
Chemistry Notes for Chapter 2 Electrochemistry Class 12 - FREE PDF Download
Electrochemistry is a vital section of chemistry that determines the function of electrodes and reactors. Vedantu’s Electrochemistry notes class 12 tries to situate the ideas behind the chemical reactions.
 Table of Content
Table of ContentClass 12 Electrochemistry Notes explain this function of electrons where two metallic electrodes are present. These metallic electrodes are immersed in an electrolytic solution for power generation. By thorough reading of Electrochemistry Class 12 Notes PDF Download, students will know that the ionic conductor is a vital part of cells.
Class 12 Chapter 2 Electrochemistry lets you quickly access and review the chapter content. For a comprehensive study experience, check out the Class 12 Chemistry Revision Notes FREE PDF here and refer to the CBSE Class 12 Chemistry syllabus for detailed coverage. Vedantu's notes offer a focused, student-friendly approach, setting them apart from other resources and providing you with the best tools for success.
Access Class 12 Chapter 2 Electrochemistry Notes
Electrochemistry
Electrochemistry is the study of generating electricity from the energy produced during a spontaneous chemical reaction, as well as the application of electrical energy to non-spontaneous chemical changes.
Electrochemical Cells
A spontaneous chemical reaction is one that can occur on its own, and in such a reaction, the system's Gibbs energy falls. This energy is then transformed into electrical energy. It is also feasible to force non-spontaneous processes to occur by providing external energy in the form of electrical energy. Electrochemical Cells are used to carry out these interconversions.
Types
Two types of electrochemical cells are present: Galvanic cells, which converts chemical energy into electrical energy and electrolytic cells which converts electrical energy into chemical energy.
Galvanic Cells
A spontaneous chemical process or reaction is used to extract cell energy, which is then transformed to electric current.
For example, a Daniell Cell is a Galvanic Cell in which the redox reaction is carried out using Zinc and Copper.
$Zn(s) + C{u^{2 + }}(aq) \to Z{n^{2 + }}(aq) + Cu(s)$
Oxidation Half: $Zn(s) \to Z{n^{2 + }}(aq) + 2{e^ - }$
Reduction Half: $C{u^{2 + }}(aq) + 2{e^ - } \to Cu(s)$
The reducing agent is $Zn$ , and the oxidising agent is $C{u^{2 + }}$ .
Electrodes are another name for half cells. The anode is the oxidation half, and Cathode is the reduction half. The cathode is a term used to describe a type of electrode. In the external circuit, electrons pass from the anode to the cathode. Negative polarity is assigned to the anode. Positive polarity is assigned to the cathode. Daniell Cell is a fictional character created by Daniell Cell. The anode is $Zn$ , while the cathode is $Cu$ .
Electrolytic Cell
These electrodes are submerged in an electrolytic solution that contains both cations and anions. When current is supplied, the ions migrate towards electrodes of opposite polarity, where they undergo simultaneous reduction and oxidation.
Preferential Discharge of Ions
When more than one cation or anion is present, the discharge process becomes competitive. Any ion that needs to be discharged requires energy, and if there are multiple ions present, the ion that requires the most energy will be discharged first.
Electrode Potential
It can be defined as an element's tendency to lose or gain electrons when in contact with its own ions, causing it to become positively or negatively charged. Depending on whether oxidation or reduction has occurred, the electrode potential will be referred to as oxidation or reduction potential.
$M(s)\underset{{{\text{Reduction}}}}{\overset{{{\text{Oxidation}}}}{\longleftrightarrow}}{M^{n + }}(aq) + n{e^ - }$
${M^{n + }}(aq) + n{e^ - }\underset{{{\text{Oxidation}}}}{\overset{{{\text{Reduction}}}}{\longleftrightarrow}}M(s)$
Characteristics
The magnitude and sign of the oxidation and reduction potentials are equal.
Because E is not a thermodynamic property, its values do not add up.
Standard Electrode Potential $({E^ \circ })$
It can be described as an electrode's electrode potential measured in comparison to a standard hydrogen electrode under standard conditions. The following are the standard conditions:
A 1M concentration of each ion in the solution.
A 298 K temperature.
Each gas has a pressure of one bar.
Electrochemical Series
The half-cell potential values are standard and are represented as standard reduction potential values in the table at the conclusion, commonly known as the Electrochemical Series.
Cell Potential or EMF of a Cell
Cell potential is the difference between the electrode potentials of two half-cells. If no current is pulled from the cell, it is known as electromotive force (EMF).
${E_{cell}} = {E_{cathode}} + {E_{anode}}$
For this equation, we take the oxidation potential of the anode and the reduction potential of the cathode.
Since the anode is put on the left and the cathode on the right, it follows therefore:
$ = {E_R} + {E_L}$
For a Daniel Cell, therefore:
$E_{cell}^ \circ = E_{C{u^{2 + }}/Cu}^ \circ - E_{Zn/Z{n^{2 + }}}^ \circ = 0.34 + (0.76) = 1.10\;V$
Cell Diagram or Representation of a Cell
In accordance with IUPAC recommendations, the following conventions or notations are used to write the cell diagram. The Daniel cell has the following representation:
$Zn(s)|Z{n^{2 + }}({C_1})||C{u^{2 + }}({C_2})|Cu(s)$
The anode half cell is written on the left, while the cathode half cell is written on the right.
The metal is separated from an aqueous solution of its own ions by a single vertical line.
Anodic Chamber: $Zn(s)|Z{n^{2 + }}(aq)$
Cathodic Chamber: $C{u^{2 + }}(aq)|Cu(s)$
A salt bridge is represented by a double vertical line.
After the formula of the corresponding ion, the molar concentration (C) is placed in brackets.
The cell's e.m.f. value is written on the cell's extreme right side. As an example:
$Zn(s)|Z{n^{2 + }}(1M)||C{u^{2 + }}(1M)|Cu$ , EMF = +1.1 V
If an inert electrode, such as platinum, is used in the cell's construction, it may be written in brackets alongside the working electrode, as when a zinc anode is coupled to a hydrogen electrode.
$Zn(s)|Z{n^{2 + }}({C_1})||{H^ + }({C_2})|{H_2}(Pt)(s)$
Salt Bridge
The salt bridge maintains charge balance and completes the circuit by allowing ions to flow freely through it. It contains a gel containing an inert electrolyte such as $N{a_2}S{O_4}$ or $KN{O_3}$ . Through the salt bridge, negative ions travel to the anode and positive ions flow to the cathode, maintaining charge balance and allowing the cell to function.
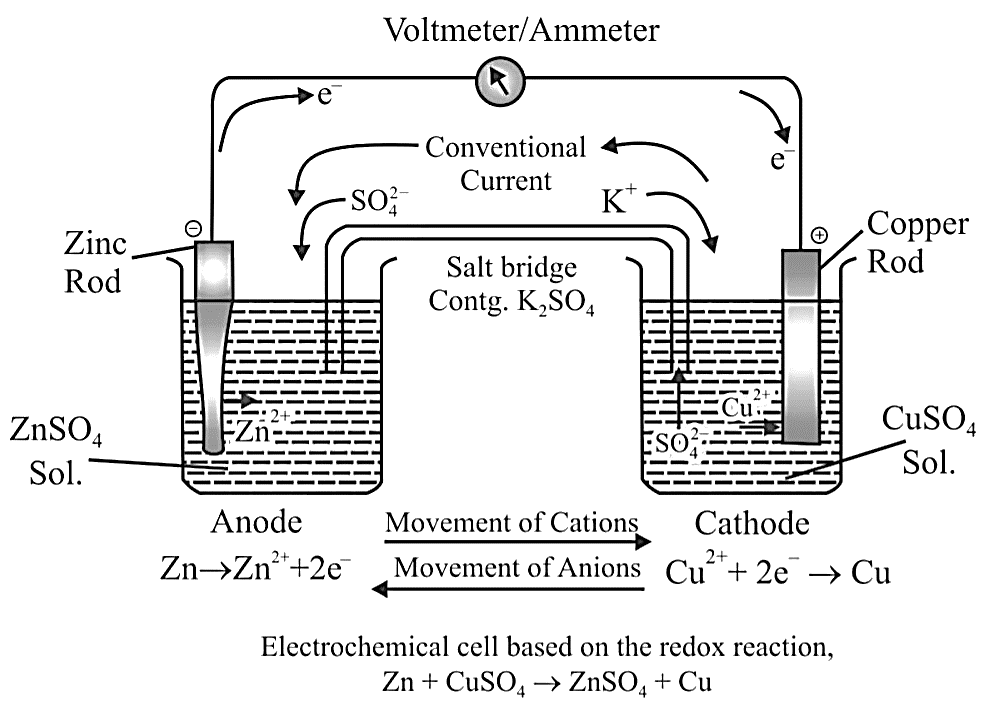
Spontaneity of a Reaction
$\Delta G = - nF{E_{cell}}$
$\Delta G$ should be negative and cell potential should be positive for a spontaneous cell reaction.
In the following equation, if we take the standard value of cell potential, we will also get the standard value of $\Delta G$ .
$\Delta {G^ \circ } = - nFE_{CELL}^ \circ $
Types of Electrodes
Metal – Metal Ion Electrodes
An electrolyte solution containing metal ions is dipped into a metal rod/plate. Because of the potential difference between these two phases, this electrode can function as both a cathode and an anode.
Anode: $M \to {M^{n + }} + n{e^ - }$
Cathode: ${M^{n + }} + n{e^ - } \to M$
Gas Electrodes
Electrode gases such as ${H_2}$ and $C{l_2}$ are used in conjunction with their respective ions. ${H_2}$ gas, for example, is utilised in conjunction with a dilute solution of $HCl$ (${H^ + }$ ions). To avoid reacting with the acid, the metal should be inert.
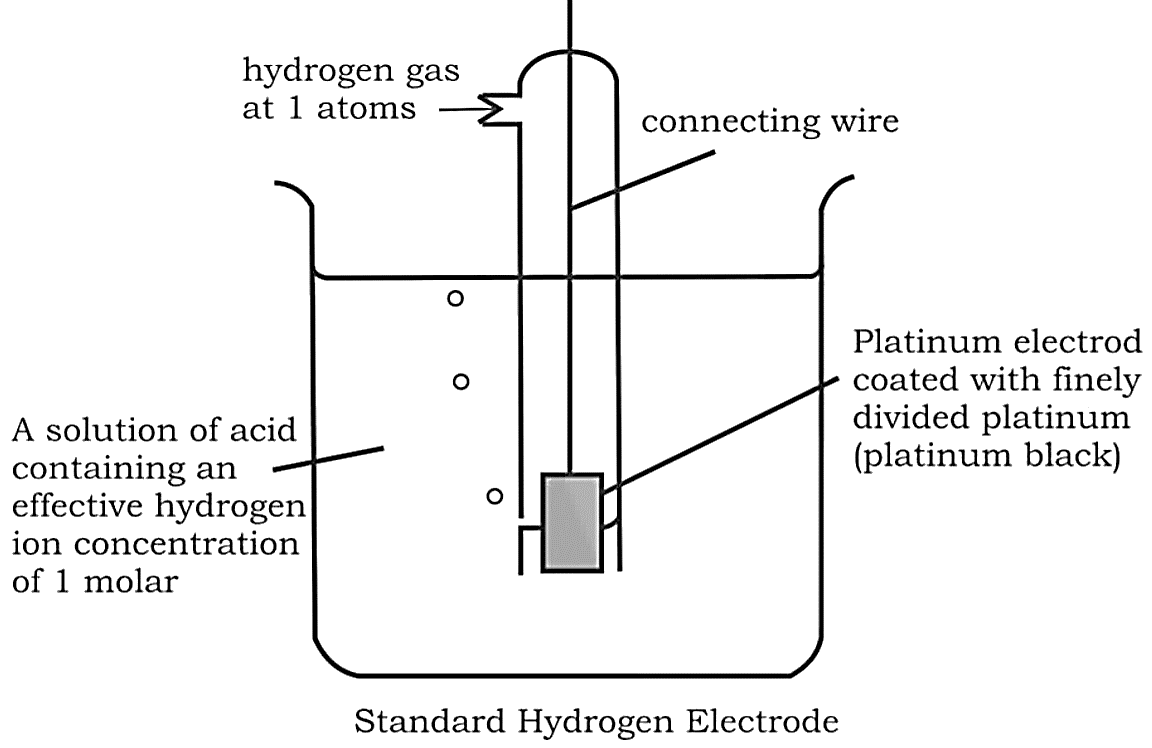
Anode: ${H_2} \to 2{H^ + } + 2{e^ - }$
Cathode: $2{H^ + } + 2{e^ - } \to {H_2}$
The hydrogen electrode is also used as a standard for measuring the potentials of other electrodes. As a reference, its own potential is set at $0\;V$ . The concentration of the HCl used as a reference is 1 M, and the electrode is known as the "Standard Hydrogen Electrode (SHE)".
Metal – Insoluble Salt Electrode
As electrodes, we use salts of several metals that are only sparingly soluble with the metal itself. When we employ $AgCl$ with $Ag$ , for example, there is a potential gap between these two phases, as seen in the following reaction:
$AgCl(s) + {e^ - } \to Ag(s) + C{l^ - }$
This electrode is made by dipping a silver rod in a solution containing $AgCl(s)$ and $C{l^ - }$ ions.
Calomel Electrode
Mercury is combined with two other phases: calomel paste $(H{g_2}C{l_2})$ and a $C{l^ - }$ ions containing electrolyte.
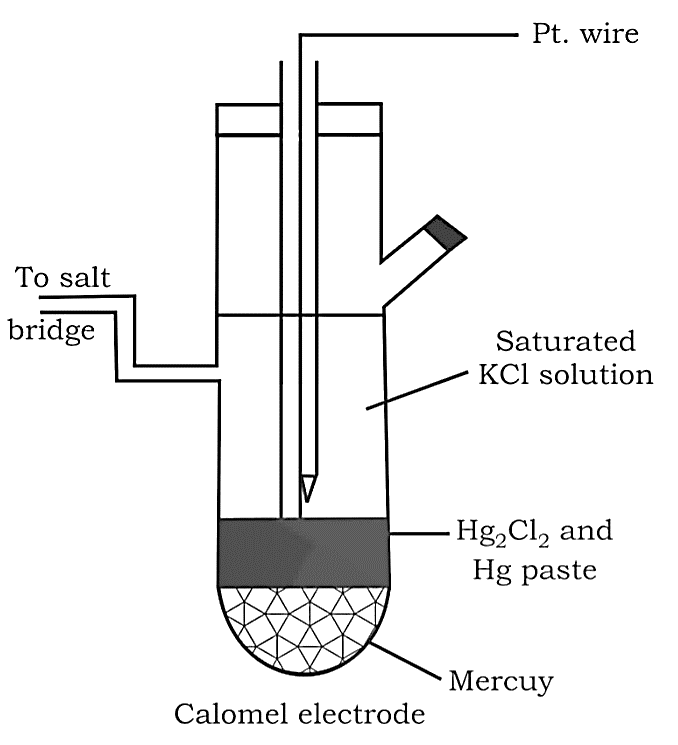
Cathode: $H{g_2}C{l_2}(s) + 2{e^ - } \to 2Hg(l) + 2C{l^ - }(aq)$
Anode: $2Hg(l) + 2C{l^ - }(aq) \to H{g_2}C{l_2}(s) + 2{e^ - }$
This electrode is also utilised as a reference point for determining other potentials. It's also known as Standard Calomel Electrode in its standard form (SCE).
Redox Electrode
Two distinct oxidation states of the same metal are used in the same half cell in these electrodes. For example, $F{e^{2 + }}$ and $F{e^{3 + }}$ are dissolved in the same container and the electron transfer is performed using a platinum inert electrode.
The following reactions may occur:
Anode: $F{e^{2 + }} \to F{e^{3 + }} + {e^ - }$
Cathode: $F{e^{3 + }} + {e^ - } \to F{e^{2 + }}$
Nernst Equation
It establishes a link between electrode voltage and ion concentration. When a result, as the concentration of ions rises, so does the reduction potential. For a type of generic electrochemical reaction.
$aA + bB\xrightarrow{{n{e^ - }}}cC + dD$
Nernst equation can be given as:
${E_{{\text{cell}}}} = E_{{\text{call}}}^0 - \dfrac{{RT}}{{nF}}\ln \dfrac{{{{[C]}^c}{{[D]}^d}}}{{{{[A]}^a}{{[B]}^b}}}$
${E_{c \in l}} = E_{cdl}^ \circ - \dfrac{{2303}}{{nF}}RT\log \dfrac{{{{[C]}^c}{{[D]}^d}}}{{{{[A]}^a}{{[B]}^b}}}$
Substituting the values of R and F we get:
${E_{{\text{cell}}}} = E_{ccll}^0 - \dfrac{{0.0591}}{n}\log \dfrac{{{{[C]}^c}{{[D]}^d}}}{{{{[A]}^a}{{[B]}^b}}}$
Applications of Nernst Equation
Equilibrium Constant from Nernst Equation
For a Daniel Cell, at equilibrium
${{E_{{\text{cell}}}} = 0 = E_{{\text{cell}}}^0 - \dfrac{{2.303{\text{RT}}}}{{2{\text{F}}}}\log \dfrac{{\left[ {{\text{Z}}{{\text{n}}^{2 + }}} \right]}}{{\left[ {{\text{C}}{{\text{u}}^{2 + }}} \right]}}}$
${{\text{E}}_{{\text{cdl}}}^{\text{o}} = \dfrac{{2.303{\text{RT}}}}{{2{\text{F}}}}\log \dfrac{{\left[ {{\text{Z}}{{\text{n}}^{2 + }}} \right]}}{{\left[ {{\text{C}}{{\text{u}}^{2 + }}} \right]}}}$
But at equilibrium:
$\dfrac{{\left[ {Z{n^{2 + }}} \right]}}{{\left[ {C{u^{2 + }}} \right]}} = {K_c}$
${{\text{E}}_{cell}^{\text{a}} = \dfrac{{2.303{\text{RT}}}}{{2{\text{F}}}}\log {{\text{K}}_{\text{c}}}}$
${{\text{E}}_{cell}^{\text{o}} = \dfrac{{2.303 \times 8.314 \times 298}}{{2 \times 96500}}\log {{\text{K}}_{\text{c}}}}$
${ = \dfrac{{0.0591}}{2}\log {{\text{K}}_{\text{c}}}}$
In general:
${{\text{E}}_{{\text{cell}}}^ \circ = \dfrac{{0.0591}}{{\text{n}}}\log {{\text{K}}_{\text{c}}}}$
${\log {{\text{K}}_{\text{c}}} = \dfrac{{{\text{n}}E_{{\text{cell}}}^ \circ }}{{0.0591}}}$
Concentration Cells
Concentration cells are formed when two electrodes of the same metal are dipped individually into two solutions of the same electrolyte with varying concentrations and the solutions are connected by a salt bridge. As an example:
${H_2}|{H^ + }({C_1})||{H^ + }({C_2})|{H_2}$
$Cu|C{u^{ + 2}}({C_1})||C{u^{2 + }}({C_2})|Cu$
These Are of Two Types:
Electrode Concentration Cells
${H_2}({P_1})|{H^ + }(C)||{H^ + }(C)|{H_2}({P_2})$
${E_{{\text{cell}}}} = 0 - \dfrac{{0.059}}{n}\log \dfrac{{{P_2}}}{{{P_1}}}$
Where, ${P_2} < {P_1}$ for spontaneous reaction.
Electrolyte Concentration Cell
The EMF of concentration cell at 298 K is given by:
$Zn|Z{n^{2 + }}({C_1})||Z{n^{2 + }}({C_2})|Zn$
${{\text{E}}_{{\text{cell}}}} = \dfrac{{0.0591}}{{{{\text{n}}_1}}}\log \dfrac{{{{\text{c}}_2}}}{{{{\text{c}}_{\text{l}}}}}$
Where, ${C_2} > {C_1}$ for spontaneous reaction
Cases of Electrolysis
Electrolysis of Molten Sodium Chloride
$2NaCl(l) \rightleftharpoons 2N{a^ + }(l) + 2C{l^ - }(l)$
The reactions occurring at the two electrodes may be shown as follows:
At cathode: $2N{a^ + } + 2{e^ - } \to 2Na$ , ${E^ \circ } = - 2.71\;V$
At anode: $2C{l^ - } \to C{l_2} + 2{e^ - }$ , ${E^ \circ } = - 1.36\;V$
Overall reaction:
$2N{a^ + }(l) + 2C{l^ - }\xrightarrow{{electrolysis}}2Na(l) + C{l_2}(g)$ OR
$2NaCl(l)\xrightarrow{{electrolysis}}2Na(l) + C{l_2}(g)$
Electrolysis of an aqueous solution of Sodium Chloride
$NaCl(aq) \to N{a^ + }(aq) + C{l^ - }(aq)$
${H_2}O(l) \rightleftharpoons {H^ + }(aq) + O{H^ - }(aq)$
At cathode:
$2N{a^ + } + 2{e^ - } \to 2Na$ , ${E^ \circ } = - 2.71\;V$
$2{H_2}O + 2{e^ - } \to {H_2} + 2O{H^ - }$ , ${E^ \circ } = - 0.83\;V$
Thus ${H_2}$ gas is evolved at cathode value $N{a^ + }$ ions remain in solution.
At Anode:
$2{H_2}O \to {O_2} + 4{H^ + } + 4{e^ - }$ , ${E^ \circ } = - 1.23\;V$
$2C{l^ - } \to C{l_2} + 2{e^ - }$ , ${E^ \circ } = - 1.36\;V$
Thus, $C{l_2}$ gas is evolved at the anode by over voltage concept while $O{H^ - }$ ions remain in the solution.
Batteries
The term "battery" refers to a configuration in which Galvanic cells are connected in series to achieve a higher voltage.
Primary Batteries
Primary cells can be employed indefinitely as long as active components are present. When they're gone, the cell stops working and can't be used again. For instance, a Dry Cell or a Leclanche Cell, as well as a Mercury Cell.
Dry Cell
Anode: Zinc container
Cathode: Carbon (graphite) rod surrounded by powdered $Mn{O_2}$ and carbon
Electrolyte: $N{H_4}Cl$ and $ZnC{l_2}$
Reaction:
Anode: $Zn \to Z{n^{2 + }} + 2{e^ - }$
Cathode: $Mn{O_1} + NH_{_4}^ + + {e^ - } \to MnO(OH) + N{H_3}$
The standard potential of this cell is 1.5 V, which decreases as the battery is repeatedly discharged, and it cannot be refilled once used.
Mercury Cells
These are used in small equipments like watches, hearing aids.
Anode: $Zn - Hg$ Amalgam
Cathode: Paste of $HgO$ and carbon
Electrolyte: Paste of $KOH$ and $ZnO$
Anode: $Zn(Hg) + 2O{H^ - } \to ZnO(s) + {H_2}O + 2{e^ - }$
Cathode: $HgO(s) + {H_2}O + 2{e^ - } \to Hg(l) + 2O{H^ - }$
Overall Reaction: $Zn(Hg) + HgO(s) \to ZnO(s) + Hg(l)$
The cell potential is approximately 1.35 V and remains constant during its life.
Secondary Batteries
Secondary batteries are rechargeable for many applications and can be recharged multiple times. Lead storage batteries and $Ni - Cd$ batteries, for example.
Lead Storage Battery
Anode: Lead $(Pb)$
Cathode: Grid of lead packed with lead oxide $(Pb{O_2})$
Electrolyte: 38% solution of ${H_2}S{O_4}$
Discharging Reaction
Anode: $Pb(s) + SO_4^{2 - }(aq) \to PbS{O_4}(s) + 2{e^ - }$
Cathode: $Pb{O_2}(s) + 4{H^ + }(aq) + SO_4^{2 - }(aq) + 2{e^ - } \to PbS{O_4}(s) + 2{H_2}O(l)$
Overall Reaction: $Pb(s) + Pb{O_2}(s) + 2{H_2}S{O_4}(aq) \to 2PbS{O_4}(s) + 2{H_2}O(l)$
To recharge the cell, it is connected to a higher-potential cell, which acts as an electrolytic cell and reverses the processes. At the relevant electrodes, $Pb(s)$ and $Pb{O_2}(s)$ are regenerated. These cells produce a voltage that is nearly constant.
Recharging Reaction: $2PbS{O_4}(s) + 2{H_2}O(l) \to Pb(s) + Pb{O_2}(s) + 2{H_2}S{O_4}(aq)$
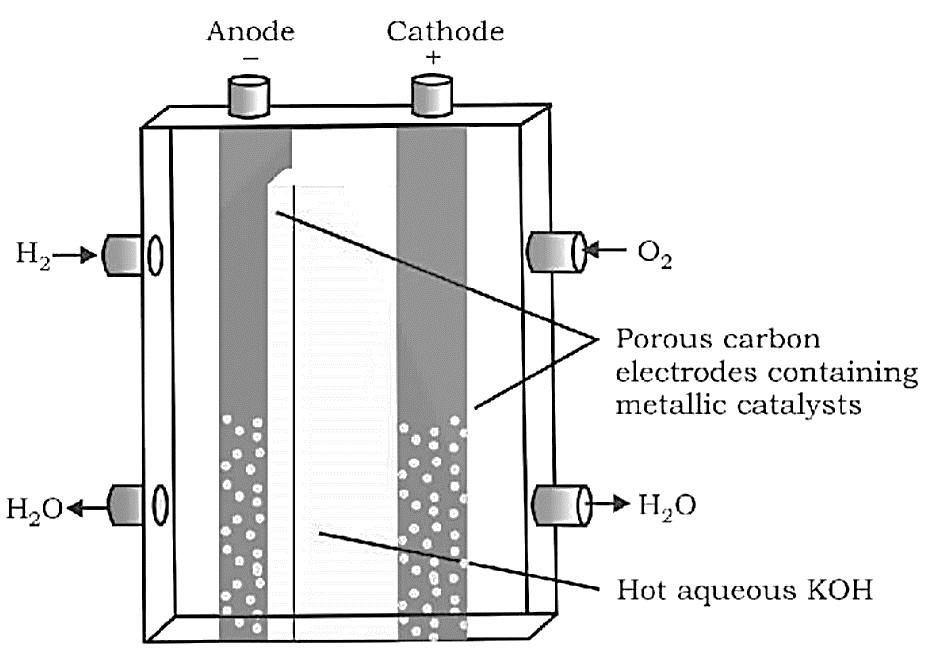
Fuel Cells
A fuel cell varies from a traditional battery in that the reactants are supplied externally from a reservoir rather than being stored inside the cell. In space vehicles, fuel cells are employed, and the two gases are supplied from external storage. The electrodes in this cell are carbon rods, and the electrolyte is $KOH$ .
Cathode: ${O_2}(g) + 2{H_2}O(l) + 4{e^ - } \to 4O{H^ - }(aq)$
Anode: $2{H_2}(g) + 4O{H^ - }(aq) \to 4{H_2}O(l) + 4{e^ - }$
Overall Reaction: $2{H_2}(g) + {O_2}(g) \to 2{H_2}O(l)$
Corrosion
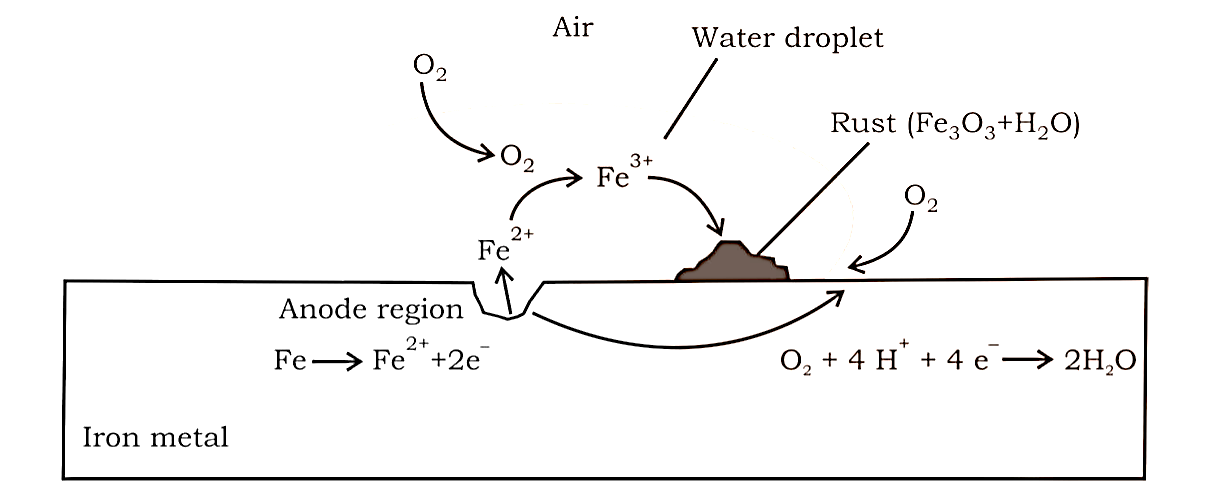
On the surface of iron or any other metal, it entails a redox process and the development of an electrochemical cell.
The oxidation of iron (anode) occurs at one point, while the reduction of oxygen to generate water occurs at another (cathode). $Fe$ is first oxidised to $F{e^{2 + }}$ , which is then converted to $F{e^{3 + }}$ in the presence of oxygen, which subsequently combines with water to generate rust, which is represented by $F{e_2}{O_3}.x{H_2}O$ .
Anode: $2Fe(s) \to 2F{e^{2 + }} + 4{e^ - }$ , ${E^ \circ } = + 0.44\;V$
Cathode: ${O_2}(g) + 4{H^ + } + 4{e^ - } \to 2{H_2}O(l)$ , ${E^ \circ } = 1.23\;V$
Overall Reaction: $2Fe(s) + {O_2}(g) + 4{H^ + } \to 2F{e^{2 + }} + 2{H_2}O$ , $E_{Cell}^ \circ = 1.67\;M$
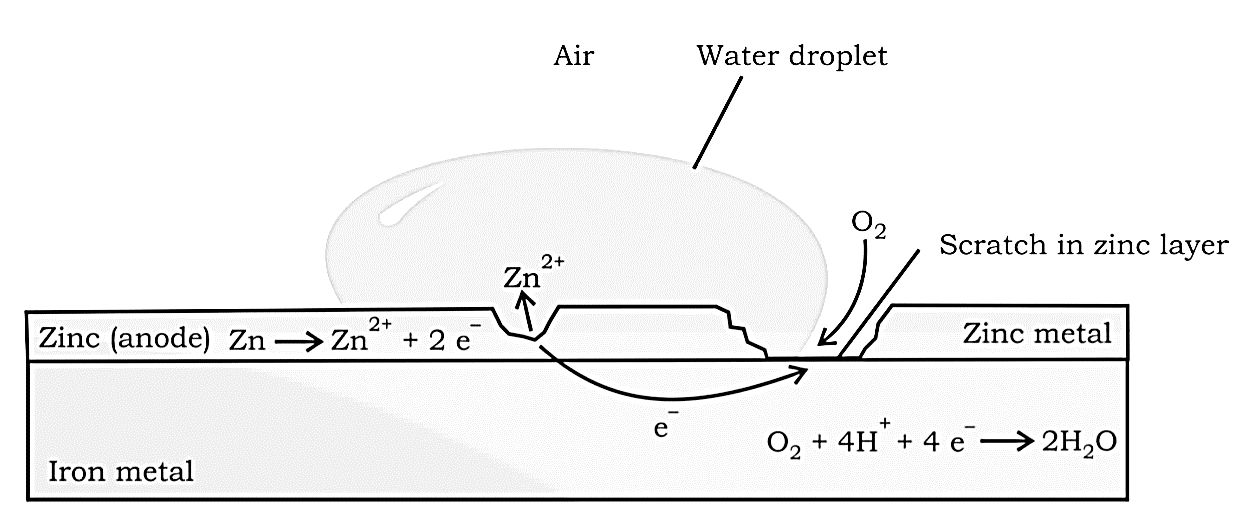
Painting or coating iron with other metals, such as zinc, helps prevent it from rusting. Galvanisation is the name for the latter procedure. Because $Zn$ has a higher potential to oxidise than iron, it is oxidised first, while iron is protected. Cathodic Protection is another name for this approach of shielding one metal by the other.
Conductance (G)
It is defined as the ease with which electric current passes through a conductor and is the reciprocal of resistance.
$G = \dfrac{1}{R}$
SI unit is Siemen (S).
$1\;S = 1\;oh{m^{ - 1}}(mho)$
Conductivity
It is the reciprocal of resistivity $(\rho )$ .
$\kappa = \dfrac{1}{\rho } = \dfrac{1}{R} \times \dfrac{\ell }{A} = G \times \dfrac{\ell }{A}$
Now is $l = 1\;cm$ and $A = 1\;c{m^2}$ , then $\kappa = G$
As a result, the conductivity of an electrolytic solution can be defined as the conductance of a $1\;cm$ long solution with a $1\;c{m^2}$ cross-sectional area.
Factors Affecting Electrolyte Conductance
Electrolyte
In a dissolved or molten form, an electrolyte is a substance that dissociates in solution to produce ions and hence conducts electricity.
Examples: $HCl,\;NaOH,\;KCl$ are strong electrolytes and $C{H_3}COOH,\;N{H_4}OH$ are weak electrolytes.
Electrolytic or ionic conductance refers to the conductance of electricity by ions present in solutions. The flow of electricity through an electrolyte solution is governed by the following factors.
Electrolyte Nature or Interionic Attractions: The lower the solute-solute interactions, the larger the freedom of ion mobility and the higher the conductance.
Ion Solvation: As the amount of solute-solvent interactions increases, the extent of solvation increases, and the electrical conductance decreases.
The Nature of the Solvent and its Viscosity: The larger the solvent-solvent interactions, the higher the viscosity, and the greater the solvent's resistance to ion flow, and thus the lower the electrical conductance.
Temperature: As the temperature of an electrolytic solution rises, solute-solute, solute-solvent, and solvent-solvent interactions diminish, causing electrolytic conductance to rise.
Measurement of Conductance
As we know, $\kappa = \dfrac{1}{{\text{R}}} \times \dfrac{\ell }{{\text{A}}}$
If we measure $l$ , $A$ , and $R$ , we can figure out what the value of $\kappa $ is. Using the ‘Wheatstones' bridge method, the resistance of the solution $R$ between two parallel electrodes is calculated.
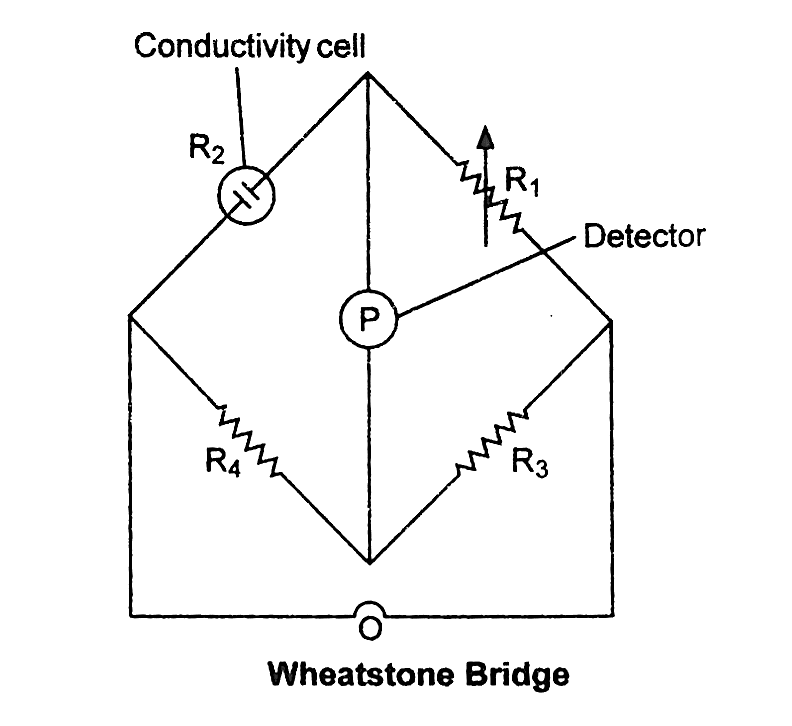
It is made up of two fixed resistances, R3 and R4, a variable resistance R1, and a conductivity cell with an unknown resistance, R2. When no current goes through the detector, the bridge is balanced. Then, in these circumstances:
$\dfrac{{{R_1}}}{{{R_2}}} = \dfrac{{{R_3}}}{{{R_4}}}$ or ${R_2} = \dfrac{{{R_1}{R_4}}}{{{R_3}}}$
Molar Conductivity
It's the total conducting power of all the ions created by dissolving one mole of an electrolyte between two big electrodes separated by one centimetre.
Mathematically,
\[\Lambda_{m} = \kappa \times V, \Lambda_{m} = \frac{\kappa \times V}{C}\]
where, V is the volume of solution in $c{m^3}$ containing 1 mole of electrolyte and C is the molar concentration.
Units: \[\Lambda_{m} = \frac{\kappa \times V}{C} = \frac{\text{S }cm^{-1}}{\text{mol } cm^{-1}}\]
${ = {\text{oh}}{{\text{m}}^{ - 1}}{\text{c}}{{\text{m}}^2}{\text{mo}}{{\text{l}}^{ - 1}}{\text{orSc}}{{\text{m}}^2}{\text{mo}}{{\text{l}}^{ - 1}}}$
Equivalent Conductivity
It is the electrical conductivity of one equivalent electrolyte placed between two big electrodes separated by one centimetre.
Mathematically:
${{{{\Lambda }}_{{\text{eq}}}} = \kappa \times {\text{v}} = }$
${{{{\Lambda }}_{{\text{eq}}}} = \dfrac{{\kappa \times 1000}}{{\text{N}}}}$
Where, v is the volume of solution in $c{m^3}$ containing 1 equivalent of electrolyte and N is normality.
Units:
${{{{\Lambda }}_{{\text{eq}}}} = \dfrac{{\kappa \times 1000}}{{\text{N}}}}$
${ = \dfrac{{{\text{Sc}}{{\text{m}}^{ - 1}}}}{{{{\;equivalent\;c}}{{\text{m}}^{ - 3}}}} = \dfrac{{{\text{Oh}}{{\text{m}}^{ - 1}}{\text{c}}{{\text{m}}^2}{{\;equivalent}}{{{\;}}^{ - 1}}}}{{{\text{Sc}}{{\text{m}}^2}{{\;equivalent}}{{{\;}}^{ - 1}}}}}$
Variation of Conductivity and Molar Conductivity with Dilution
Because the number of ions per unit volume that carry the current in the solution reduces as concentration lowers, conductivity drops. With a decrease in concentration, molar conductivity rises. This is due to an increase in the total volume V of a solution containing one mole of electrolyte. The drop in $\kappa $ as a result of dilution of a solution has been found to be more than compensated by increases in its volume.
Graphical representation of the variation of ${\Lambda _m}$ vs $\sqrt c $ .
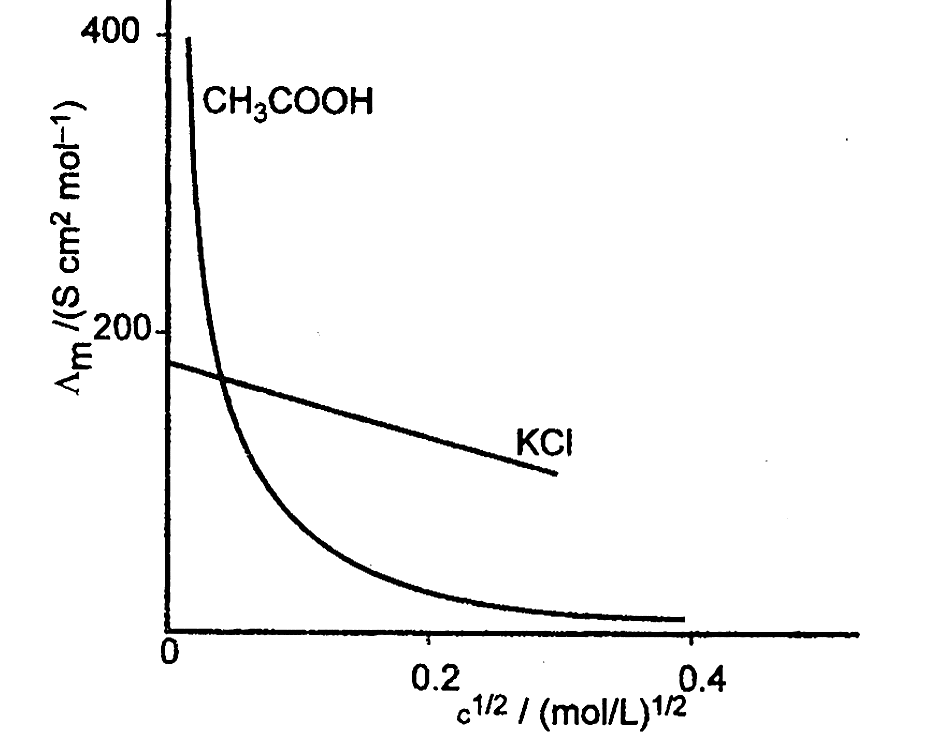
Limiting Molar Conductivity $({\Lambda _m})$
Limiting molar conductivity, also known as molar conductivity at infinite dilution, is the value of molar conductivity as the concentration approaches zero. In the case of a strong electrolyte, extrapolation of the ${\Lambda _m}$ vs $\sqrt c $ curve can be used to derive the molar conductivity at infinite dilution. Extrapolation of the curve, on the other hand, cannot be used to calculate the value of molar conductivity of a weak electrolyte at infinite dilution since the curve becomes practically parallel to the y-axis as concentration approaches zero.
The mathematical relationship between ${\Lambda _m}$ and $\Lambda _m^ \circ $ for a strong electrolyte was developed by Debye, Huckel and Onsagar.
In simplified form the equation can be given as:
${{t{\Lambda }}_{\text{m}}} = {{\Lambda }}_{\text{m}}^\infty - {\text{b}}{{\text{c}}^{1/2}}$
Kohlrausch’s Law
It asserts that an electrolyte limiting molar conductivity can be described as the total of the individual contributions of the electrolyte's anion and cation.
In general, if an electrolyte produces ${v_ + }$ cations and ${v_ - }$ anions upon dissociation, its limiting molar conductivity is given by:
${{\Lambda }}_{\text{m}}^\infty = {{\text{v}}_ + }\lambda _ + ^ \circ + {{\text{v}}_ - }\lambda _ - ^ \circ $
Applications of Kohlrausch’s Law
Calculation of molar conductivities of weak electrolyte at infinite dilution
For example, the molar conductivity of acetic acid at infinite dilution can be calculated using the molar conductivities of strong electrolytes like $HCl$ , $C{H_3}COONa$ , and $NaCl$ at infinite dilution, as shown below.
${{\Lambda }}_{{\text{m}}\left( {{\text{C}}{{\text{H}}_3} - {\text{COOH}}} \right)}^{\text{o}} = {{\Lambda }}_{{\text{m}}\left( {{\text{C}}{{\text{H}}_3} - {\text{cooNa}}} \right)}^{\text{o}} + {{\Lambda }}_{{\text{m}}({\text{HCl}})}^{\text{o}} - {{\Lambda }}_{{\text{m}}({\text{NaCl}})}^ \circ $
Determination of Degree of Dissociation of Weak Electrolytes
Degree of dissociation $\alpha = \dfrac{{\Lambda _m^c}}{{\Lambda _m^ \circ }}$
Determination of Dissociation Constant of Weak Electrolytes:
${{\text{K}} = \dfrac{{{\text{c}}{\alpha ^2}}}{{1 - \alpha }}}$
${\alpha = \dfrac{{{{\Lambda }}_{\text{m}}^{\text{c}}}}{{{{\Lambda }}_{\text{m}}^\infty }}}$
${{\text{K}} = \dfrac{{{\text{c}}{{\left( {{{\Lambda }}_{\text{m}}^{\text{c}}/{{\Lambda }}_{\text{m}}^\infty } \right)}^2}}}{{1 - {{\Lambda }}_{\text{m}}^c/{{\Lambda }}_{\text{m}}^\infty }} = \dfrac{{{\text{C}}{{\left( {{{\Lambda }}_{\text{m}}^{\text{c}}} \right)}^2}}}{{{{\Lambda }}_{\text{m}}^\infty \left( {{{\Lambda }}_{\text{m}}^ * - {{\Lambda }}_{\text{m}}^{\text{c}}} \right)}}}$
Use of $\Delta G$ in Relating EMF values of Half Cell Reactions
When we have two half-cell reactions that produce another half-cell reaction when we combine them, their emfs cannot be mixed directly. However, thermodynamic functions such as $\Delta G$ can be added and EMF values can be connected through them in any scenario. Take a look at the three half-cell responses below:
$F{e^{2 + }} + 2{e^ - } \to Fe$ , ${E_1}$
$F{e^{3 + }} + 3{e^ - } \to Fe$ , ${E_2}$
$F{e^{3 + }} + {e^ - } \to F{e^{2 + }}$ , ${E_3}$
We can clearly see that subtracting the first reaction from the second yields the third reaction. However, the same relationship does not hold true for EMF values.
That is: ${E_3} \ne {E_2} - {E_1}$ . But the $\Delta G$ values can be related according to the reactions:
${{{\Delta }}{{\text{G}}_3} = {\text{\Delta }}{{\text{G}}_2} - {{\Delta }}{{\text{G}}_1}}$
${ - {{\text{n}}_3}{\text{F}}{{\text{E}}_3} = - {{\text{n}}_2}{\text{F}}{{\text{E}}_2} + {{\text{n}}_1}{\text{F}}{{\text{E}}_1}}$
${ - {{\text{E}}_3} = - 3{{\text{E}}_2} + 2{{\text{E}}_1}}$
${ \Rightarrow {{\mathbf{E}}_3} = 3{{\mathbf{E}}_2} - {\mathbf{2}}{{\mathbf{E}}_1}}$
Formula:
${\text{R}} = \rho \left( {\dfrac{\ell }{{\text{A}}}} \right) = \rho \times {\text{Cell constant}}$
Where, $R$ = Resistance,
$A$ = Area of cross-section of the electrodes
$\rho $ = Resistivity
$\kappa = \dfrac{1}{{\text{R}}} \times {\text{\;cell constant\;}}$
Where, $\kappa $ = Conductivity or specific conductance
${{{\Lambda }}_{\text{m}}} = \dfrac{{\kappa \times 1000}}{{\text{M}}}$
Where, ${\Lambda _m}$ = Molar conductivity
$M$ = Molarity of the solution.
${{\Lambda }}_m^\infty \left( {{A_x}{B_y}} \right) = x{{\Lambda }}_m^\infty \left( {{A^y}} \right) + y{\text{\Lambda }}_m^\infty \left( {{B^{x - }}} \right)$
$\alpha = \dfrac{{\Lambda _m^c}}{{\Lambda _m^ \circ }}$
Where, $\alpha $ = Degree of dissociation
$\Lambda _m^c$ = Molar conductivity at a given concentration
For a weak binary electrolyte AB
${\text{K}} = \dfrac{{{\text{c}}{\alpha ^2}}}{{1 - \alpha }} = \dfrac{{{\text{c}}{{\left( {{{\Lambda }}_{\text{m}}^{\text{c}}} \right)}^2}}}{{{{\Lambda }}_{\text{m}}^\infty \left( {{{\Lambda }}_{\text{m}}^\infty - {{\Lambda }}_{\text{m}}^{\text{c}}} \right)}}$
Where, K is the Dissociation constant
${{\text{E}}_{{\text{edl}}}^ \circ = {\text{E}}_{{\text{cathode}}}^ \circ + {\text{E}}_{{\text{anode}}}^ \circ }$
${ = {{\text{E}}^ \circ }{\text{Right}} + {{\text{E}}^{\text{o}}}{\text{left}}}$
Nernst equation for a generation electrochemical reation
${E_{{\text{ofll}}}} = E_{{\text{cell}}}^ \circ - \dfrac{{0.059}}{n}\log \dfrac{{{{[A]}^2}{{[B]}^b}}}{{{{[C]}^c}{{[D]}^d}}}$
$\log {{\text{K}}_{\text{c}}} = \dfrac{{\text{n}}}{{0.0591}}{\text{E}}_{{\text{cell}}}^ \circ $
Where, ${K_c}$ = Equilibrium constant.
${{{\Delta }}_r}{{\text{G}}^ \circ } = - {\text{nFE}}_{{\text{cell}}}^ \circ $
${{{\Delta }}_{\text{r}}}{{\text{G}}^ \circ } = - 2.303{\text{RT}}\log {{\text{K}}_{\text{c}}}$
${\Delta _r}{G^ \circ }$ = Standard Gibbs energy of a reaction
$Q = I \times t$
Where, $Q$ = Quantity of charge in coulombs
$I$ = Current in amperes
$t$ = Time in seconds
$m = Z \times I \times t$
Where, $m$ = mass of the substance liberated at the electrodes
$Z$ = Electrochemical equivalent
Standard Reduction Potential At 298 K. In Electrochemical Order
$\mathrm{H}_{4} \mathrm{XeO}_{6}+2 \mathrm{H}^{+}+2 \mathrm{e}^{-} \rightarrow \mathrm{XeO}_{3}+3 \mathrm{H}_{2} \mathrm{O} \quad\quad\quad\quad+3.0$
$\mathrm{~F}_{2}+2 \mathrm{e}^{-} \rightarrow 2 \mathrm{~F}^{-} \quad\quad\quad\quad+2.87$
$\mathrm{O}_{3}+2 \mathrm{H}^{+}+2 \mathrm{e}^{-} \rightarrow \mathrm{O}_{2}+\mathrm{H}_{2} \mathrm{O} \quad\quad\quad\quad +2.07$
$\mathrm{~S}_{2} \mathrm{O}_{8}^{2-}+2 \mathrm{e}^{-} \rightarrow 2 \mathrm{SO}_{4}^{2-} \quad\quad\quad\quad +2.05$
$\mathrm{Ag}^{2+}+\mathrm{c}^{-} \rightarrow \mathrm{Ag}^{+} \quad\quad\quad\quad +1.98$
$\mathrm{Co}^{3+}+\mathrm{e}^{-} \rightarrow \mathrm{Co}^{2+} \quad\quad\quad\quad +1.81$
$\mathrm{H}_{2} \mathrm{O}_{2}+2 \mathrm{H}^{+}+2 \mathrm{e}^{-} \rightarrow 2 \mathrm{H}_{2} \mathrm{O} \quad\quad\quad\quad +1.78$
$\mathrm{Au}^{+}+\mathrm{e}^{-} \rightarrow \mathrm{Au} \quad\quad\quad\quad +1.69$
$\mathrm{~Pb}^{4+}+2 \mathrm{e}^{-} \rightarrow \mathrm{Pb}^{2+} \quad\quad\quad\quad +1.67$
$2 \mathrm{HClO}+2 \mathrm{H}^{+}+2 \mathrm{e}^{-} \rightarrow \mathrm{Cl}_{2}+2 \mathrm{H}_{2} \mathrm{O} \quad\quad\quad\quad +1.63$
$\mathrm{Ce}^{4+}+\mathrm{e}^{-} \rightarrow \mathrm{Ce}^{3+} \quad\quad\quad\quad +1.61$
$2 \mathrm{H} \mathrm{IBrO}+2 \mathrm{H}^{+}+2 \mathrm{e}^{-} \rightarrow \mathrm{Br}_{2}+2 \mathrm{H}_{2} \mathrm{O} \quad\quad\quad\quad +1.60$
$\mathrm{MnO}_{4}^{-}+8 \mathrm{H}^{+}+5 \mathrm{e}^{-} \rightarrow \mathrm{Mn}^{2+}+4 \mathrm{H}_{2} \mathrm{O} \quad\quad\quad\quad +1.51$
$\mathrm{Mn}^{3+}+\mathrm{e}^{-} \rightarrow \mathrm{Mn}^{2+} \quad\quad\quad\quad +1.51$
$\mathrm{Au}^{3+}+3 \mathrm{e}^{-} \rightarrow \mathrm{Au} \quad\quad\quad\quad +1.40$
$\mathrm{Cl}_{2}+2 \mathrm{e}^{-} \rightarrow 2 \mathrm{Cl}^{-} \quad\quad\quad\quad +1.36$
$\mathrm{Cr}_{2} \mathrm{O}_{7}^{2-}+14 \mathrm{H}^{+}+6 \mathrm{e}^{-} \rightarrow 2 \mathrm{Cr}^{3+}+7 \mathrm{H}_{2} \mathrm{O} \quad\quad\quad\quad +1.33$
$\mathrm{O}_{3}+\mathrm{H}_{2} \mathrm{O}+2 \mathrm{e}^{-} \rightarrow \mathrm{O}_{2}+2 \mathrm{OH}^{-} \quad\quad\quad\quad +1.24$
$\mathrm{O}_{2}+4 \mathrm{H}^{+} 4 \mathrm{e}^{-} \rightarrow 2 \mathrm{H}_{2} \mathrm{O} \quad\quad\quad\quad +1.23$
$\mathrm{Hg}_{2} \mathrm{SO}_{4}+2 \mathrm{e}^{-} \rightarrow 2 \mathrm{Hg}+\mathrm{SO}_{4}^{2-} \quad \quad \quad \quad +0.62$
$\mathrm{MnO}_{4}^{2-}+2 \mathrm{H}_{2} \mathrm{O}+2 \mathrm{e}^{-} \rightarrow \mathrm{MnO}_{2}+4 \mathrm{OH}^{-} \quad \quad \quad \quad +0.60$
$\mathrm{MnO}_{4}^{-}+\mathrm{e}^{-} \rightarrow \mathrm{MnO}_{4}^{2-} \quad \quad \quad \quad +0.56$
$\mathrm{I}_{2}+2 \mathrm{e}^{-} \rightarrow 2 \mathrm{I}^{-} \quad \quad \quad \quad +0.54$
$\mathrm{Cu}^{+}+\mathrm{e}^{-} \rightarrow \mathrm{Cu} \quad \quad \quad \quad +0.52$
$\mathrm{I}_{3}^{-}+2 \mathrm{e}^{-} \rightarrow 3 \mathrm{I}^{-} \quad \quad \quad \quad +0.53$
$\mathrm{NiOOH}+\mathrm{H}_{2} \mathrm{O}+\mathrm{e}^{-} \rightarrow \mathrm{Ni}(\mathrm{OH})_{2}+\mathrm{OH}^{-} \quad \quad \quad \quad +0.49$
$\mathrm{Ng}_{2} \mathrm{CrO}_{4}+2 \mathrm{e}^{-} \rightarrow 2 \mathrm{Ag}+\mathrm{CrO}_{4}^{2-} \quad \quad \quad \quad +0.45$
$\mathrm{O}_{2}+2 \mathrm{H}_{2} \mathrm{O}+4 \mathrm{e}^{-} \rightarrow 4 \mathrm{OH}^{-} \quad \quad \quad \quad +0.40$
$\mathrm{ClO}_{4}^{-}+\mathrm{H}_{2} \mathrm{O}+2 \mathrm{e}^{-} \rightarrow \mathrm{ClO}_{3}^{-}+2 \mathrm{OH}^{-} \quad \quad \quad \quad +0.36$
${\left[\mathrm{Fe}(\mathrm{CN})_{6}^{3-}+\mathrm{e}^{-} \rightarrow\left[\mathrm{Fe}(\mathrm{CN})_{6}\right]^{+}\right.} \quad \quad \quad \quad +0.36$
$\mathrm{Cu}^{2+}+2 \mathrm{e}^{-} \rightarrow \mathrm{Cu} \quad \quad \quad \quad +0.34$
$\mathrm{Hg}_{2} \mathrm{Cl}_{2}+2 \mathrm{e}^{-} \rightarrow 2 \mathrm{Hg}+2 \mathrm{Cl}^{-} \quad \quad \quad \quad +0.27$
$\mathrm{AgCl}+\mathrm{e}^{-} \rightarrow \mathrm{Ag}+\mathrm{Cl}^{-} \quad \quad \quad \quad +0.22$
$\mathrm{Bi}+3 \mathrm{e}^{-} \rightarrow \mathrm{Bi} \quad \quad \quad \quad +0.20$
$\mathrm{Cu}^{2+}+\mathrm{e}^{-} \rightarrow \mathrm{Cu}^{+} \quad \quad \quad \quad +0.16$
$\mathrm{Sn}^{4+}+2 \mathrm{e}^{-} \rightarrow \mathrm{Sn}^{2+} \quad \quad \quad \quad +0.15$
$\mathrm{AgBr}+\mathrm{e}^{-} \rightarrow \mathrm{Ag}+\mathrm{Br}^{-} \quad \quad \quad \quad +0.07$
$\mathrm{ClO}_{4}^{-}+2 \mathrm{H}^{+}+2 \mathrm{e}^{-} \rightarrow \mathrm{ClO}_{3}^{-}+\mathrm{H}_{2} \mathrm{O} \quad \quad \quad \quad +1.23$
$\mathrm{MNO}_{2}+4 \mathrm{H}^{+}+2 \mathrm{e}^{-} \rightarrow \mathrm{Mn}^{2+}+2 \mathrm{H}_{2} \mathrm{O} \quad \quad \quad \quad +1.23$
$\mathrm{Br}_{2}+2 \mathrm{e}^{-} \rightarrow 2 \mathrm{Br}^{-} \quad \quad \quad \quad +1.09$
$\mathrm{Pu}^{4+}+\mathrm{e}^{-} \rightarrow \mathrm{Pu}^{3+} \quad \quad \quad \quad +0.97$
$\mathrm{NO}_{3}^{-}+4 \mathrm{H}^{+}+3 \mathrm{e}^{-} \rightarrow \mathrm{NO}+2 \mathrm{H}_{2} \mathrm{O} \quad \quad \quad \quad +0.96$
$2 \mathrm{Hg}^{2+}+2 \mathrm{e}^{-} \rightarrow \mathrm{Hg}_{2}^{2+} \quad \quad \quad \quad +0.92$
$\mathrm{ClO}^{-}+\mathrm{H}_{2} \mathrm{O}+2 \mathrm{e}^{-} \rightarrow \mathrm{Cl}^{-}+2 \mathrm{OH}^{-} \quad \quad \quad \quad +0.89$
$\mathrm{Hg}^{2+}+2 \mathrm{e}^{-} \rightarrow \mathrm{Hg} \quad \quad \quad \quad +0.86$
$\mathrm{NO}_{3}^{-}+2 \mathrm{H}^{+}+\mathrm{e}^{-} \rightarrow \mathrm{NO}_{2}+\mathrm{H}_{2} \mathrm{O} \quad \quad \quad \quad +0.80$
$\mathrm{Ag}^{+}+\mathrm{e}^{-} \rightarrow \mathrm{Ag} \quad \quad \quad \quad +0.80$
$\mathrm{Hg}_{2}^{2+}+2 \mathrm{e}^{-} \rightarrow 2 \mathrm{Hg} \quad \quad \quad \quad +0.79$
$\mathrm{Fe}^{3+}+\mathrm{e}^{-} \rightarrow \mathrm{Fe}^{2+} \quad \quad \quad \quad +0.77$
$\mathrm{BrO}^{-}+\mathrm{H}_{2} \mathrm{O}+2 \mathrm{e}^{-} \rightarrow \mathrm{Br}^{-}+2 \mathrm{OH}^{-} \quad \quad \quad \quad +0.76$
$\mathrm{Ti}^{4+}+\mathrm{e}^{-} \rightarrow \mathrm{Ti}^{3+} \quad \quad \quad \quad 0.00 2 \mathrm{H}^{+}+2 \mathrm{e}^{-} \rightarrow \mathrm{H}_{2} \quad \quad \quad \quad 0, \text { by definition }$
$\mathrm{Fe}^{3-}+3 \mathrm{e}^{-} \rightarrow \mathrm{Fe} \quad \quad \quad \quad -0.04$
$\mathrm{O}_{2} \mathrm{H}_{2} \mathrm{O}+2 \mathrm{e}^{-} \rightarrow \mathrm{HO}_{2}^{-}+\mathrm{OH}^{-} \quad \quad \quad \quad -0.08$
$\mathrm{~Pb}^{2+}+2 \mathrm{e}^{-} \rightarrow \mathrm{Pb} \quad \quad \quad \quad -0.13$
$\mathrm{In}^{+}+\mathrm{e}^{-} \rightarrow \mathrm{In} \quad \quad \quad \quad -0.14$
$\mathrm{Sn}^{2+}+2 \mathrm{e}^{-} \rightarrow \mathrm{Sn} \quad \quad \quad \quad -0.14$
$\mathrm{AgI}+\mathrm{e}^{-} \rightarrow \mathrm{Ag}+\mathrm{F}^{-} \quad \quad \quad \quad -0.15$
$\mathrm{Ni}^{2+}+2 \mathrm{e}^{-} \rightarrow \mathrm{Ni} \quad \quad \quad \quad -0.23$
$\mathrm{Co}^{2+}+2 \mathrm{e}^{-} \rightarrow \mathrm{Co} \quad \quad \quad \quad -0.28$
$\mathrm{In}^{3+}+3 \mathrm{e}^{-} \rightarrow \mathrm{In} \quad \quad \quad \quad -0.34$
$\mathrm{Tl}^{+}\mathrm{e}^{-} \rightarrow \mathrm{Tl} \quad \quad \quad \quad -0.34$
$\mathrm{PbSO}_{4}+2 \mathrm{e}^{-} \rightarrow \mathrm{Pb}+\mathrm{SO}_{4}^{2-} \quad \quad \quad \quad -0.36$
$\mathrm{Ti}^{3+}+\mathrm{e}^{-} \rightarrow \mathrm{Ti}^{2+} \quad \quad \quad \quad -0.37$
$\mathrm{Cd}^{2+}+2 \mathrm{e}^{-} \rightarrow \mathrm{Cd} \quad \quad \quad \quad -0.40$
$\mathrm{In}^{2+}+\mathrm{e}^{-} \rightarrow \mathrm{In}^{+} \quad \quad \quad \quad -0.40$
$\mathrm{Cr}^{3+}+\mathrm{e}^{-} \rightarrow \mathrm{Cr}^{2+} \quad \quad \quad \quad -0.41$
$\mathrm{Fe}^{2+}+2 \mathrm{e}^{-} \rightarrow \mathrm{Fe} \quad \quad \quad \quad -0.44$
$\mathrm{In}^{3+}+2 \mathrm{e}^{-} \rightarrow \mathrm{In}^{+} \quad \quad \quad \quad -0.44$
$\mathrm{~S}+2 \mathrm{e}^{-} \rightarrow \mathrm{S}^{2-} \quad \quad \quad \quad -0.48$
$\mathrm{In}^{3+}+\mathrm{e}^{-} \rightarrow \mathrm{In}^{2+} \quad \quad \quad \quad -0.49$
$\mathrm{U}^{4+}+\mathrm{e}^{-} \rightarrow \mathrm{U}^{3+} \quad \quad \quad \quad -0.61$
$\mathrm{Cr}^{3+}+3 \mathrm{e}^{-} \rightarrow \mathrm{Cr} \quad \quad \quad \quad -0.74$
$\mathrm{Zn}^{2+}+2 \mathrm{e}^{-} \rightarrow \mathrm{Zn} \quad \quad \quad \quad -0.76$
$\mathrm{Cd}(\mathrm{OH})_{2}+2 \mathrm{e}^{-} \rightarrow \mathrm{Cd}+2 \mathrm{OH}^{-} \quad \quad \quad \quad -0.81$
$2 \mathrm{H}_{2} \mathrm{O}+2 \mathrm{e}^{-} \rightarrow \mathrm{H}_{2}+2 \mathrm{OH}^{-} \quad \quad \quad \quad -0.83$
$\mathrm{Cr}^{2+}+2 \mathrm{e}^{-} \rightarrow \mathrm{Cr} \quad \quad \quad \quad -0.91$
$\mathrm{Mn}^{2+}+2 \mathrm{e}^{-} \rightarrow \mathrm{Mn} \quad \quad \quad \quad -1.18$
$\mathrm{V}^{2+}+2 \mathrm{e}^{-} \rightarrow \mathrm{V} \quad \quad \quad \quad -1.19$
$\mathrm{Ti}^{2+}+2 \mathrm{e}^{-} \rightarrow \mathrm{Ti} \quad \quad \quad \quad -1.63$
$\mathrm{Al}^{3+}+3 \mathrm{e}^{-} \rightarrow \mathrm{Al} \quad \quad \quad \quad -1.66$
$\mathrm{U}^{3+}+3 \mathrm{e}^{-} \rightarrow \mathrm{U} \quad \quad \quad \quad -1.79$
$\mathrm{Sc}^{3+}+3 \mathrm{e}^{-} \rightarrow \mathrm{Sc} \quad \quad \quad \quad -2.09$
$\mathrm{Mg}^{2+}+2 \mathrm{e}^{-} \rightarrow \mathrm{Mg} \quad \quad \quad \quad -2.36$
$\mathrm{Ce}^{3+}+3 \mathrm{e}^{-} \rightarrow \mathrm{Ce} \quad \quad \quad \quad -2.48$
$\mathrm{La}^{3+}+3 \mathrm{e}^{-} \rightarrow \mathrm{La} \quad \quad \quad \quad -2.52$
$\mathrm{Na}^{+}+\mathrm{e}^{-} \rightarrow \mathrm{Na} \quad \quad \quad \quad -2.71$
$\mathrm{Ca}^{2+}+2 \mathrm{e}^{-} \rightarrow \mathrm{Ca} \quad \quad \quad \quad -2.87$
$\mathrm{Sr}^{2+}+2 \mathrm{e}^{-} \rightarrow \mathrm{Sr} \quad \quad \quad \quad -2.89$
$\mathrm{Ba}^{2+}+2 \mathrm{e}^{-} \rightarrow \mathrm{Ba} \quad \quad \quad \quad -2.91$
$\mathrm{Ra}^{2+}+2 \mathrm{e}^{-} \rightarrow \mathrm{Ra} \quad \quad \quad \quad -2.92$
$\mathrm{Cs}^{+}+\mathrm{e}^{-} \rightarrow \mathrm{Cs} \quad \quad \quad \quad -2.92$
$\mathrm{Rb}^{+}+\mathrm{e}^{-} \rightarrow \mathrm{Rb} \quad \quad \quad \quad -2.93$
$\mathrm{~K}^{+}+\mathrm{e}^{-} \rightarrow \mathrm{K} \quad \quad \quad \quad -2.93$
$\mathrm{Li}^{+}+\mathrm{e}^{-} \rightarrow \mathrm{Li} \quad \quad \quad \quad -3.05$
Reduction Potential in Alphabetical Order:
$\mathrm{Ag}^{+}+\mathrm{e}^{-} \rightarrow \mathrm{Ag}$
$\mathrm{Ag}^{2+}+\mathrm{e}^{-} \rightarrow \mathrm{Ag}^{+}$
$\mathrm{AgBr}+\mathrm{e}^{-} \rightarrow \mathrm{Ag}+\mathrm{Br}^{-}$
$\mathrm{AgCl}+\mathrm{e}^{-} \rightarrow \mathrm{Ag}+\mathrm{Cl}^{-}$
$\mathrm{Ag}_{2} \mathrm{CrO}_{4}+2 \mathrm{e}^{-} \rightarrow 2 \mathrm{Ag}+\mathrm{CrO}_{4}^{2-}$
$\mathrm{AgF}+\mathrm{e}^{-} \rightarrow \mathrm{Ag}+\mathrm{F}^{-}$
$\mathrm{Agl}+\mathrm{e}^{-} \rightarrow \mathrm{Ag}+\mathrm{I}^{-}$
$\mathrm{Al}^{3+}+3 \mathrm{e}^{-} \rightarrow \mathrm{Al}$
$\mathrm{Au}^{+}+\mathrm{e}^{-} \rightarrow \mathrm{Au}$
$\mathrm{Au}^{3+}+3 \mathrm{e}^{-} \rightarrow \mathrm{Au}$
$\mathrm{Ba}^{2+}+2 \mathrm{e}^{-} \rightarrow \mathrm{Ba}$
$\mathrm{Be}^{2+}+2 \mathrm{e}^{-} \rightarrow \mathrm{Be}$
$\mathrm{Bi}^{-3+}+3 \mathrm{e}^{-} \rightarrow \mathrm{Bi}$
$\mathrm{Br}_{2}+2 \mathrm{e}^{-} \rightarrow 2 \mathrm{Br}^{-}$
$\mathrm{BrO}^{-}+\mathrm{H}_{2} \mathrm{O}+2 \mathrm{e}^{-} \rightarrow \mathrm{Br}^{-}+2 \mathrm{OH}^{-}$
$\mathrm{Ca}^{2+}+2 \mathrm{e}^{-} \rightarrow \mathrm{Ca} \quad \quad \quad \quad -2.87$
$\mathrm{Cd}(\mathrm{OH})_{2}+2 \mathrm{e}^{-} \rightarrow \mathrm{Cd}+2 \mathrm{OH}^{-} \quad \quad \quad \quad -0.81$
$\mathrm{Cd}^{2+}+2 \mathrm{e}^{-} \rightarrow \mathrm{Cd} \quad \quad \quad \quad -0.40$
$\mathrm{Ce}^{3+}+3 \mathrm{e}^{-} \rightarrow \mathrm{Ce} \quad \quad \quad \quad -2.48$
$\mathrm{Ce}^{4+}+\mathrm{e}^{-} \rightarrow \mathrm{Ce}^{3+} \quad \quad \quad \quad +1.61$
$\mathrm{Cl}_{2}+2 \mathrm{e}^{-} \rightarrow 2 \mathrm{Cl}^{-} \quad \quad \quad \quad +1.36$
$\mathrm{ClO}^{-}+\mathrm{H}_{2} \mathrm{O}+2 \mathrm{e}^{-} \rightarrow \mathrm{Cl}^{-}+2 \mathrm{OH}^{-} \quad \quad \quad \quad +0.89$
$\mathrm{ClO}_{4}^{-}+2 \mathrm{H}^{+}+2 \mathrm{e}^{-} \rightarrow \mathrm{ClO}_{3}^{-}+\mathrm{H}_{2} \mathrm{O} \quad \quad \quad \quad +1.23$
$\mathrm{ClO}_{4}^{-}+\mathrm{H}_{2} \mathrm{O}+2 \mathrm{e}^{-} \rightarrow \mathrm{ClO}_{3}^{-}+2 \mathrm{OH}^{-} \quad \quad \quad \quad +0.36$
$\mathrm{Co}^{2+}+2 \mathrm{e}^{-} \rightarrow \mathrm{Co} \quad \quad \quad \quad -0.28$
$\mathrm{Co}^{3+}+\mathrm{e}^{-} \rightarrow \mathrm{Co}^{2+} \quad \quad \quad \quad +1.81$
$\mathrm{Cr}^{2+}+2 \mathrm{e}^{-} \rightarrow \mathrm{Cr} \quad \quad \quad \quad -0.91$
$\mathrm{Cr}_{2} \mathrm{O}_{7}^{2-}+14 \mathrm{H}^{+}+6 \mathrm{e}^{-} \rightarrow 2 \mathrm{Cr}^{3+}+7 \mathrm{H}_{2} \mathrm{O} \quad \quad \quad \quad +1.33$
$\mathrm{Cr}^{3+}+3 \mathrm{e}^{-} \rightarrow \mathrm{Cr} \quad \quad \quad \quad -0.74$
$\mathrm{Cr}^{3+}+\mathrm{e}^{-} \rightarrow \mathrm{Cr}^{2+} \quad \quad \quad \quad -0.41$
$\mathrm{Cs}^{+} \mathrm{e}^{-} \rightarrow \mathrm{Cs} \quad \quad \quad \quad -2.92$
$\mathrm{Cu}^{+}+\mathrm{e}^{-} \rightarrow \mathrm{Cu} \quad \quad \quad \quad +0.52$
$\mathrm{Cu}^{2+}+2 \mathrm{e}^{-} \rightarrow \mathrm{Cu} \quad \quad \quad \quad +0.34$
$\mathrm{Cu}^{2+}+\mathrm{e}^{-} \rightarrow \mathrm{Cu}^{+} \quad \quad \quad \quad +0.16$
$\mathrm{~F}_{2}+2 \mathrm{e}^{-} \rightarrow 2 \mathrm{~F}^{-} \quad \quad \quad \quad +2.87$
$\mathrm{Fe}^{2+}+2 \mathrm{e}^{-} \rightarrow \mathrm{Fe} \quad \quad \quad \quad -0.44$
$\mathrm{Fe}^{3+}+3 \mathrm{e}^{-} \rightarrow \mathrm{Fe} \quad \quad \quad \quad -0.04$
$\mathrm{Fe}^{3+}+\mathrm{e}^{-} \rightarrow \mathrm{Fe}^{2+} \quad \quad \quad \quad +0.77$
${\left[\mathrm{Fe}(\mathrm{CN})_{6}\right]^{3}+\mathrm{e}^{-} \rightarrow\left[\mathrm{Fe}(\mathrm{CN})_{6}\right]^{+}} \quad \quad \quad \quad +0.36$
$2 \mathrm{H}^{+}+2 \mathrm{e}^{-} \rightarrow \mathrm{H}_{2} \quad \quad \quad \quad 0, \text { by definition }$
$2 \mathrm{H}_{2} \mathrm{O}+2 \mathrm{e}^{-} \rightarrow \mathrm{H}_{2}+2 \mathrm{OH}^{-} \quad \quad \quad \quad -0.83$
$2 \mathrm{HBrO}+2 \mathrm{H}^{+}+2 \mathrm{e}^{-} \rightarrow \mathrm{Br}_{2}+2 \mathrm{H}_{2} \mathrm{O} \quad \quad \quad \quad +1.60$
$2 \mathrm{HClO}+2 \mathrm{H}^{+}+2 \mathrm{e}^{-} \rightarrow \mathrm{Cl}_{2}+2 \mathrm{H}_{2} \mathrm{O} \quad \quad \quad \quad +1.63$
$\mathrm{H}_{2} \mathrm{O}_{2}+2 \mathrm{H}^{+}+2 \mathrm{e}^{-} \rightarrow 2 \mathrm{H}_{2} \mathrm{O} \quad \quad \quad \quad +1.78$
$\mathrm{H}_{4} \mathrm{XeO}_{6}+2 \mathrm{H}^{+}+2 \mathrm{e}^{-} \rightarrow \mathrm{XeO}_{3}+3 \mathrm{H}_{2} \mathrm{O} \quad \quad \quad \quad +3.0$
$\mathrm{MnO}_{4}^{-}+2 \mathrm{H}_{2} \mathrm{O}+2 \mathrm{e}^{-} \rightarrow \mathrm{MnO}_{2}+4 \mathrm{OH}^{-} \quad \quad \quad \quad +0.60$
$\mathrm{Na}^{-}+\mathrm{e}^{-} \rightarrow \mathrm{Na} \quad \quad \quad \quad -2.71$
$\mathrm{Ni}^{2+}+2 \mathrm{e}^{-} \rightarrow \mathrm{Ni} \quad \quad \quad \quad -0.23$
$\mathrm{NiOOH}+\mathrm{H}_{2} \mathrm{O}+\mathrm{e}^{-} \rightarrow \mathrm{Ni}(\mathrm{OH})_{2}+\mathrm{OH}^{-} \quad \quad \quad \quad +0.49$
$\mathrm{NO}_{3}^{-}+2 \mathrm{H}^{+}+\mathrm{e}^{-} \rightarrow \mathrm{NO}_{2}+\mathrm{H}_{2} \mathrm{O} \quad \quad \quad \quad -0.80$
$\mathrm{NO}_{3}^{-}+4 \mathrm{H}^{+}+3 \mathrm{e}^{-} \rightarrow \mathrm{NO}+2 \mathrm{H}_{2} \mathrm{O} \quad \quad \quad \quad +0.96$
$\mathrm{NO}_{3}^{-}+\mathrm{H}_{2} \mathrm{O}+2 \mathrm{e}^{-} \rightarrow \mathrm{NO}_{2}^{-}+2 \mathrm{OH}^{-} \quad \quad \quad \quad +0.10$
$\mathrm{O}_{2}+2 \mathrm{H}_{2} \mathrm{O}+4 \mathrm{e}^{-} \rightarrow 4 \mathrm{OH}^{-} \quad \quad \quad \quad +0.40$
$\mathrm{O}_{2}+4 \mathrm{H}^{+}+4 \mathrm{e}^{-} \rightarrow 2 \mathrm{H}_{2} \mathrm{O} \quad \quad \quad \quad +1.23$
$\mathrm{O}_{2}+\mathrm{e}^{-} \rightarrow \mathrm{O}_{2}^{-} \quad \quad \quad \quad -0.56$
$\mathrm{O}_{2}+\mathrm{H}_{2} \mathrm{O}+2 \mathrm{e}^{-} \rightarrow \mathrm{HO}_{2}^{-}+\mathrm{OH}^{-} \quad \quad \quad \quad -0.08$
$\mathrm{O}_{3}+2 \mathrm{H}^{+}+2 \mathrm{e}^{-} \rightarrow \mathrm{O}_{2}+\mathrm{H}_{2} \mathrm{O} \quad \quad \quad \quad +2.07$
$\mathrm{O}_{3}+\mathrm{H}_{2} \mathrm{O}+2 \mathrm{e}^{-} \rightarrow \mathrm{O}_{2}+2 \mathrm{OH}^{-} \quad \quad \quad \quad +1.24$
$\mathrm{H}_{4} \mathrm{XeO}_{6}+2 \mathrm{H}^{+}+2 \mathrm{e}^{-} \rightarrow \mathrm{XeO}_{3}+3 \mathrm{H}_{2} \mathrm{O} \quad \quad \quad \quad +3.0$
$\mathrm{Hg}_{2}^{2+}+2 \mathrm{e}^{-} \rightarrow 2 \mathrm{Hg} \quad \quad \quad \quad +0.79$
$\mathrm{Hg}_{2} \mathrm{Cl}_{2}+2 \mathrm{e}^{-} \rightarrow 2 \mathrm{Hg}+2 \mathrm{Cl}^{-} \quad \quad \quad \quad +0.27$
$\mathrm{Hg}^{2+}+2 \mathrm{e}^{-} \rightarrow \mathrm{Hg} \quad \quad \quad \quad +0.86$
$2 \mathrm{Hg}^{2+}+2 \mathrm{e}^{-} \rightarrow \mathrm{Hg}_{2}^{2+} \quad \quad \quad \quad +0.92$
$\mathrm{Hg}_{2} \mathrm{SO}_{4}+2 \mathrm{e}-\rightarrow 2 \mathrm{Hg}+\mathrm{SO}_{4}^{2-} \quad \quad \quad \quad +0.62$
$\mathrm{I}_{2}+2 \mathrm{e}^{-} \rightarrow 2 \mathrm{I}^{-} \quad \quad \quad \quad +0.54$
$\mathrm{I}_{3}^{-}+2 \mathrm{e}^{-} \rightarrow 3 \mathrm{I}^{-} \quad \quad \quad \quad +0.53$
$\mathrm{In}^{+}+\mathrm{e}^{-} \rightarrow \mathrm{In} \quad \quad \quad \quad -0.14$
$\mathrm{In}^{2+}+\mathrm{e}^{-} \rightarrow \mathrm{In}^{+} \quad \quad \quad \quad -0.40$
$\mathrm{In}^{3+}+2 \mathrm{e}^{-} \rightarrow \mathrm{In}^{+} \quad \quad \quad \quad -0.44$
$\mathrm{In}^{3+}+3 \mathrm{e}^{-} \rightarrow \mathrm{In} \quad \quad \quad \quad -0.34$
$\mathrm{In}^{3+}+\mathrm{c}^{-} \rightarrow \mathrm{In}^{2+} \quad \quad \quad \quad -0.49$
$\mathrm{~K}^{+}+\mathrm{e}^{-} \rightarrow \mathrm{K} \quad \quad \quad \quad -2.93$
$\mathrm{La}^{3+}+3 \mathrm{e}^{-} \rightarrow \mathrm{La} \quad \quad \quad \quad -2.52$
$\mathrm{Li}+\mathrm{e}^{-} \rightarrow \mathrm{Li} \quad \quad \quad \quad -3.05$
$\mathrm{Mg}^{2+}+2 \mathrm{e}^{-} \rightarrow \mathrm{Mg} \quad \quad \quad \quad -2.36$
$\mathrm{Mn}^{2+}+2 \mathrm{e}^{-} \rightarrow \mathrm{M} \quad \quad \quad \quad -1.18$
$\mathrm{Mn}^{3+}+\mathrm{e}^{-} \rightarrow \mathrm{Mn}^{2+} \quad \quad \quad \quad +1.51$
$\mathrm{MnO}_{2}+4 \mathrm{H}^{+}+2 \mathrm{e}^{-} \rightarrow \mathrm{Mn}^{2+}+2 \mathrm{H}_{2} \mathrm{O} \quad \quad \quad \quad +1.23$
$\mathrm{MnO}_{4}^{-}+8 \mathrm{H}^{+}+5 \mathrm{e}^{-} \rightarrow \mathrm{Mn}^{2+}+4 \mathrm{H}_{2} \mathrm{O} \quad \quad \quad \quad +1.51$
$\mathrm{MnO}_{4}^{-}+\mathrm{e}^{-} \rightarrow \mathrm{MnO}_{4}^{2-} \quad \quad \quad \quad +0.56$
$\mathrm{O}_{3}+\mathrm{H}_{2} \mathrm{O}+2 \mathrm{e}^{-} \rightarrow \mathrm{O}_{2}+2 \mathrm{OH}^{-} \quad \quad \quad \quad +1.24$
$\mathrm{~Pb}^{2+}+2 \mathrm{e}^{-} \rightarrow \mathrm{Pb} \quad \quad \quad \quad -0.13$
$\mathrm{~Pb}^{4+}+2 \mathrm{e}^{-} \rightarrow \mathrm{Pb}^{2+} \quad \quad \quad \quad +1.67$
$\mathrm{PbSO}_{4}+2 \mathrm{e}^{-} \rightarrow \mathrm{Pb}+\mathrm{SO}_{4}^{2-} \quad \quad \quad \quad -0.36$
$\mathrm{Pt}^{2+}+2 \mathrm{e}^{-} \rightarrow \mathrm{Pt} \quad \quad \quad \quad +1.20$
$\mathrm{Pu}^{4+}+\mathrm{e}^{-} \rightarrow \mathrm{Pu}^{3+} \quad \quad \quad \quad +0.97$
$\mathrm{Ra}^{2+}+2 \mathrm{e}^{-} \rightarrow \mathrm{Ra} \quad \quad \quad \quad -2.92$
$\mathrm{Rb}^{+}+\mathrm{e}^{-} \rightarrow \mathrm{Rb} \quad \quad \quad \quad -2.93$
$\mathrm{~S}+2 \mathrm{e}^{-} \rightarrow \mathrm{S}^{2-} \quad \quad \quad \quad -0.48$
$\mathrm{~S}_{2} \mathrm{O}_{8}^{2-}+2 \mathrm{e}^{-} \rightarrow 2 \mathrm{SO}_{4}^{2-} \quad \quad \quad \quad +2.05$
$\mathrm{SC}^{3+}+3 \mathrm{e}^{-} \rightarrow \mathrm{Sc} \quad \quad \quad \quad -2.09$
$\mathrm{Sn}^{2+}+2 \mathrm{e}^{-} \rightarrow \mathrm{Sn} \quad \quad \quad \quad -0.14$
$\mathrm{Sn}^{4+}+2 \mathrm{e}^{-} \rightarrow \mathrm{Sn}^{2+} \quad \quad \quad \quad +0.15$
$\mathrm{Sr}^{2+}+2 \mathrm{e}^{-} \rightarrow \mathrm{Sr} \quad \quad \quad \quad -2.89$
$\mathrm{Ti}^{2+}+2 \mathrm{e}^{-} \rightarrow \mathrm{Ti} \quad \quad \quad \quad -1.63$
$\mathrm{Ti}^{3+}+\mathrm{e}^{-} \rightarrow \mathrm{Ti}^{2+} \quad \quad \quad \quad -0.37$
$\mathrm{Ti}^{4+}+\mathrm{e}^{-} \rightarrow \mathrm{Ti}^{3+} \quad \quad \quad \quad 0.00$
$\mathrm{Tl}^{+}+\mathrm{e}^{-} \rightarrow \mathrm{Tl} \quad \quad \quad \quad -0.34$
$\mathrm{U}^{3+}+3 \mathrm{e}^{-} \rightarrow \mathrm{U} \quad \quad \quad \quad -1.79$
$\mathrm{U}^{4+}+\mathrm{e}^{-} \rightarrow \mathrm{U}^{3+} \quad \quad \quad \quad -0.61$
$\mathrm{~V}^{2+}+2 \mathrm{e}^{-} \rightarrow \mathrm{V} \quad \quad \quad \quad -1.19$
$\mathrm{~V}^{3+}+\mathrm{e}^{-} \rightarrow \mathrm{V}^{2+} \quad \quad \quad \quad -0.26$
$\mathrm{Zn}^{2+}+2 \mathrm{e}-\rightarrow \mathrm{Zn} \quad \quad \quad \quad -0.76$
Some Important Questions on Electrochemistry
1. What is the meaning of the negative sign in the expression $\dfrac{E_0 Zn^{2+}}{Zn} = – 0.76 V$?
Ans. The negative sign in the given expression implies that Zn is more reactive than hydrogen or that it is a stronger reducing agent than hydrogen. Zinc will be oxidised to $Zn^{2+}$ ions, while the $H^+$ ions will get reduced to hydrogen in a cell that contains a zinc electrode and a standard hydrogen electrode present in two half-cells.
2. What are the conditions under which $E_0$ cell = 0 and $\Delta rG_0 = 0$?
Ans. When at equilibrium, $E_0$cell = 0 and $\Delta rG_0 = 0$.
3. Can we measure the absolute electrode potential of an electrode?
Ans. No, it is not possible to measure the absolute potential of an electrode since the half-cell that contains a single electrode cannot work on its own, it can only work in combination with another half-cell.
Class 12 Chemistry Chapter 2: Details, Formulas and Concepts.
Nernst Equation: This equation relates the equilibrium potential of an electrochemical cell to the concentrations of the reactants and products involved. It's given as:
\[ E = E^\circ - \dfrac{0.0592}{nlog Q}\]
Gibbs Free Energy Change (ΔG): In electrochemistry, this concept is crucial as it determines whether a reaction is spontaneous or not. The relationship between Gibbs free energy change, cell potential, and temperature is given by:\[ \Delta G = -nFE \]
Class 12 Chemistry Chapter 2 Important Topics and Subtopics Covered
S. No | Topic |
1 | Redox Reactions |
2 | EMF of a Cell |
3 | Standard Electrode Potential |
4 | Nernst Equation and Its Application to Chemical Cells |
5 | Relation Between Gibbs Energy Change and EMF of a Cell |
6 | Conductance in Electrolytic Solutions |
7 | Specific and Molar Conductivity |
8 | Variations of Conductivity with Concentration |
9 | Kohlrausch's Law |
10 | Electrolysis and Law of Electrolysis (Elementary Idea) |
11 | Dry Cell, Electrolytic Cells, and Galvanic Cells |
12 | Lead Accumulator |
13 | Fuel Cells |
14 | Corrosion |
Importance of Revision Notes for Class 12 Chemistry Chapter 2
Summarises Key Points: Condenses important concepts for quick review.
Saves Time: Provides a fast way to revise before exams.
Highlights Essentials: Focuses on crucial topics and definitions.
Improves Memory: Helps in better retention of information.
Enhances Exam Prep: Targets weak areas for more effective study.
Clarifies Concepts: Simplifies complex ideas for easier understanding.
Includes Visuals: Uses diagrams and charts for better grasp.
Boosts Confidence: Prepares students thoroughly for exams.
Tips for Learning the Class 12 Chemistry Chapter 2
Focus on core processes with illustrations and examples.
Draw and label diagrams for clarity.
Create summaries of each process.
Connect concepts to everyday examples.
Solve past exam questions to test understanding.
Explain concepts to others to reinforce learning.
Revisit material frequently to retain information.
Utilise platforms like Vedantu for additional support.
Conclusion
Vedantu's Electrochemistry Class 12 Revision Notes for CBSE Chemistry Chapter 2 provides a comprehensive and effective resource for students studying this topic. The Electrochemistry Revision notes cover all the important concepts outlined in the NCERT textbook, ensuring that students have a clear understanding of electrochemistry. Vedantu's notes are well-structured and easy to follow, making it easier for students to grasp complex concepts and solve problems.
Related Study Materials for Class 12 Chapter 2 Electrochemistry
S. No | Important Study Material Links for Class 12 Chemistry Chapter 2 |
1. | |
2. | |
3. |
Revision Notes Links for Class 12 Chemistry Revision Notes
S.No. | Chapter-wise Links for Class 12 Chemistry Revision Notes |
1 | |
3 | |
4 | |
5 | |
6 | |
7 | |
8 | |
9 | |
10 |
Related Study Material Links for Class 12 Chemistry
S.No. | Related Study Materials for Class 12 Chemistry |
1. | |
2. | |
3. | |
4. | |
5. | |
6. |
FAQs on CBSE Notes Class 12 Chemistry Chapter 2 - Electrochemistry - 2025-26
1. What key topics are essential for a quick revision of Class 12 Electrochemistry?
For an effective revision of the Electrochemistry chapter, your summary should focus on these core concepts as per the CBSE 2025-26 syllabus:
- Electrochemical Cells: The fundamental differences between Galvanic (Voltaic) and Electrolytic cells.
- Nernst Equation: Understanding how to apply it to calculate cell potential under non-standard conditions.
- Conductance in Electrolytic Solutions: The concepts of specific and molar conductivity and their variation with concentration.
- Kohlrausch's Law: Its application in determining the limiting molar conductivity for weak electrolytes.
- Batteries: Key reactions at the anode and cathode for primary (e.g., Leclanche cell) and secondary (e.g., Lead-acid battery) batteries.
- Corrosion: The electrochemical mechanism behind the rusting of iron.
2. How can I make learning Electrochemistry easier for my Class 12 exams?
To simplify your revision of Electrochemistry, focus on understanding the logic rather than just memorising. Start by clearly distinguishing between the anode (oxidation) and cathode (reduction). Create a concept map linking cell potential (Ecell), Gibbs free energy (ΔG), and the equilibrium constant (K). Practise numerical problems related to the Nernst equation and Kohlrausch's law to build confidence. Relating concepts to real-world examples like batteries and rusting also makes them easier to remember.
3. What is the main difference between molar conductivity and conductivity, and how do they change with dilution?
This is a crucial concept for revision.
- Conductivity (κ) is the conductance of 1 cm³ of an electrolyte solution. It decreases on dilution because the number of ions per unit volume decreases.
- Molar Conductivity (Λm) is the conductance of a solution containing one mole of electrolyte. It increases on dilution because the total volume of the solution containing one mole of the electrolyte increases, leading to greater ionic mobility.
4. Why is a salt bridge essential for the functioning of a Galvanic cell?
A salt bridge plays two critical roles in a Galvanic cell, which are vital to revise:
- It completes the electrical circuit by allowing the flow of ions between the two half-cells.
- It maintains electrical neutrality in the half-cells. It prevents the accumulation of positive charge in the anode compartment and negative charge in the cathode compartment by supplying counter-ions (anions to the anode half-cell and cations to the cathode half-cell). Without it, the cell would stop working almost instantly.
5. How does Kohlrausch's Law help in revising the concept of molar conductivity for weak electrolytes?
Kohlrausch's Law is a key revision tool because the limiting molar conductivity (Λ°m) of a weak electrolyte cannot be determined by graphical extrapolation. The law states that the limiting molar conductivity of an electrolyte is the sum of the individual contributions of its cations and anions. This allows us to calculate Λ°m for a weak electrolyte (like acetic acid) by using the Λ°m values of strong electrolytes (like HCl, NaCl, and CH₃COONa).
6. What is the core difference between a primary and a secondary battery in a revision context?
For revision purposes, the core difference is rechargeability.
- Primary Batteries (e.g., Dry cell, Mercury cell) involve irreversible chemical reactions. Once the reactants are consumed, the battery is dead and cannot be recharged.
- Secondary Batteries (e.g., Lead-acid storage battery, Nickel-cadmium cell) involve reversible reactions. They can be recharged by passing an electric current through them, which reverses the cell reactions and regenerates the original reactants.
7. Why is the Nernst equation so important for understanding electrochemical cells?
The Nernst equation is fundamental because it connects the ideal Standard Electrode Potential (E°), measured under standard conditions (1M concentration, 298K, 1 bar pressure), to the actual cell potential (Ecell) under non-standard conditions. This is crucial for real-world applications, as cells rarely operate under perfectly standard conditions. It helps us understand how changes in concentration or temperature affect the EMF of a cell.
8. What key formulas from Electrochemistry should be on a revision summary sheet?
For a quick and effective revision, your formula sheet for Electrochemistry must include:
- Nernst Equation: E_cell = E°_cell - (0.0591/n) log Q
- Gibbs Free Energy: ΔG° = -nFE°_cell
- Relation to Equilibrium Constant: E°_cell = (0.0591/n) log K_c
- Molar Conductivity: Λm = (κ × 1000) / M
- Kohlrausch's Law: Λ°m(AxBy) = xλ°(Aʸ⁺) + yλ°(Bˣ⁻)
- Degree of Dissociation (α): α = Λm / Λ°m
9. For Class 12 board exams, is revising only the NCERT textbook sufficient for the Electrochemistry chapter?
While the NCERT textbook is the absolute foundation and essential for building concepts, relying solely on it for revision might not be enough for scoring top marks. Revision notes are crucial for quickly summarising formulas and key reaction mechanisms. For a thorough preparation, it's highly recommended to also solve a wide variety of numerical problems from past papers and sample papers to master the application of concepts like the Nernst equation and conductivity calculations, as expected in the CBSE board exams.
10. How is corrosion an electrochemical phenomenon? What should I focus on for revision?
Corrosion, particularly the rusting of iron, is an electrochemical process because it involves the formation of a miniature Galvanic cell on the surface of the metal. For revision, focus on:
- Anode: The site where iron is oxidised: Fe(s) → Fe²⁺(aq) + 2e⁻.
- Cathode: The site where oxygen is reduced in the presence of H⁺ ions (from dissolved CO₂ in water): O₂(g) + 4H⁺(aq) + 4e⁻ → 2H₂O(l).
- The overall redox reaction and the formation of hydrated ferric oxide (Fe₂O₃.xH₂O), which is rust.


























