




How Is Triphenylphosphine Prepared and Why Is It Important?
PPh3 ligand name is triphenylphosphine which is also known as triphenylphosphine (IUPAC name) is a common organophosphorus chemical compound with the formula (C6H5)3 which is most commonly used in the synthesis of organometallic and organic compounds. Due to its efficiency as a reducing agent and neutrality as a ligand, it is frequently utilised in the synthesis of organic and organometallic compounds. PPh3 crystals are largely air-stable and colourless at ambient temperature. It dissolves in non-polar chemical solvents like benzene and diethyl ether.
Structure of Triphenylphosphine
There are a total of 36 bonds in the triphenylphosphine molecule. There are 18 multiple bonds, 3 rotatable bonds, 18 aromatic bonds, 3 six-membered rings, and 1 phosphine in addition to the 21 non-H bonds. Both the triclinic and monoclinic forms are formed when triphenylphosphine crystallises. In both instances, the molecule is composed of three phenyl groups that are arranged like propellers and have a pyramidal structure.
The phosphorus atom at the centre of triphenylphosphine is sp3 hybridised. P-C sigma bond directly links the phosphorus atom to three phenyl groups, and an electron pair is present in one sp3 hybridised orbital as a lone pair.

PPh3 Structure
Properties of PPh3
Triphenylphosphine is a white crystalline solid which is odourless and largely air-stable at room temperature. It has a density of 1.1 g/cm3 and a dipole moment of 1.42D. Its polarity is very low hence it is soluble in non-polar solvents like benzene, diethyl ether, etc.
Its chemical formula is ${{({{C}_{6}}{{H}_{5}})}_{3}}P$ and its molecular mass is about 262.3 g/mol
Melting point = 80 ℃
Boiling point = 377 ℃
Solubility in water = 0.09 mg/L at 25 ℃ (insoluble in water)
Vapour pressure = 0.017 Pa at 50 ℃
It breaks down when heated, producing phosphine and phosphorus oxides; an allergen that causes irritation to the skin, eyes, and respiratory system; the powerful reducing agent that induces convulsions in lethal doses, is very harmful if consumed.
Preparation of PPh3
By treating phosphorus trichloride with phenylmagnesium bromide or phenyl lithium, triphenylphosphine can be synthesised in a laboratory. Commercial synthesis involves the reaction of sodium, chlorobenzene, and phosphorus trichloride.
$PC{{l}_{3}}\,+\,3PhCl\,+\,6Na\,\to \,PP{{h}_{3}}\,+\,6NaCl$
Reactions of Triphenylphosphine
Quaternization: Alkyl halides and $PP{{h}_{3}}$ react at elevated temperatures with the help of metal catalysts to form phosphonium salts. Benzylic and allylic halides undergo an extremely rapid quaternization process.
$PP{{h}_{3}}\,+\,C{{H}_{3}}I\,\to \,{{\left[ C{{H}_{3}}PP{{h}_{3}} \right]}^{+}}{{I}^{-}}$
Mitsunobu Reaction: A mixture of triphenylphosphine and diisopropyl azodicarboxylate ("DIAD" or its diethyl counterpart, DEAD) transforms alcohol and a carboxylic acid into an ester in the Mitsunobu reaction. The $PP{{h}_{3}}$ is oxidised to $OPP{{h}_{3}}$ and the DIAD is reduced while acting as the hydrogen acceptor.
Oxidation: Slow air oxidation of triphenylphosphine results in triphenylphosphine oxide.
$2PP{{h}_{3}}\,+\,{{O}_{2}}\,\to \,2OPP{{h}_{3}}$
Chlorination: Triphenylphosphine dichloride, which occurs as the moisture-sensitive phosphonium halide, is produced when Chlorine reacts with $PP{{h}_{3}}$. In organic synthesis, this reagent is used to transform alcohols into alkyl chlorides.
$2P{{h}_{3}}PC{{l}_{2}}\,+\,N{{H}_{2}}OH.HCl\,+\,P{{h}_{3}}P\,\to \,\left\{ {{\left[ P{{h}_{3}}P \right]}_{2}}N \right\}Cl\,+\,3HCl\,+\,P{{h}_{3}}PO$
Protonation: Being a weak base $PP{{h}_{3}}$ produces isolable triphenylphosphonium salts when combined with strong acids like HBr.
$P{{\left( {{C}_{6}}{{H}_{5}} \right)}_{3}}\,+\,HBr\,\to \,{{\left[ HP{{\left( {{C}_{6}}{{H}_{5}} \right)}_{3}} \right]}^{+}}B{{r}^{-}}$
Metal - Phosphine Complex
A coordination compound with one or more phosphine ligands is known as a metal-phosphine complex. The phosphine is almost usually an organophosphine of type ${{R}_{3}}P$ (R = alkyl, aryl). There is a vast application of metal phosphine compounds in homogeneous catalysis.
Many metal phosphine complexes are made by reacting metal halides with phosphines that have already been produced. For instance, when palladium chloride is suspended in ethanol and treated with triphenylphosphine, monomeric bis(triphenylphosphine)palladium(II) chloride units are produced.
$\left[ PdC{{l}_{2}} \right]n\,+\,2nPP{{h}_{3}}\,\to \,nPdC{{l}_{2}}{{(PP{{h}_{3}})}_{2}}$
Bonding in Metal - Phosphine Complex
Phosphines are L-type ligands which means that $PP{{h}_{3}}$ is a neutral ligand and can donate 2 electrons to the metal centre. Metal phosphine complexes often exhibit strong solubility in organic solvents and are lipophilic, when compared to the majority of metal ammine complexes. Additionally, phosphine ligands function as π -acceptors. Their π-acidity results from the overlap of P-C σ* anti-bonding orbitals with filled metal orbitals. Alkyl Phosphines are weaker π-acceptors than aryl phosphines and fluorophosphate.
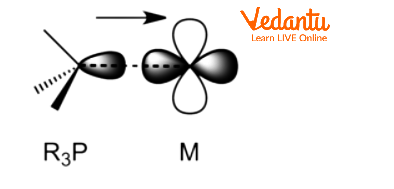
${{R}_{3}}P$–M σ Bonding
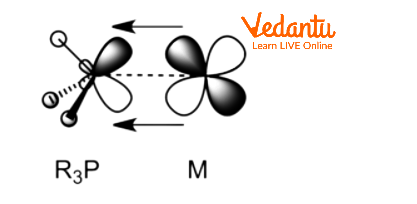
${{R}_{3}}P$–M π Back Bonding
Uses of Triphenylphosphine
Making of sensitizers, heat stabilisers, light stabilisers, antioxidants, flame retardants, antistatic agents, rubber antiozonants, and analytical reagents are just a few of the uses for triphenylphosphine.
Chlorambucil, which has been demonstrated to be cytotoxic in breast and pancreatic tumours, is made using triphenylphosphine.
In the production of vitamin D2, vitamin A, clindamycin, and other medicines, triphenylphosphine is utilised.
In addition, triphenylphosphine is employed as a transition metal-ligand in the Suzuki, Mitsunobu, and Appel reactions as well as in the polymerisation of olefins and it is a standard ligand used in homogeneous catalysis.
Important Questions
Triphenylphosphine is what kind of ligand?
Ans: Triphenylphosphine is an L-type ligand which means that it is a neutral ligand and can donate 2 electrons to the metal centre. Metal phosphine complexes, in contrast to the majority of metal ammine complexes, are lipophilic and have high solubility in organic solvents.
Comment on the toxicity of triphenylphosphine.
Ans: Triphenylphosphine is a toxic compound found in nature. Our skin, respiratory system, or eyes may become irritated when it comes in contact with them.
Conclusion
Triphenylphosphine is a white crystalline solid which is odourless, cheaper and air-stable than any other phosphines. Due to its efficiency as a reducing agent and neutrality as a ligand, the Metal-Phosphine complex is frequently utilised in the synthesis of organic and organometallic compounds. It has a total of 36 bonds and a pyramidal structure. It undergoes various reactions like quaternization, mitsunobu reaction, oxidation, chlorination, and protonation reactions. It is widely used for making analytical reagents, drugs like chlorambucil, vitamins like Vitamin D2, Vitamin A, etc.
Practice Questions
A total of how many bonds are present in triphenylphosphine?
20
36
42
51
What is the structure of triphenylphosphine?
Pyramidal
Tetrahedral
Angular
Planar
Answers
(b)
(a)
FAQs on Triphenylphosphine Explained: Structure, Properties & Applications
1. What is the molecular structure and geometry of triphenylphosphine (PPh₃)?
Triphenylphosphine, with the chemical formula P(C₆H₅)₃ or PPh₃, consists of a central phosphorus atom bonded to three phenyl groups. Due to the presence of a lone pair of electrons on the phosphorus atom, the molecule adopts a trigonal pyramidal geometry, as predicted by VSEPR theory. The three bulky phenyl groups are arranged in a propeller-like fashion around the central phosphorus atom.
2. What are the key physical and chemical properties of triphenylphosphine?
Triphenylphosphine exhibits several distinct properties that are important for its use in chemistry.
Physical Properties:
It is a white crystalline solid at room temperature.
It is generally soluble in non-polar organic solvents like ether and benzene but is insoluble in water.
Chemical Properties:
It is relatively air-stable but can be slowly oxidized by air to form triphenylphosphine oxide (TPPO).
It acts as a weak Lewis base due to the lone pair on the phosphorus atom.
It is an excellent nucleophile and a good reducing agent.
3. Why is triphenylphosphine (PPh₃) considered a weak base but a good nucleophile?
This difference arises from the electronic structure of the molecule. PPh₃ is a weak base because the lone pair on the phosphorus atom is delocalised into the π-systems of the three attached phenyl rings through resonance. This delocalisation reduces the availability of the lone pair to accept a proton. However, it is a good nucleophile because phosphorus is a large, highly polarisable atom. This 'softness' allows its electron cloud to be easily distorted to attack soft electrophilic centres, like the carbon in an alkyl halide, making it highly effective in nucleophilic substitution reactions.
4. What are the most important applications of triphenylphosphine in organic synthesis?
Triphenylphosphine is a versatile reagent used in several fundamental organic reactions. Its main applications include:
Wittig Reaction: Used to synthesise alkenes from aldehydes and ketones.
Appel Reaction: Used for converting alcohols into alkyl chlorides.
Mitsunobu Reaction: Enables the conversion of primary and secondary alcohols to esters, ethers, and other functional groups with an inversion of stereochemistry.
Staudinger Reaction: Used to prepare amines from azides.
Ligand in Catalysis: It serves as a ligand in important organometallic catalysts, such as Wilkinson's catalyst for the hydrogenation of alkenes.
5. What is the role of triphenylphosphine in the Wittig Reaction?
In the Wittig reaction, triphenylphosphine's primary role is to form the key intermediate, the phosphonium ylide. First, PPh₃ acts as a nucleophile and attacks an alkyl halide to form a phosphonium salt. A strong base then removes a proton from the carbon adjacent to the phosphorus, creating the ylide. This ylide then reacts with a carbonyl compound (aldehyde or ketone). The entire process is driven by the formation of the highly stable triphenylphosphine oxide (P=O) as a byproduct, which has a very strong double bond.
6. Is triphenylphosphine (PPh₃) a monodentate or bidentate ligand in coordination chemistry?
Triphenylphosphine is a classic example of a monodentate ligand. This means it binds to a central metal atom at only one site. The coordination occurs through the donation of the single lone pair of electrons located on the phosphorus atom. It does not have any other donor atoms to form a second bond with the metal.
7. Is PPh₃ considered a strong-field or a weak-field ligand, and why?
Triphenylphosphine is generally classified as a strong-field ligand. While it acts as a σ-donor through its lone pair, its strength comes from its ability to act as a π-acceptor. The phosphorus atom has empty, accessible d-orbitals that can accept electron density from the filled d-orbitals of the transition metal. This process, known as π-backbonding, strengthens the metal-ligand bond and increases the crystal field splitting energy (Δ), which is characteristic of strong-field ligands.
8. How does the steric hindrance of triphenylphosphine influence its behaviour in chemical reactions?
The three bulky phenyl groups in PPh₃ create significant steric hindrance around the central phosphorus atom. This steric bulk has important consequences:
It influences the selectivity of reactions by controlling which molecules can approach a metal centre it is coordinated to.
It affects the coordination number of metal complexes, often favouring lower numbers to minimise steric repulsion.
Its size is quantified by the Tolman cone angle, a concept used in organometallic chemistry to measure the steric bulk of phosphine ligands and predict their effect on catalysis.
9. What is triphenylphosphine oxide (TPPO), and why is its formation so significant?
Triphenylphosphine oxide, or TPPO (Ph₃P=O), is the product formed when triphenylphosphine is oxidised. Its formation is a powerful thermodynamic driving force for many reactions like the Wittig, Appel, and Mitsunobu reactions. The reason for this is the exceptional stability of the phosphorus-oxygen double bond (P=O), which has a very high bond energy. The release of this energy upon forming TPPO makes the overall reaction highly favourable and often irreversible.























