 oxidation state of Co the configuration is $\text{3}{{\text{d}}^{\text{6}}}$.
So, the configuration will be $\text{t}_{\text{2g}}^{\text{6}}\text{ e}_{\text{g}}^{\text{0}}$
(ii) ${{\text{(N}{{\text{H}}_{\text{4}}}\text{)}}_{\text{2}}}\text{ }\!\![\!\!\text{ Co}{{\text{F}}_{\text{4}}}\text{ }\!\!]\!\!\text{ }$
Ans: The complex will be ${{\text{ }\!\![\!\!\text{ Co}{{\text{F}}_{4}}\text{ }\!\!]\!\!\text{ }}^{\text{2-}}}$.
Oxidation state of cobalt will be = x – 4 = - 2
The oxidation state is = +2
Coordination number = 4
In +2 oxidation state of Co the configuration is $\text{3}{{\text{d}}^{7}}$.
So, the configuration will be
(iii) $\text{cis- }\!\![\!\!\text{ Cr(en}{{\text{)}}_{\text{2}}}\text{C}{{\text{l}}_{\text{2}}}\text{ }\!\!]\!\!\text{ Cl}$
Ans: The complex will be ${{\text{ }\!\![\!\!\text{ Cr(en}{{\text{)}}_{2}}\text{C}{{\text{l}}_{\text{2}}}\text{ }\!\!]\!\!\text{ }}^{+}}$.
Oxidation state of chromium will be = x + 0 – 2 = + 1
The oxidation state is = + 3
Coordination number = 6
In +3 oxidation state of Cr the configuration is $\text{3}{{\text{d}}^{3}}$.
So, the configuration will be $\text{t}_{\text{2g}}^{3}$
(iv) $\text{ }\!\![\!\!\text{ Mn(}{{\text{H}}_{\text{2}}}\text{O}{{\text{)}}_{\text{6}}}\text{ }\!\!]\!\!\text{ S}{{\text{O}}_{\text{4}}}$
Ans: The complex will be ${{\text{ }\!\![\!\!\text{ Mn(}{{\text{H}}_{\text{2}}}\text{O}{{\text{)}}_{6}}\text{ }\!\!]\!\!\text{ }}^{2+}}$.
Oxidation state of manganese will be = x + 0 = + 2
The oxidation state is = + 2
Coordination number = 6
In +2 oxidation state of Mn the configuration is $\text{3}{{\text{d}}^{5}}$.
So, the configuration will be $\text{t}_{\text{2g}}^{3}\text{ e}_{\text{g}}^{2}$
24. Write down the IUPAC name for each of the following complexes and indicate the oxidation state, electronic configuration and coordination number. Also give stereochemistry and magnetic moment of the complex:
(i) $\text{K }\!\![\!\!\text{ Cr(}{{\text{H}}_{\text{2}}}\text{O}{{\text{)}}_{\text{2}}}{{\text{(}{{\text{C}}_{\text{2}}}{{\text{O}}_{\text{4}}}\text{)}}_{\text{2}}}\text{ }\!\!]\!\!\text{ }\text{.3}{{\text{H}}_{\text{2}}}\text{O}$
Ans: Its IUPAC name is Potassium diaquadioxalatochromate(III) hydrate.
Oxidation state of chromium = +3
Electronic configuration = $\text{3}{{\text{d}}^{\text{3}}}\text{ = t}_{\text{2g}}^{\text{3}}$
Coordination number = 6
Shape = octahedral
Stereochemistry:
Number of unpaired electrons = 3
Magnetic moment =
\[\text{ }\!\!\mu\!\!\text{ = }\sqrt{\text{n(n+2)}}\]
\[\text{ }\!\!\mu\!\!\text{ = }\sqrt{\text{3(3+2)}}\]
\[\text{ }\!\!\mu\!\!\text{ = }\sqrt{15}\]
\[\text{ }\!\!\mu\!\!\text{ =3}\text{.87 BM}\]
(ii) $\text{ }\!\![\!\!\text{ CrC}{{\text{l}}_{\text{3}}}\text{p}{{\text{y}}_{\text{3}}}\text{ }\!\!]\!\!\text{ }$
Ans: Its IUPAC name is Trichloridotripyridinechromium(III).
Oxidation state of chromium = +3
Electronic configuration = $\text{3}{{\text{d}}^{\text{3}}}\text{ = t}_{\text{2g}}^{\text{3}}$
Coordination number = 6
Shape = octahedral
Stereochemistry:
Number of unpaired electrons = 3
Magnetic moment =
\[\text{ }\!\!\mu\!\!\text{ =}\sqrt{\text{n(n+2)}}\]
\[\text{ }\!\!\mu\!\!\text{ =}\sqrt{\text{3(3+2)}}\]
\[\text{ }\!\!\mu\!\!\text{ =}\sqrt{15}\]
\[\text{ }\!\!\mu\!\!\text{ =3}\text{.87 BM}\]
(iii) ${{\text{K}}_{\text{4}}}\text{ }\!\![\!\!\text{ Mn(CN}{{\text{)}}_{\text{6}}}\text{ }\!\!]\!\!\text{ }$
Ans: Its IUPAC name is Potassium hexacyanomanganate(II).
Oxidation state of manganese = +2
Electronic configuration = $\text{3}{{\text{d}}^{5}}\text{ = t}_{\text{2g}}^{5}$
Coordination number = 6
Shape = octahedral
Stereochemistry: optically inactive
Number of unpaired electrons = 1
Magnetic moment =
\[\text{ }\!\!\mu\!\!\text{ =}\sqrt{\text{n(n+2)}}\]
\[\text{ }\!\!\mu\!\!\text{ =}\sqrt{\text{1(1+2)}}\]
\[\text{ }\!\!\mu\!\!\text{ =}\sqrt{3}\]
\[\text{ }\!\!\mu\!\!\text{ =1}\text{.73 BM}\]
(iv) $\text{ }\!\![\!\!\text{ Co(N}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{5}}}\text{Cl }\!\!]\!\!\text{ C}{{\text{l}}_{\text{2}}}$
Ans: Its IUPAC name is Pentaamminechloridecobalt(III) chloride.
Oxidation state of manganese = +3
Electronic configuration = $\text{3}{{\text{d}}^{6}}\text{ = t}_{\text{2g}}^{6}$
Coordination number = 6
Shape = octahedral
Stereochemistry:
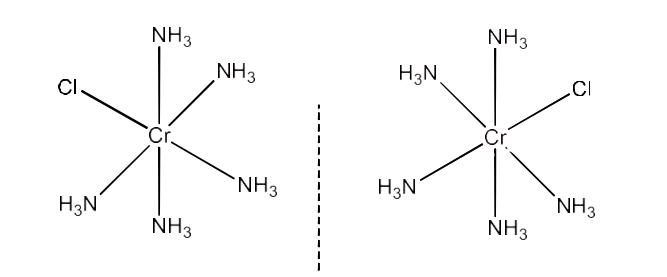
Number of unpaired electrons = 0
Magnetic moment = 0
(v) $\text{Cs }\!\![\!\!\text{ FeC}{{\text{l}}_{\text{4}}}\text{ }\!\!]\!\!\text{ }$
Ans: Its IUPAC name is Caesium tetrachloridoferrate(II).
Oxidation state of manganese = +3
Electronic configuration = $\text{3}{{\text{d}}^{\text{5}}}\text{ = }{{\text{e}}^{\text{2}}}\text{ t}_{\text{2}}^{\text{3}}$
Coordination number = 4
Shape = tetrahedral
Stereochemistry: optically inactive
Number of unpaired electrons = 5
Magnetic moment =
\[\text{ }\!\!\mu\!\!\text{ =}\sqrt{\text{n(n+2)}}\]
\[\text{ }\!\!\mu\!\!\text{ =}\sqrt{\text{5(5+2)}}\]
\[\text{ }\!\!\mu\!\!\text{ =}\sqrt{35}\]
\[\text{ }\!\!\mu\!\!\text{ =5}\text{.92 BM}\]
25. What is meant by stability of a coordination compound in solution? State the factors which govern stability of complexes.
Ans: The stability of the complex in a solution relates to the extent to which the two species are in balance. In terms of stability or formation constant , stability may be expressed numerically.
\[\text{M + 3L }\rightleftharpoons \text{M}{{\text{L}}_{\text{3}}}\]
Stability constant, $\beta \text{ }=\text{ }\frac{[M{{L}_{3}}]}{[M]{{[L]}^{3}}}$
The higher the stability constant value, the more the percentage of $\text{M}{{\text{L}}_{\text{3}}}$ in the solution is for this reaction.
Stability can be of two types:
Thermodynamic stability:
Thermodynamic stability determines to what extent the compound at the point of balance is produced or converted into another species.
Kinetic stability:
This helps to determine how quickly the change is to achieve the condition of balance.
Factors that affect the stability of a complex are:
Charge on the central metal ion: The greater the charge on the central metal ion, the greater is the stability of the complex.
Basic nature of the ligand: A more basic ligand will form a more stable complex.
Presence of chelate rings: Chelation increases the stability of complexes.
26. What is meant by the chelate effect? Give an example.
Ans: When a ligand binds to the metal ion in a way that creates a ring, the interaction of a metal ligand is more stable. In other terms, we may argue that chelate ring complexes are more stable than ring-free complexes. This is known as the Chelate effect.
For example,
\[\text{N}{{\text{i}}^{\text{2+}}}\text{(aq) + 6 N}{{\text{H}}_{\text{3}}}\text{(aq) }\rightleftharpoons \text{ }\!\![\!\!\text{ Ni(N}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{6}}}{{\text{ }\!\!]\!\!\text{ }}^{\text{2+}}}\]
For this reaction, $\text{log }\!\!\beta\!\!\text{ = 8}\text{.61}$
\[\text{N}{{\text{i}}^{\text{2+}}}\text{(aq) + 3 en(aq) }\rightleftharpoons \text{ }\!\![\!\!\text{ Ni(en}{{\text{)}}_{3}}{{\text{ }\!\!]\!\!\text{ }}^{\text{2+}}}\]
For this reaction, $\text{log }\!\!\beta\!\!\text{ = 18}\text{.28}$
27. Discuss briefly giving an example in each case the role of coordination compounds in:
(i) biological system
Ans: The presence of the chlorophyll pigment enables photosynthesis. The pigment is a magnesium co-ordinating molecule. Multiple coordinated chemicals perform essential functions in the human biological system. An iron coordination complex is, for example, the oxygen transporter of blood i.e. haemoglobin.
The cobalt coordination compound is vitamin B12, cyanocobalamine The anaemia anti-pernicious factor. Enzymes such as carboxypeptidase A and carbon dioxide are among other biologically important molecules containing co-ordinated metal ions.
(ii) medicinal chemistry
Ans: Certain platinum coordinating chemicals (e.g., cis-platinum) suppress tumour development.
Chelating ligands D-penicillamine, and desferrioxime B by forming coordination compounds, eliminate the excess of metal ions existing in a hazardous proportion in plant and animal systems such as copper or iron. EDTA is used in lead poisoning therapy.
(iii) Analytical Chemistry
Ans: A number of basic radicals are identified in the salt analysis using the colour changes with different reagents. These colour shift is due to the coordination of molecules or complexes formed with various ligands by the basic radicals.
For example: EDTA, DMG, etc.
(iv) Extraction/Metallurgy of Metals
Ans: The extraction process includes the production of complexes of many metals from their ores. In an aqueous solution, for instance, gold mixes to create $\text{Au(CN}{{\text{)}}_{\text{2}}}$ with cyanide ions. Gold is then recovered from this solution by adding zinc.
28. How many ions are produced from the complex $\text{Co(N}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{6}}}\text{C}{{\text{l}}_{\text{2}}}$ in solution?
(i) 6
(ii) 4
(iii) 3
(iv) 2
Ans: (iii) 3
The coordination number of cobalt is 6, therefore, the complex will be $\text{ }\!\![\!\!\text{ Co(N}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{6}}}\text{ }\!\!]\!\!\text{ C}{{\text{l}}_{\text{2}}}$. When this ionizes, three ions are formed. The reaction is given below:\[\text{ }\!\![\!\!\text{ Co(N}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{6}}}\text{ }\!\!]\!\!\text{ C}{{\text{l}}_{\text{2}}}\text{ }\xrightarrow{\text{aq}}\text{ }\!\![\!\!\text{ Co(N}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{6}}}{{\text{ }\!\!]\!\!\text{ }}^{\text{2+}}}\text{ + 2 C}{{\text{l}}^{\text{-}}}\]
29. Amongst the following ions which one has the highest magnetic moment value?
(i) ${{\text{ }\!\![\!\!\text{ Cr(}{{\text{H}}_{\text{2}}}\text{O}{{\text{)}}_{\text{6}}}\text{ }\!\!]\!\!\text{ }}^{\text{3+}}}$
Ans: The number of unpaired electrons in this compound is 3.
Magnetic moment =
\[\text{ }\!\!\mu\!\!\text{ =}\sqrt{\text{n(n+2)}}\]
\[\text{ }\!\!\mu\!\!\text{ =}\sqrt{\text{3(3+2)}}\]
\[\text{ }\!\!\mu\!\!\text{ =}\sqrt{15}\]
\[\text{ }\!\!\mu\!\!\text{ =3}\text{.87 BM}\]
(ii) ${{\text{ }\!\![\!\!\text{ Fe(}{{\text{H}}_{\text{2}}}\text{O}{{\text{)}}_{\text{6}}}\text{ }\!\!]\!\!\text{ }}^{\text{2+}}}$
Ans: The number of unpaired electrons in this compound is 4.
Magnetic moment =
\[\text{ }\!\!\mu\!\!\text{ =}\sqrt{\text{n(n+2)}}\]
\[\text{ }\!\!\mu\!\!\text{ =}\sqrt{\text{4(4+2)}}\]
\[\text{ }\!\!\mu\!\!\text{ =}\sqrt{24}\]
\[\text{ }\!\!\mu\!\!\text{ =4}\text{.89 BM}\]
(iii) ${{\text{ }\!\![\!\!\text{ Zn(}{{\text{H}}_{\text{2}}}\text{O}{{\text{)}}_{\text{6}}}\text{ }\!\!]\!\!\text{ }}^{\text{2+}}}$
Ans: The number of unpaired electrons in this compound is 0
Magnetic moment = 0
So, ${{\text{ }\!\![\!\!\text{ Fe(}{{\text{H}}_{\text{2}}}\text{O}{{\text{)}}_{\text{6}}}\text{ }\!\!]\!\!\text{ }}^{\text{2+}}}$ is the compound which has the highest magnetic moment.
30. The oxidation number of cobalt in$\text{K }\!\![\!\!\text{ Co(CO}{{\text{)}}_{\text{4}}}\text{ }\!\!]\!\!\text{ }$ is
(i) +1
(ii) +3
(iii) –1
(iv) –3
Ans: The complex will be ${{\text{ }\!\![\!\!\text{ Co(CO}{{\text{)}}_{\text{4}}}\text{ }\!\!]\!\!\text{ }}^{\text{-}}}$
The oxidation state of Cobalt will be = x + 0 = - 1
The oxidation number of Co = -1
Therefore, the answer is an option (iii) -1.
31. Amongst the following, the most stable complex is
(i) ${{\text{ }\!\![\!\!\text{ Fe(}{{\text{H}}_{\text{2}}}\text{O}{{\text{)}}_{\text{6}}}\text{ }\!\!]\!\!\text{ }}^{\text{3+}}}$
(ii) ${{\text{ }\!\![\!\!\text{ Fe(N}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{6}}}\text{ }\!\!]\!\!\text{ }}^{\text{3+}}}$
(iii) ${{\text{ }\!\![\!\!\text{ Fe(}{{\text{C}}_{\text{2}}}{{\text{O}}_{\text{4}}}{{\text{)}}_{\text{3}}}\text{ }\!\!]\!\!\text{ }}^{\text{3+}}}$
(iv) ${{\text{ }\!\![\!\!\text{ FeC}{{\text{l}}_{\text{6}}}\text{ }\!\!]\!\!\text{ }}^{\text{3-}}}$
Ans: In the compounds, Fe is the central metal ion and its oxidation state +3. As ${{\text{C}}_{\text{2}}}\text{O}_{\text{4}}^{\text{2-}}$ is a didentate chelating ligand, it can form chelating ring and hence is the most stable complex.
Therefore, the answer is an option (iii).
32. What will be the correct order for the wavelengths of absorption in the visible region for the following: ${{\text{ }\!\![\!\!\text{ Ni(N}{{\text{O}}_{\text{2}}}{{\text{)}}_{\text{6}}}\text{ }\!\!]\!\!\text{ }}^{\text{4-}}}\text{, }\!\![\!\!\text{ Ni(N}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{6}}}{{\text{ }\!\!]\!\!\text{ }}^{\text{2+}}}\text{, }\!\![\!\!\text{ Ni(}{{\text{H}}_{\text{2}}}\text{O}{{\text{)}}_{\text{6}}}{{\text{ }\!\!]\!\!\text{ }}^{\text{2+}}}$
Ans: In all three complexes, the core metal ion is identical. The absorption depends thus on the ligands in the visible area. In the spectrochemical series, the sequence in which the CFSE values of the ligands are increasing is: ${{\text{H}}_{\text{2}}}\text{O}$ < $\text{N}{{\text{H}}_{\text{3}}}$ < $\text{N}{{\text{O}}_{\text{2}}}$.
Therefore, the amount of crystal-field splitting observed will be in the following order:
$\text{ }{{\text{ }\!\!\Delta\!\!\text{ }}_{\text{0 (}{{\text{H}}_{2}}\text{O)}}}$ < $\text{ }{{\text{ }\!\!\Delta\!\!\text{ }}_{\text{0 (N}{{\text{H}}_{3}}\text{)}}}$ < $\text{ }{{\text{ }\!\!\Delta\!\!\text{ }}_{\text{0 (NO}_{2}^{-}\text{)}}}$
So, the wavelength of absorption in the visible region will be in the order: ${{\text{ }\!\![\!\!\text{ Ni(}{{\text{H}}_{\text{2}}}\text{O}{{\text{)}}_{\text{6}}}\text{ }\!\!]\!\!\text{ }}^{\text{2+}}}$ > ${{\text{ }\!\![\!\!\text{ Ni(N}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{6}}}\text{ }\!\!]\!\!\text{ }}^{\text{2+}}}$ > ${{\text{ }\!\![\!\!\text{ Ni(N}{{\text{O}}_{\text{2}}}{{\text{)}}_{\text{6}}}\text{ }\!\!]\!\!\text{ }}^{\text{2+}}}$.
Class 12 Chemistry Chapter 5 Quick Overview of Topics
Chemistry class 12 chapter 5 NCERT Solutions - Quick Overview of Detailed Structure of Topics and Subtopics Covered.
Topics | Subtopics |
Introduction to Coordination Compounds | - Definition and characteristics of coordination compounds |
- Ligands and coordination number | |
Nomenclature of Coordination Compounds | - IUPAC rules for naming coordination compounds |
- Isomerism in coordination compounds | |
Bonding in Coordination Compounds | - Werner's theory of coordination compounds |
- Valence bond theory | |
- Crystal field theory | |
Isomerism in Coordination Compounds | - Structural isomerism |
- Geometric isomerism | |
- Optical isomerism | |
Bonding in Metal Carbonyls | - σ and π bonding in metal carbonyls |
- Metal-metal bond in dinuclear carbonyls | |
Importance and Applications | - Industrial and biological importance of coordination compounds |
- Role in catalysis and medicinal chemistry |
Some Important Concepts for Class 12 Chapter 5- Coordination Compounds
Class 12 NCERT solutions help the students to go through the Concepts easily. Find the important topics in Chapter 5- Coordination Compounds to crack your exams.
Coordination Number (CN): The coordination number of a central metal ion in a complex is the total number of ligands attached to it. It is determined experimentally or by the nature of the complex.
Werner's Coordination Theory: Werner proposed the theory of coordination compounds, stating that metal ions exhibit two types of valencies - primary and secondary. Primary valency determines the oxidation state of the metal ion, while secondary valency determines the coordination number and the number of ligands attached to the metal ion.
Stability Constant (Kₛ): The stability constant (also known as formation constant) is a measure of the stability of a complex ion in solution. It is defined as the equilibrium constant for the formation of the complex ion from its constituent ions.
Isomerism: Coordination compounds exhibit various types of isomerism, including structural isomerism (geometric isomerism, linkage isomerism) and stereoisomerism (optical isomerism, geometrical isomerism).
Crystal Field Theory (CFT): CFT explains the electronic structure and properties of transition metal complexes by considering the interaction between the d orbitals of the metal ion and the ligand's electron pairs.
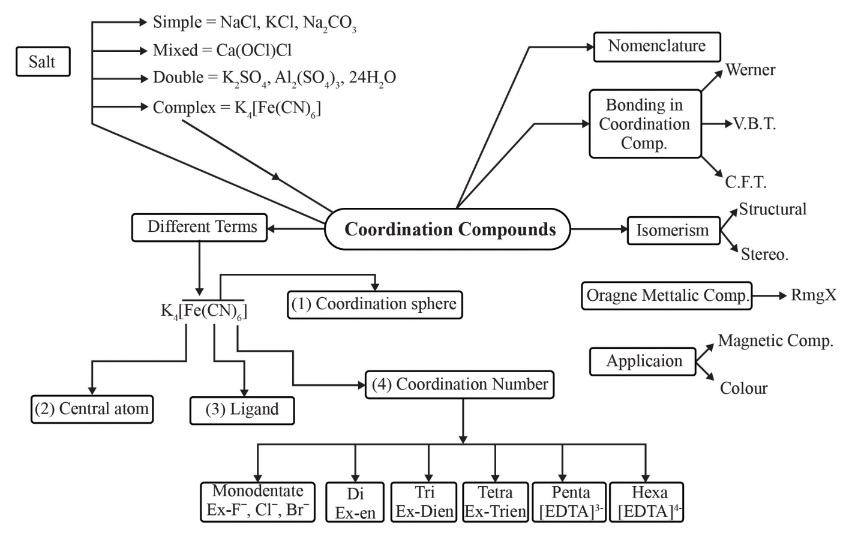
Below is the Concept Map for the chapter Coordination Compounds :
Benefits of Referring to Vedantu’s NCERT Solutions for Class 12 Chemistry Chapter 5
The Vedantu’s class 12 NCERT Solutions of Chemistry Chapter 5- Coordination Compounds provided in PDFs offer various benefits, including:
The answers provided here are straightforward topics like CFT explains the electronic structure and properties of transition metal complexes by considering the interaction between the d orbitals of the metal ion and the ligand's electron pairs
It provides the Concise Notes and saves a lot of time for Revision.
To facilitate comprehension, topics like isomerism and ligands the best explained are best explained.
All of the questions from each chapter are answered.
For effective preparations, comprehend all of the processes outlined in the answers.
Related Study Materials for Class 12 Chemistry Chapter 5 NCERT solutions
Students can access extra study materials in Chapter 5- Coordination Compounds. These resources are available for download and offer additional support for your studies.
S.No | Related Links for Class 12 Chemistry Chapter 5: Coordination Compounds |
1. | |
2. | |
3. |
Conclusion
Vedantu's Class 12 Chemistry Coordination Compounds NCERT Solutions offer a comprehensive and reliable resource for students studying this topic. The solutions are well-structured and cover all the important concepts and questions outlined in the NCERT textbook. They provide clear explanations, step-by-step solutions, and relevant examples, aiding students in understanding the subject matter effectively. Vedantu's solutions also ensure that students grasp the underlying principles of coordination compounds, their nomenclature, bonding, and various types of isomerism. With these solutions, students can enhance their problem-solving skills, build a strong foundation in coordination chemistry, and confidently prepare for their Class 12 Chemistry examinations.
NCERT Solutions Class 12 Chemistry | Chapter-wise Links
Access Vedantu’s chapter-wise NCERT Chemistry Class 12 Solutions PDFs below for all other chapters.
S.No. | NCERT Solutions Class 12 Chemistry Chapter-wise List |
1 | |
2 | |
3 | |
4 | |
5 | |
6 | |
7 | Chapter 8 - Aldehydes, Ketones and Carboxylic Acids Solutions |
8 | |
9 |
NCERT Solutions Class 12 Chemistry - Related Links
S.No | Important Resources Links for Class 12 Chemistry |
1 | |
2 | |
3 | |
4 | |
5 |
FAQs on NCERT Solutions For Class 12 Chemistry Chapter 5 Coordination Chemistry - 2025-26
1. Where can I find stepwise NCERT Solutions for Class 12 Chemistry Chapter 5 Coordination Compounds as per CBSE 2025–26 syllabus?
You can access the NCERT Solutions for Class 12 Chemistry Chapter 5 – Coordination Compounds in a stepwise format that strictly follows the official CBSE 2025–26 NCERT textbook on Vedantu’s NCERT Solutions page. Each exercise and intext question is solved using the latest NCERT answer structure and provides clear steps so that students can easily follow the CBSE-approved methods.
2. Are the answers in Class 12 Chemistry Chapter 5 NCERT Solutions explained using proper NCERT answer format?
Yes, all solutions for Class 12 Chemistry Chapter 5 are presented in the official NCERT answer format with step-by-step explanations as per the updated CBSE guidelines. This ensures full alignment with the marking scheme used in board examinations and helps students secure maximum marks.
3. How do you solve the intext questions of Class 12 Chemistry Chapter 5 using the NCERT pattern?
To solve the intext questions in Chapter 5 – Coordination Compounds, carefully read each question, apply the theoretical concepts and rules stated in the NCERT textbook, and answer using a clear, stepwise explanation. Each solution should start with the correct chemical formula or principle and end with a precise, to-the-point answer aligned with CBSE’s marking pattern.
4. Is the Class 12 Chemistry Chapter 5 solution PDF available for download in both English and Hindi?
Yes, NCERT Solutions for Class 12 Chemistry Chapter 5 are available for both English and Hindi mediums in downloadable PDF format. These PDFs contain accurately solved back exercises and intext questions as per CBSE 2025–26 NCERT syllabus, making it easy for students to study offline.
5. What is the correct NCERT answer for Class 12 Chemistry Chapter 5 Exercise 5.3 Question 4?
The correct answer to Exercise 5.3 Question 4 of Class 12 Chemistry Chapter 5 is given in the official NCERT Solutions, providing a stepwise explanation involving coordination number, geometry, and the application of rules for writing IUPAC names or drawing structures, as required by the question in the CBSE NCERT textbook.
6. Does the NCERT Solutions for Chapter 5 Chemistry cover all exercise and intext questions from the 2025–26 NCERT book?
Yes, the NCERT Solutions for Class 12 Chemistry Chapter 5 comprehensively cover every exercise and intext question included in the CBSE 2025–26 NCERT textbook. Each answer follows the latest academic guidelines and helps students prepare thoroughly for board exams.
7. How can I download the NCERT Solutions for Class 12 Chemistry Chapter 5 PDF for offline use?
You can download the NCERT Solutions for Class 12 Chemistry Chapter 5 PDF directly from Vedantu’s official website. The solutions are organized in an easy-to-navigate manner and follow the CBSE and NCERT approved answer format for the current academic year.
8. Are the Class 12 Chemistry Chapter 5 NCERT Solutions suitable for quick revision before board exams?
Yes, these solutions provide precise, stepwise answers for every question as per the NCERT textbook, making them ideal for last-minute revision. The clarity and accuracy help reinforce key concepts and ensure that students are prepared according to CBSE standards for board exams.
9. Do these NCERT Solutions clarify common mistakes or misconceptions in Coordination Compounds questions?
Absolutely. The solutions are structured to address frequently made errors, such as confusion in coordination number, naming ligands, or writing correct formulas, by providing stepwise justification for each answer according to NCERT and CBSE norms.
10. Are stepwise explanations given for drawing structures and writing IUPAC names for complex compounds in Chapter 5?
Yes, every question that requires structure drawing or IUPAC nomenclature in Class 12 Chemistry Chapter 5 is solved with a detailed, stepwise explanation. This includes the reasoning based on textbook conventions and ensures students learn the NCERT-approved solving method.
11. How do the intext solution answers for Class 12 Chemistry Chapter 5 differ from those of the exercise questions?
Intext solution answers focus on conceptual clarity and are generally more concise, while exercise solutions tend to involve longer, stepwise reasoning and application of theory. Both use the NCERT-approved answer formats and are solved based on the latest CBSE guidelines.
12. What should I do if the NCERT answer for a coordination compound differs from my teacher’s answer?
Always refer first to the CBSE-approved NCERT textbook solution. The answers provided in NCERT Solutions strictly follow CBSE marking guidelines and official NCERT answer patterns, which are preferred and accepted in board exam evaluation.
13. Are solutions also available for application-based or HOTS questions in Chapter 5 NCERT Chemistry?
The main focus of NCERT Solutions for Class 12 Chemistry Chapter 5 is on exercise and intext questions directly from the NCERT textbook, including application-based questions wherever provided in the book. Each is answered in a logical and NCERT-aligned stepwise format suitable for board preparation.


























