




What is the Structure of Dimethylformamide? – Detailed Explanation
DMF is an organic solvent which is hygroscopic in nature. The DMF full form is dimethylformamide. It shares structural similarities with formamide, as suggested by its name. DMF can be easily absorbed through the skin, inhaled, or consumed. Serious liver toxin. Abdominal pain, constipation, vomiting and nausea, headache, fatigue, disorientation, skin problems, and alcohol intolerance are all possible side effects of DMF. The current data linking DMF to cancer in humans is inconclusive.
What is DMF?
Dimethylformamide is a chemical compound with the most common abbreviation as DMF and the DMF full form. The general dimethylformamide formula is (CH3)2NC(O)H. It's a colourless liquid that mixes with water and other organic solvents. It is widely used as a solvent in chemical processes. It is a formic acid amide derivative of formamide. DMF is a polar aprotic solvent hence DMF boiling point is high at 153 °C. It contributes to the accelaration of SN2 reactions. This solvent is recommended for the production of acrylic fibres.
Production of DMF
It is synthesized by reacting methyl formate with dimethylamine or by mixing dimethylamine with carbon monoxide. It can also be produced employing supercritical carbon monoxide and a Ru-based catalyst. A combination of dimethylamine hydrochloride and potassium formate distillation was used in this approach.
Dimethylformamide Structure and Properties
The DMF structure is given below:
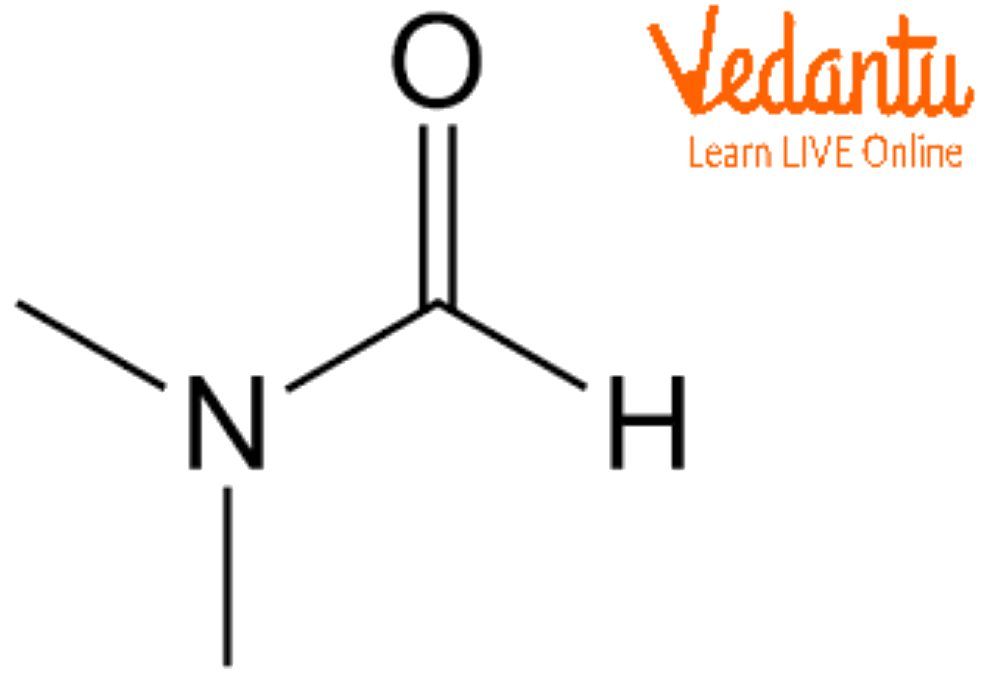
DMF Structure
For most amides, spectroscopic investigations have revealed a partial double bond nature for C-N and C-O bonds. C=O stretches at 1675 cm-1 in the IR spectrum, while ketone absorption is at 1700 cm-1.
At high temperatures, DMF gets easily hydrolyzed in the influence of strong acids and bases. It is transformed to format and dimethylamine when it reacts with NaOH. Decarboxylation occurs at its boiling point, resulting in the synthesis of dimethylamine.
As the DMF density (0.95 g cm-3 at 20°C) is comparable to that of water, considerable floating or stratification in surface waters is not predicted in the event of unintentional losses.
Exothermic decompositions have been observed at temperatures as low as 26 °C in reactions involving the utilization of sodium hydride in DMF as a solvent. On a lab level, any high temperature is (generally) easily detected and controlled using an ice bath, and this stays a preferred reagent mixture. On the contrary, some disasters have indeed been documented on a pilot plant scale.
Dimethylformamide Uses
Dimethylformamide is widely utilised as a solvent in industry. Dimethylformamide solutions are employed to treat polymer fibres, films, and surface coatings; make acrylic fibres easier to spin; make wire enamels, and as a crystallisation media in the pharmaceutical sector. Some of the other dimethylformamide uses are as follows:
Dimethylformamide undergoes several organic, organometallic, and bioorganic conversions.
Nucleophilic aprotic dipolar solvent dimethylformamide serves several uses, particularly in the production of polymers. Although it is hepatotoxic, its toxic effect for mammals is minimal.
The manufacture of metal colloids uses DMF as it is a potent reducing agent for metallic compounds.
DMF is thermally decomposed to CO, which interacts with water to create hydrogen when CuFe2O4 is used as a catalyst.
The creation and processing of polymers are significant uses. It is employed in the creation of synthetic leather as well as the spinning of polyacrylonitrile and polyurethane (Spandex) fibres.
It is additionally utilized in a variety of steps in the manufacturing of medications. DMF's exceptional solvent qualities allow it to be used in numerous pharmaceutical activities as a reaction and crystallising solvent.
DMF is additionally applied in several industrial paint removing processes. However, safety issues prevent its application in these kinds of consumer devices. DMF's strong solvent strength also influences its employment as an ink and dye carrier in a variety of printing and fiber-dyeing uses.
Interesting Facts
DMF can operate as a metal colloids' stabilising agent to provide efficient and recyclable catalysts.
It is impossible to compress and preserve pure acetylene gas without running the risk of an explosion. In the context of dimethylformamide, which creates a secure, concentrated solution, industrial acetylene can be compressed without risk.
Key Features to Remember
The high dielectric constant, the aprotic character of the solvent, and its broad range of liquids of DMF make its solvent characteristics especially appealing.
DMF is a widely used reagent in research labs since it is inexpensive and accessible.
DMF's main application is as a solvent with a slow evaporation rate. Plastics and acrylic fibres are produced using DMF.
The manufacturing of acrylic fibres prefers using DMF as the solvent because of the high solubility of polyacrylonitrile in it and the solvent's good miscibility with water. Additionally, polyurethane-based elastomer spinning is carried out using DMF-based solutions.
List of Related Articles
FAQs on Dimethylformamide Structure: Key Concepts & Applications
1. What exactly is Dimethylformamide (DMF)?
Dimethylformamide, often abbreviated as DMF, is an organic compound with the chemical formula (CH₃)₂NC(O)H. It is a colorless liquid that is miscible with water and most organic liquids. Due to its properties, it's widely used as a common polar aprotic solvent in chemical reactions.
2. What functional group is found in the Dimethylformamide structure?
The key functional group in Dimethylformamide is an amide. Specifically, it is a disubstituted amide, where two methyl groups (-CH₃) are attached to the nitrogen atom of the amide group.
3. How does resonance affect the structure and properties of DMF?
Resonance plays a crucial role in DMF's structure. The lone pair of electrons on the nitrogen atom can delocalise to form a double bond with the carbonyl carbon. This results in two main resonance structures. This delocalisation gives the C-N bond partial double-bond character, making it shorter and stronger than a typical C-N single bond and restricting rotation around it. This also contributes to its high boiling point (153 °C).
4. Is DMF a solvent or a reagent?
Dimethylformamide is primarily used as a solvent. Its high dielectric constant and aprotic nature make it excellent for facilitating reactions, especially SN2 reactions. However, it can also act as a reagent in specific reactions, such as the Vilsmeier-Haack reaction, where it serves as a source of a formyl group.
5. What are the main uses of Dimethylformamide in industry?
DMF has several important industrial applications. Its main uses include:
- As a versatile solvent for producing polymers like polyacrylonitrile and polyurethanes.
- In the manufacturing of pesticides and pharmaceuticals.
- As a catalyst in certain chemical syntheses.
- For separating and refining gases in the petrochemical industry.
6. Why is Dimethylformamide considered a polar aprotic solvent?
DMF is classified as a polar aprotic solvent for two reasons:
- Polar: It has a large dipole moment due to the highly polar carbonyl group (C=O).
- Aprotic: It does not have any acidic protons. The hydrogen atoms are attached to carbon, not oxygen or nitrogen, so they cannot participate in hydrogen bonding as a donor.
7. Is DMF harmful or flammable?
Yes, Dimethylformamide has safety concerns. It is considered flammable, with a flash point of 58 °C. It is also toxic and can be harmful if inhaled, ingested, or absorbed through the skin, potentially causing liver damage with prolonged exposure. For this reason, its use is regulated in many regions.
8. How does the structure of DMF compare to that of DMSO (Dimethyl sulfoxide)?
While both DMF and DMSO are common polar aprotic solvents, their structures are different. DMF is an amide with a central carbonyl group (C=O). In contrast, DMSO (Dimethyl sulfoxide) is a sulfoxide, containing a sulfur atom double-bonded to an oxygen atom (S=O). This difference in the central functional group leads to variations in their reactivity and solvent properties.
























