NCERT Exemplar for Class 12 Chemistry - Electrochemistry - Free PDF Download
Free PDF download of NCERT Exemplar for Class 12 Chemistry Chapter 3 - Electrochemistry solved by expert Chemistry teachers on Vedantu.com as per NCERT (CBSE) Book guidelines. All Chapter 3 - Electrochemistry Exercise questions with solutions to help you to revise the complete syllabus and score more marks in your examinations.
Access NCERT Exemplar Solutions for Class 12 Chemistry Chapter 3 - Electrochemistry
Exercise
Multiple Choice Questions (Type-I):
1. Which cell will measure standard electrode potential of copper electrode?
(i) $Pt(s)|{H_2}\left( {g,0.1} \right.bar)|{H^ + }(aq.,1M)||C{u^{2 + }}(aq.,1M)|Cu$
ii) $Pt(s)|{H_2}\left( {g,1} \right.bar)|{H^ + }(1M)||C{u^{2 + }}(aq.,2M)|Cu$
(iii) $Pt(s)|{H_2}\left( {g,1} \right.bar)|{H^ + }(aq.,1M)||C{u^{2 + }}(aq.,1M)|Cu$
(iv)$Pt(s)|{H_2}\left( {g,1} \right.bar)|{H^ + }(aq.,0.1M)||C{u^{2 + }}\left( {aq., 1M} \right)|Cu$
Ans: (i) For measurement of electrode potential standard conditions must be met which is 1 bar pressure and $1{\text{M}}$ concentration which is not here so this option is incorrect.
(ii) For measurement of electrodes potential standard conditions must be met which is 1 bar pressure and $1{\text{M}}$ concentration which is not here so this option is incorrect.
(iii) When copper electrode is connected to SHE it acts as cathode and its standard electrode potential can be measured as follows
${E^0} = {E^0}_R - {E^0}_L$
For calculation of standard potential of a given cell it should be coupled with SHE and standard conditions such as 1 bar pressure and $1{\text{M}}$ concentration and the concentrations of oxidised and reduced forms is one so this option is correct.
(iv) For measurement of electrode potential standard conditions must be met which is 1 bar pressure and $1{\text{M}}$ concentration which is not here so this option is incorrect.
Correct Option: (iii)
2. Electrode potential for Mg electrode varies according to the equation
${E_{M{g^{2 + }}/Mg}} = {E^0}_{M{g^{2 + }}/Mg} - \frac{{0.059}}{2}log\frac{1}{{M{g^{2 + }}}}$. The graph of ${E_{M{g^{2 + }}/Mg}}$ vs $log[M{g^{2 + }}]$ is
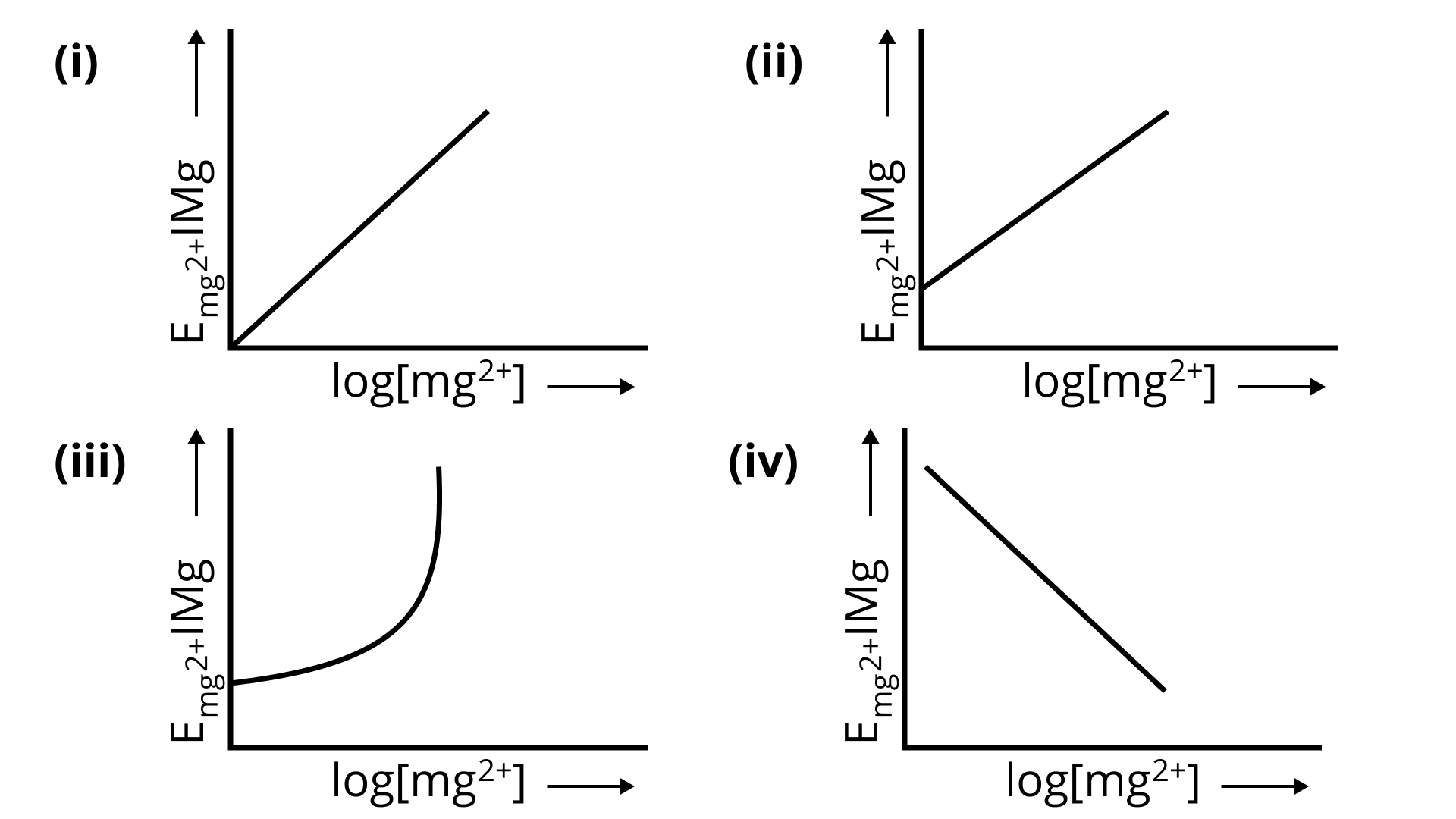
Ans: Comparing this equation${E_{{\text{M}}{{\text{g}}^{2 + }}/{\text{Mg}}}} = {E^0}_{{\text{M}}{{\text{g}}^{2 + }}/{\text{Mg}}} - \frac{{0.059}}{2}\log \frac{1}{{{\text{M}}{{\text{g}}^{2 + }}}}$ with equation of straight line,
${E_{{\text{M}}{{\text{g}}^{2 + }}/{\text{Mg}}}} = {E^0}_{{\text{M}}{{\text{g}}^{2 + }}/{\text{Mg}}} - \frac{{0.059}}{2}\log \frac{1}{{{\text{M}}{{\text{g}}^{2 + }}}}$
${E_{{\text{M}}{{\text{g}}^{2 + }}/{\text{Mg}}}} = E_{{\text{M}}{{\text{g}}^{2 + }}/{\text{Mg}}}^0 + \frac{{0.059}}{2}\log [{\text{M}}{{\text{g}}^{2 + }}]$
${E_{{\text{M}}{{\text{g}}^{2 + }}/{\text{Mg}}}} = \frac{{0.059}}{2}\log [{\text{M}}{{\text{g}}^{2 + }}] + E_{{\text{M}}{{\text{g}}^{2 + }}/{\text{Mg}}}^0$
Comparing it with $y = mx + c$ we get,
The slope is positive and the intercept is ${E_{{\text{M}}{{\text{g}}^{2 + }}/{\text{Mg}}}}$therefore the graph satisfying the conditions is (ii).
Correct Option: (ii)
3. Which of the following statements is correct?
(i) ${E_{cell}}$and${\Delta _r}G$ of cell reactions both have extensive properties.
(ii) ${E_{cell}}$and${\Delta _r}G$ of cell reactions both are intensive properties.
(iii) ${E_{cell}}$is an intensive property while ${\Delta _r}G$ Cell reaction is an extensive property.
(iv) ${E_{cell}}$ is an extensive property while ${\Delta _r}G$ of cell reaction is an intensive property
Ans: (i) The properties which don’t depend on the mass of species are termed intensive properties. ${E_{cell}}$ is an intensive property that doesn’t depend on the mass of the species. ${\Delta _r}G$ is an extensive property as it depends on the mass of the species. Therefore, this option is incorrect
(ii) ${E_{cell}}$ is an intensive property but ${\Delta _r}G$ is an extensive property so this option is incorrect
(iii) The properties which don’t depend on the mass of species are termed intensive properties. ${E_{cell}}$ is an intensive property that doesn’t depend on the mass of the species. ${\Delta _r}G$ is an extensive property as it depends on the mass of the species. Therefore, this option is correct
(iv) ${E_{cell}}$ is an intensive property but ${\Delta _r}G$ is an extensive property so this option is incorrect.
Correct Option: (iii)
4. The difference between the electrode potentials of two electrodes when no current is drawn through the cell is called ___________.
(i) Cell potential
(ii) Cell emf
(iii) Potential difference
(iv) Cell voltage
Ans: The difference between electrode potential potentials of two electrodes when no current is drawn through the cell is termed as cell emf. It is the energy provided by a cell per coulomb of charge passing through it. It is the maximum potential difference between the electrodes of a cell.
Correct Option: (iii)
5. Which of the following statements is not correct about an inert electrode in a cell?
(i) It does not participate in the cell reaction.
(ii) It provides a surface either for oxidation or for reduction reaction.
(iii) It provides a surface for conduction of electrons.
(iv) It provides a surface for redox reaction.
Ans: (i) Inert electrodes like graphite are a source of electrons in a reaction. It doesn’t participate in a chemical reaction. Therefore, this option is incorrect
(ii) Inert electrode chemically doesn’t participate in the reaction but it provides a surface either for oxidation and reduction reaction. Therefore, this option is incorrect
(iii) Inert electrodes provide surface for conduction of electrons so this option is incorrect.
(iv) Inert electrodes cease transfer of electrons for oxidation or reduction but not for redox reaction. Therefore, this option is correct.
Correct Option: (iv)
6. An electrochemical cell can behave like an electrolytic cell when ____________.
(i) ${E_{cell}} = 0$
(ii) ${E_{cell}} > {E_{ext}}$
(iii) ${E_{ext}} > {E_{cell}}$
(iv)${E_{cell}} = {E_{ext}}$
Ans:On the application of external potential, an increased reaction starts till the opposing voltage becomes $1.1{\text{V}}$.
However, further increase in the external potential leads the reaction to start in an opposite direction functioning as an electrolytic cell which is a device that uses electrical energy for carrying out electrochemical reactions.
Correct Option: (iii)
7. Which of the statements about solutions of electrolytes is not correct?
(i) Conductivity of solution depends upon the size of ions.
(ii) Conductivity depends upon the viscosity of the solution.
(iii) Conductivity does not depend upon the solvation of ions present in the solution.
(iv) Conductivity of solution increases with temperature
Ans: (i) Conductivity of solution depends upon the size of ions as more is the size of ion, mobility decreases, and conductivity decreases so this option is incorrect.
(ii) Viscosity is the measure of the resistance of a liquid to flow. Greater will be the viscosity of solvent, less will be the flow of electrons and lesser will be the conductivity. Therefore, this option is incorrect.
(iii) Conductivity depends on the solvation of ions in the solution. Greater is the solvation, lesser will be the conductivity of the solution. Therefore, this option is correct.
(iv) On increasing temperature the moving ions acquire higher kinetic energy and move with higher speeds thus increasing conductivity. Therefore, this option is incorrect
Correct Option: (iii)
8. Using the data given below find out the strongest reducing agent.
${E^0}_{C{r_2}{O_7}^{2 - }/C{r^{3 + }}} = 1.33V$
${E^0}_{C{l_2}/C{l^ - }} = 1.36V$
${E^0}_{Mn{O_4}^ - /M{n^{2 + }}} = 1.51V$
${E^0}_{C{r^{3 + }}/Cr} = \, - 0.74V$
(i)$C{l^ - }$
(ii)$Cr$
(iii)$C{r^{3 + }}$
(iv)$M{n^{2 + }}$
Ans: According to the electrochemical series and standard reduction potential of metal, the higher the negative value of standard reduction potential stronger will be the reducing agent
In the given options, the standard reduction potential of chromium has the highest value so it is the strongest reducing agent.
Correct Option: (ii)
9. Use the data given in Q.8 and find out which of the following is the strongest oxidizing agent.
(i)\[C{l^ - }\]
(ii)$M{n^{2 + }}$
(iii)$MnO_4^ - $
(iv)$C{r^{3 + }}$
Ans: According to the electrochemical series higher the positive value of standard reduction potential of metal ion, the higher is the oxidizing power
Therefore, ${\text{MnO}}_4^ - $ is the strongest oxidizing agent.
Correct Option: (iii)
10. Using the data given in Q.8 find out in which option the order of reducing power is correct.
(i)$C{r^{3 + }} < C{l^ - } < M{n^{2 + }} < Cr$
(ii)$M{n^{2 + }} < C{l^ - } < C{r^{3 + }} < Cr$
(iii)$C{r^{3 + }} < C{l^ - } < C{r_2}O_7^{2 - } < Mn{O_4}^ - $
(iv)\[{\text{M}}{{\text{n}}^{2 + }} < {\text{C}}{{\text{r}}^{3 + }} < {\text{C}}{{\text{l}}^ - } < {\text{Cr}}\]
Ans:According to the electrochemical series, the lower the reduction potential higher is the reducing power.
Therefore, the order of reducing power is ${\text{M}}{{\text{n}}^{2 + }} < {\text{C}}{{\text{l}}^ - } < {\text{C}}{{\text{r}}^{3 + }} < {\text{Cr}}$.
Correct Option: (iii)
11. Use the data given in Q.8 and find out the most stable ion in its reduced form
(i) \[C{l^ - }\]
(ii) $C{r^{3 + }}$
(iii) $Cr$
(iv)$M{n^{2 + }}$
Ans: ${E^0}_{{\text{Mn}}{{\text{O}}_4}^ - /{\text{M}}{{\text{n}}^{2 + }}} = 1.51{\text{V}}$ is the highest positive value among the given species. So ${\text{M}}{{\text{n}}^{2 + }}$ is the most stable ion in its reduced form.
Correct Option: (iv)
12. Use the data of Q.8 and find out the most stable oxidized species.
(i) $C{r^{3 + }}$
(ii) $MnO_4^ - $
(iii)$C{r_2}O_7^{2 - }$
(iv)$M{n^{2 + }}$
Ans: According to the given table, ${E^0}_{{\text{Mn}}{{\text{O}}_4}^ - /{\text{M}}{{\text{n}}^{2 + }}} = - 0.74{\text{V}}$ has the most negative value among the given species. So ${\text{C}}{{\text{r}}^{{\text{3 + }}}}$ is the most stable oxidized species.
Correct Option: (i)
13. The quantity of charge required to obtain one mole of aluminium from $A{l_2}{O_3}$ is
(i) $1F$
(ii) $6F$
(iii) $3F$
(iv)$2F$
Ans: Given: One mole of aluminium
Calculate the number of electrons required to convert ${\text{A}}{{\text{l}}_{\text{2}}}{{\text{O}}_{\text{3}}}$ to ${\text{Al}}$
It is the charge required to obtain one mole of aluminium from ${\text{A}}{{\text{l}}_{\text{2}}}{{\text{O}}_{\text{3}}}$
The dissociation of aluminium oxide is as follows;
\[{\text{A}}{{\text{l}}_{\text{2}}}{{\text{O}}_{\text{3}}} \to 2{\text{A}}{{\text{l}}^{3 + }} + 3{{\text{O}}^{2 - }}\]
${\text{A}}{{\text{l}}^{3 + }} + 3{e^ - } \to {\text{Al}}$
Therefore $3{\text{F}}$charge is required to obtain one mole of aluminum from ${\text{A}}{{\text{l}}_{\text{2}}}{{\text{O}}_{\text{3}}}$.
Correct Option: (iii)
14. The cell constant of a conductivity cell _____________.
(i) changes with the change of electrolyte.
(ii) changes with the change of concentration of electrolyte.
(iii) changes with the temperature of the electrolyte.
(iv) remains constant for a cell.
Ans: The cell constant is described as the ratio of the length of the object and the area of cross-section. It can be written as follows
$G = \frac{l}{A}$
As $l$and $A$ remains constant for an object it can be inferred that the cell constant for a conductivity cell remains constant for a cell.
Correct Option: (iv)
15. While charging the lead storage battery ______________.
(i) $PbS{O_4}$anode is reduced to $Pb$
(ii) $PbS{O_4}$cathode is reduced to $Pb$
(iii) $PbS{O_4}$cathode is oxidized to $Pb$
(iv) $PbS{O_4}$anode is oxidized to $Pb{O_2}$
Ans:In the lead storage battery, a reverse reaction occurs and lead sulfate is formed at the anode, and at the cathode, it is converted into lead and lead oxide respectively. The reaction is as follows
At the cathode, the reaction goes this way,
${\text{PbS}}{{\text{O}}_{\text{4}}}(s) + 2{e^ - } \to {\text{Pb}}(s) + {\text{S}}{{\text{O}}_{\text{4}}}^{2 - }(aq)$ , Reduction process
At the anode, the reaction goes this way,
${\text{PbS}}{{\text{O}}_{\text{4}}}(s) + 2{{\text{H}}_{\text{2}}}{\text{O}} \to {\text{Pb}}{{\text{O}}_{\text{2}}}(s) + {\text{SO}}_4^{2 - } + 2{{\text{H}}^ + } + 2e$, oxidation process
The overall reaction is as follows;
$2{\text{PbS}}{{\text{O}}_{\text{4}}}(s) + 2{{\text{H}}_{\text{2}}}{\text{O}} \to {\text{Pb}}(s) + {\text{Pb}}{{\text{O}}_{\text{2}}}(s) + 4{{\text{H}}^ + }(aq) + 2{\text{SO}}_4^{2 - }(aq)$
Therefore, at anode lead sulfate is reduced to lead.
Correct Option: (i)
16. ${\Lambda ^0}_{m(N{H_4}OH)}$is equal to _____
(i)\[{\Lambda ^0}_{m(N{H_4}OH)} + {\Lambda ^0}_{m(N{H_4}Cl)} - {\Lambda ^0}_{m(HCl)}\]
(ii)${\Lambda ^0}_{m(N{H_4}OH)} + {\Lambda ^0}_{m(NaOH)} - {\Lambda ^0}_{m(NaCl)}$
(iii)${\Lambda ^0}_{m(N{H_4}Cl)} - {\Lambda ^0}_{m(NaCl)} - {\Lambda ^0}_{m(NaOH)}$
(iv)${\Lambda ^0}_{m(NaOH)} + {\Lambda ^0}_{m(NaCl)} - {\Lambda ^0}_{m(N{H_4}OH)}$
Ans:Given: Salt ammonium hydroxide
Apply Kohlrausch Law
Kohlrausch law of states that limiting molar conductivity of any salt species is equal to the sum of the limiting molar conductivity of cations and anions of the electrolyte
${\Lambda ^0}_{m({\text{N}}{{\text{H}}_{\text{4}}}{\text{OH}})} = {\Lambda ^0}_{m({\text{N}}{{\text{H}}_4}^ + )} + {\Lambda ^0}_{m({\text{O}}{{\text{H}}^ - })} + {\Lambda ^0}_{m({\text{N}}{{\text{a}}^ + })} + {\Lambda ^0}_{m({\text{O}}{{\text{H}}^ - })} - {\Lambda ^0}_{m({\text{N}}{{\text{a}}^ + })} - {\Lambda ^0}_{m({\text{C}}{{\text{l}}^ - })}$
${\Lambda ^0}_{m({\text{N}}{{\text{H}}_{\text{4}}}{\text{OH}})} = {\Lambda ^0}_{m({\text{N}}{{\text{H}}_{\text{4}}}{\text{OH}})} + {\Lambda ^0}_{m({\text{NaOH}})} - {\Lambda ^0}_{m({\text{NaCl}})}$
Correct Option: (ii)
17. In the electrolysis of aqueous sodium chloride solution, which half-cell reaction will occur at the anode?
(i) $\mathrm{Na}^{+}(\mathrm{aq})^{+\mathrm{e}^{-}} \longrightarrow \mathrm{Na}_{(\mathrm{s})} ; \mathrm{E}^{\mathrm{O}} \text { cell }=-2.71 \mathrm{~V}$
(ii) $2 \mathrm{H}_{2} \mathrm{O}_{(\mathrm{f}} \longrightarrow \mathrm{O}_{(\mathrm{g})}+4 \mathrm{H}^{+}{ }_{(\mathrm{aq})}+4 \mathrm{e}^{-} ; \mathrm{E}^{\mathrm{o}} \text { cell=1.23V }$
(iii) $\mathrm{H}_{(\mathrm{aq})}^{+}+\mathrm{e}^{-} \longrightarrow \frac{1}{2} \mathrm{H}_{2(\mathrm{~g})} ; \mathrm{E}_{\text {cell }}^{\mathrm{o}}=0.00 \mathrm{~V}$
(iv) $\mathrm{Cl}_{(\mathrm{aq})}^{-} \longrightarrow \frac{1}{2} \mathrm{Cl}_{2(\mathrm{~g})}+\mathrm{e}^{-} ; \mathrm{E}_{\text {cell }}^{\mathrm{o}}=1.36 \mathrm{~V}$
Ans:The electrolysis of aqueous sodium chloride is as follows
${\text{NaCl}} \to {\text{N}}{{\text{a}}^ + } + {\text{C}}{{\text{l}}^ - }$
${{\text{H}}_{\text{2}}}{\text{O}} \to {{\text{H}}^ + } + {\text{O}}{{\text{H}}^ - }$
At the cathode, the reaction goes this way,
${{\text{H}}_{\text{2}}}{\text{O}} + {e^ - } \to \frac{1}{2}{{\text{H}}_{\text{2}}} + {\text{O}}{{\text{H}}^ - }$
At the anode, the reaction goes this way,
${\text{C}}{{\text{l}}^ - }(aq) \to \frac{1}{2}{\text{C}}{{\text{l}}_{\text{2}}}(g) + {e^ - };{E^0}_{cell} = 1.36{\text{V}}$
$2{{\text{H}}_{\text{2}}}{\text{O}}(l) \to {{\text{O}}_{\text{2}}}(g) + {\text{4}}{{\text{H}}^ + }(aq) + 4{e^ - };{E^0}_{cell} = 1.23{\text{V}}$
At the anode, the reaction with a lower ${E^0}$ value will be preferred and oxidation of oxygen is a slow process and requires high voltage so the first reaction will take place.
Correct Option: (iv)
18. The positive value of the standard electrode potential of $C{u^{2 + }}/Cu$ indicates that ____________.
(i) this redox couple is a stronger reducing agent than the ${H^ + }/{H_2}$ couple.
(ii) this redox couple is a stronger oxidizing agent than ${H^ + }/{H_2}$
(iii) $Cu$can displace ${H_2}$ from acid.
(iv) $Cu$cannot displace ${H_2}$ from acid
Ans: The lesser the ${E^0}$value of the redox couple higher is the reducing power
For ${\text{C}}{{\text{u}}^{2 + }} + 2{e^ - } \to {\text{Cu}}$; ${E^0} = 0.34{\text{V}}$
For ${\text{2}}{{\text{H}}^ + } + 2{e^ - } \to {{\text{H}}_{\text{2}}}$;${E^0} = 0.00{\text{V}}$
Since the second redox couple has less standard reduction potential than the first so it can be concluded that the redox couple is a stronger oxidizing agent than ${{\text{H}}^ + }/{{\text{H}}_{\text{2}}}$ and copper cannot displace ${{\text{H}}_{\text{2}}}$ from acid.
Correct Option: (ii, iv)
19.${E^0}_{cell}$for some half-cell reactions are given below. On the basis of these mark the correct answer
(a) ;$E_{cell}^0 = 0.00V$
(b) ;$E_{cell}^0 = 1.23V$
(c) ;$E_{cell}^0 = 1.96V$
(i) In dilute sulphuric acid solution, hydrogen will be reduced at the cathode.
(ii) In concentrated sulphuric acid solution, water will be oxidized at the anode.
(iii) In dilute sulphuric acid solution, water will be oxidized at the anode.
(iv) In dilute sulphuric acid solution, ${\text{SO}}_4^{2 - }$ ion will be oxidized to tetrathionate ion at the anode
Ans: In the electrolysis of sulphuric acid, the following reactions occur
$2{\text{SO}}_4^{2 - }(aq) \to {{\text{S}}_{\text{2}}}{\text{O}}_8^{2 - }(aq) + 2{e^ - }$; \[E_{cell}^0 = 1.96{\text{V}}\]
$2{{\text{H}}_{\text{2}}}{\text{O}}(l) \to {{\text{O}}_{\text{2}}}(g) + 4{{\text{H}}^ + }(aq) + 4{e^ - }$;$E_{cell}^0 = 1.23{\text{V}}$
The reaction will lower the value of ${E^0}_{cell}$ is preferred at anode so the second reaction is feasible.
${{\text{H}}^ + } + {e^ - } \to \frac{1}{2}{{\text{H}}_{\text{2}}}$; $E_{cell}^0 = 0.00{\text{V}}$
At the cathode, reduction of water occurs. Therefore, in dilute sulphuric acid solution, hydrogen will be reduced at the cathode.
Correct Option: Option (i, iii)
20. $E_{cell}^0 = 1.1V$for Daniel's cell. Which of the following expressions are the correct description of the state of equilibrium in this cell?
(i)$1.1 = {K_c}$
(ii)$\frac{{2.303RT}}{{2F}}log{K_{_C}} = 1.1$
(iii)$log{K_C} = \frac{{2.2}}{{0.059}}$
(iv)$log{K_C} = 1.1V$
Ans: At equilibrium,$\Delta {G^0} = - 2.303RT\log {K_C}$
$ - nF{E^0} = - 2.303RT\log {K_C}$
${E^0} = \frac{{2.303RT}}{{nF}}\log {K_C}$
For Daniel cell,$n = 2$
${E^0} = \frac{{2.303RT}}{{2F}}\log {K_C}$
At equilibrium ${E^0} = 1.1{\text{V}}$
$1.1{\text{V}} = \frac{{2.303RT}}{{2F}}\log {K_C}$
$\log {K_C} = \frac{{2.2}}{{0.059}}$
(i) Since $\log {K_C} = \frac{{2.2}}{{0.059}}$ this option is incorrect
(ii) As derived $1.1{\text{V}} = \frac{{2.303RT}}{{2F}}\log {K_C}$ so this option is correct
(iii) As derived $\log {K_C} = \frac{{2.2}}{{0.059}}$ so this option is correct
(iv) As $\log {K_C} = \frac{{2.2}}{{0.059}}$ so this option is incorrect
Correct Option: Option (ii, iii)
21. Conductivity of an electrolytic solution depends on ____________.
(i) nature of electrolyte.
(ii) concentration of electrolyte.
(iii) power of AC source.
(iv) distance between the electrode
Ans: Conductivity is due to the movement of ions in the solution. The conductivity of ions depends on the following factors:
(i) nature of electrolyte added
(ii) size of ion produced
(iii) concentration of electrolyte
(iv) nature of the solvent
(v) temperature
Distance between electrodes does not affect the conductivity of an electrolytic solution.
Correct Option: Option (i, ii)
22. ${\Lambda ^0}_{m({H_2}O)}$is equal to _____
(i)\[{\Lambda ^0}_{m(HCl)} + {\Lambda ^0}_{m(NaOH)} - {\Lambda ^0}_{m(NaCl)}\]
(ii)\[{\Lambda ^0}_{m(HN{O_3})} + {\Lambda ^0}_{m(NaN{O_3})} - {\Lambda ^0}_{m(NaOH)}\]
(iii) \[{\Lambda ^0}_{m(HN{O_3})} + {\Lambda ^0}_{m(NaOH)} - {\Lambda ^0}_{m(NaN{O_3})}\]
(iv)\[{\Lambda ^0}_{m(N{H_4}OH)} + {\Lambda ^0}_{m(HCl)} - {\Lambda ^0}_{m(N{H_4}Cl)}\]
Ans: Kohlrausch law of states that limiting molar conductivity of any salt species is equal to the sum of the limiting molar conductivity of cations and anions of the electrolyte
${\Lambda ^0}_{m({H_2}O)}$$ = $${\Lambda ^0}_{m(HCl)} + {\Lambda ^0}_{m(NaOH)} - {\Lambda ^0}_{m(NaCl)}$
${\Lambda ^0}_{m({H^ + })} + {\Lambda ^0}_{m(O{H^ - })} = {\Lambda ^0}_{m({H^ + })} + {\Lambda ^0}_{m(C{l^ - })} + {\Lambda ^0}_{m(N{a^ + })} + {\Lambda ^0}_{m(OH)} - {\Lambda ^0}_{m(N{a^ + })} - {\Lambda ^0}_{m(C{l^ - })}$
${\Lambda ^0}_{m({H_2}O)}$$ = $${\Lambda ^0}_{m(HN{O_3})} + {\Lambda ^0}_{m(NaOH)} - {\Lambda ^0}_{m(NaN{O_3})}$
${\Lambda ^0}_{m({H^ + })} + {\Lambda ^0}_{m(O{H^ - })} = {\Lambda ^0}_{m({H^ + })} + {\Lambda ^0}_{m(N{O_3}^ - )} + {\Lambda ^0}_{m(N{a^ + })} + {\Lambda ^0}_{m(O{H^ - })} - {\Lambda ^0}_{m(N{a^ + })} - {\Lambda ^0}_{m(N{O_3}^ - )}$
Correct Option: Option (i, iii)
23. What will happen during the electrolysis of an aqueous solution of $CuS{O_4}$by using platinum electrodes?
(i) Copper will deposit at the cathode.
(ii) Copper will deposit at the anode.
(iii) Oxygen will be released at anode.
(iv) Copper will dissolve at the anode
Ans: Electrolysis of copper sulfate solution is as follows
${\text{CuS}}{{\text{O}}_{\text{4}}} \rightleftharpoons {\text{C}}{{\text{u}}^{2 + }} + {\text{SO}}_4^{2 - }$
${{\text{H}}_{\text{2}}}{\text{O}} \rightleftharpoons {{\text{H}}^ + } + {\text{O}}{{\text{H}}^ - }$
At the cathode, the reaction goes this way,
${\text{C}}{{\text{u}}^{2 + }} + 2{e^ - } \to {\text{Cu}}$; $E_{cell}^0 = 0.34{\text{V}}$
${{\text{H}}^ + } + {e^ - } \to \frac{1}{2}{{\text{H}}_{\text{2}}}$; $E_{cell}^0 = 0.00{\text{V}}$
At the anode, the reaction goes this way,
$2{\text{SO}}_4^{2 - } - 2{e^ - } \to {{\text{S}}_{\text{2}}}{\text{O}}_8^{2 - }$; $E_{cell}^0 = 1.96{\text{V}}$
$2{{\text{H}}_{\text{2}}}{\text{O}} \to {{\text{O}}_{\text{2}}} + 4{{\text{H}}^ + } + 4{e^ - }$; $E_{cell}^0 = 1.23{\text{V}}$
The reaction will lower the value of ${E^0}_{cell}$ is preferred at anode so the second reaction is feasible.
Correct Option: Option (i, iii)
24. What will happen during the electrolysis of an aqueous solution of $CuS{O_4}$ in the presence of ${\text{Cu}}$electrodes?
(i) Copper will deposit at the cathode.
(ii) Copper will dissolve at the anode.
(iii) Oxygen will be released at anode.
(iv) Copper will deposit at the anode
Ans: In the electrolysis process of ${\text{CuS}}{{\text{O}}_{\text{4}}}$ the following reactions occur at the half cell which is as follows:
At the cathode, the reaction goes this way,
${\text{C}}{{\text{u}}^{2 + }} + 2{e^ - } \to {\text{Cu}}(s)$
At the anode, the reaction goes this way,
${\text{Cu}}(s) \to {\text{C}}{{\text{u}}^{2 + }} + 2{e^ - }$
Here copper will be deposited at the cathode and copper will dissolve at the anode.
Correct Option: Option (i, ii)
25. Conductivity $\kappa $ , is equal to ____________.
(i) $\frac{1}{R}\frac{l}{A}$
(ii) $\frac{{{G^*}}}{R}$
(iii) ${\Lambda _m}$
(iv)$\frac{l}{A}$
Ans: Conductance is the reciprocal of resistance and conductivity is the reciprocal of resistivity. It can be written as follows
$\kappa = \frac{1}{\rho }$
$R = \rho \frac{l}{A}$
$\rho = \frac{{RA}}{l}$
$\kappa = \frac{1}{{\frac{{RA}}{l}}}$
$\kappa = \frac{1}{R}\frac{l}{A}$
$\kappa = \frac{1}{R}{G^*}$
$\kappa = \frac{{{G^*}}}{R}$
${G^*}$is cell constant
Correct Option: Option (i, ii)
26. Molar conductivity of ionic solution depends on ___________.
(i) temperature.
(ii) distance between electrodes.
(iii) concentration of electrolytes in solution.
(iv) surface area of electrodes.
Ans: The conductivity in an ionic solution is due to the ions in the solution. It depends on the following factors;
(a)Temperature: On an increase in temperature, the molar conductivity of ionic solutions increases.
(b)Concentration of Electrolytes: An increase in the concentration of electrolytes decreases the molar conductivity as the number of ions per unit volume decreases.
Therefore, options (i,iii) are correct
Correct Option: Option (i, iii)
27.For the given cell, $Mg|M{g^{2 + }}||C{u^{2 + }}|Cu$
(i)$Mg$is cathode
(ii)$Cu$is anode
(iii)The cell reaction is
(iv) ${\text{Cu}}$is an oxidizing agent
Ans:In the given representation of the cell, the left side represents the oxidation half cell and the right side of the cell represents the reduction half cell
The electrode at which oxidation occurs is called anode and oxidation of magnesium occurs at anode so magnesium is the anode.
Similarly, the electrode at which the reduction occurs is called the cathode, and the reduction of copper occurs at the cathode.
The cell reaction is as follows
${\text{Mg}} + {\text{C}}{{\text{u}}^{2 + }} \to {\text{M}}{{\text{g}}^{2 + }} + {\text{Cu}}$
Correct Option: Option (ii, iii)
28. Can the absolute electrode potential of an electrode be measured?
Ans:No, the difference in potentials of the electrodes is measured. A reference electrode is to be taken while measuring the electrode potential of the electrode.
29.Can ${E^0}_{cell}$or ${\Delta _r}{G^0}$for cell reactions to ever be equal to zero?
Ans: ${E^0}_{cell}$ can never be zero. For a feasible reaction ${E^0}_{cell}$ should be positive or ${\Delta _r}{G^0}$ should be negative and at the stage of equilibrium, both of these parameters are zero.
30. Under what condition is ${E_{cell}} = 0$or ${\Delta _r}G = 0$ ?
Ans: At the stage of equilibrium ${E_{cell}} = 0$. So according to the relation,
${\Delta _r}G = - nF{E_{cell}}$
${\Delta _r}G = - n \times F \times 0$
${\Delta _r}G = 0$
31. What does the negative sign in the expression ${E^0}_{Z{n^{2 + }}/Zn} = - 0.76V$mean?
Ans:${E^0}_{{\text{Z}}{{\text{n}}^{2 + }}/{\text{Zn}}} = - 0.76{\text{V}}$ infers that the reducing ability of zinc is greater than that of hydrogen. The greater the negative value of the reduction potential, the greater is its reactivity.
32. Aqueous copper sulfate solution and aqueous silver nitrate solution are electrolyzed by 1 ampere current for 10 minutes in separate electrolytic cells. Will the mass of copper and silver deposited on the cathode be the same or different? Explain your answer
Ans: According to Faraday’s second law of electrolysis, the amount of different substances liberated keeping the electricity flow same through the electrolytic solution is directly proportional to the equivalent weight.
$\frac{{{w_1}}}{{{w_2}}} = \frac{{{E_1}}}{{{E_2}}}$
${E_1}$and${E_2}$ have different values. Therefore, the mass of copper and silver deposited will be different
33. Depict the galvanic cell in which the cell reaction is
Ans: Galvanic cell consists of two electrodes in which anode and cathode are present as electrodes. The anode is present on the left side at which oxidation occurs. The cathode is present on the right side at which reduction occurs and in the middle, there is a salt bridge which is depicted by parallel lines. Therefore, the galvanic cell is ${\text{Cu|C}}{{\text{u}}^{{\text{2 + }}}}{\text{||A}}{{\text{g}}^{\text{ + }}}{\text{|Ag}}$.
34. Value of standard electrode potential for the oxidation of $C{l^ - }$ ions is more positive than that of water, even then in the electrolysis of aqueous sodium chloride, why is $C{l^ - }$oxidized at anode instead of water?
Ans: In the electrolysis process of sodium chloride, oxidation of water at anode requires more potential. So ${\text{C}}{{\text{l}}^ - }$ is oxidized at anode instead of water.
35. What is electrode potential?
Ans: The potential difference between the metal and its solution is termed electrode potential.
36. Consider the following diagram in which an electrochemical cell is coupled to an electrolytic cell. What will be the polarity of electrodes ‘A’ and ‘B’ in the electrolytic cell?
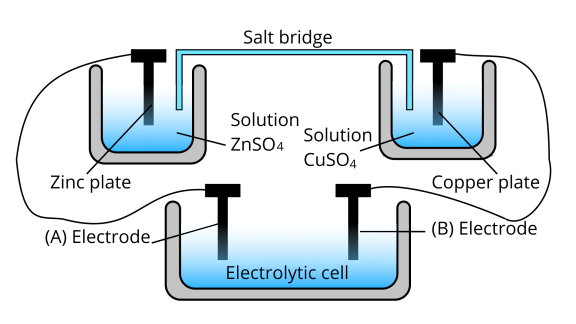
Ans: The cell represented above shows an electrochemical cell in which two different electrodes are present and the cell at the bottom represents the electrolytic cell.
In this zinc is losing electrons moving towards electrode A and copper is accepting an electron from electrode B. Therefore, the polarity of electrode A is positive and electrode B is negative.
37. Why is alternating current used for measuring the resistance of an electrolytic solution?
Ans: Alternating current is used to stop electrolysis so that the concentration of the ions in the solution remains constant.
38. A galvanic cell has an electrical potential of\[1.1V\]. If an opposing potential of \[1.1V\] is applied to this cell, what will happen to the cell reaction and current flowing through the cell?
Ans: When the opposing potential becomes equal to the electrical potential there is no current flowing in the cell and the cell reaction stops and there is no chemical reaction in the cell
39. How will the pH of brine ($aq.NaCl$ solution) be affected when it is electrolyzed?
Ans: Electrolysis of brine solution is as follows
${\text{NaCl}}(aq) \to {\text{N}}{{\text{a}}^ + }(aq) + {\text{C}}{{\text{l}}^ - }(aq)$
At the cathode, the reaction goes this way,
${{\text{H}}_{\text{2}}}{\text{O}}(l) + {e^ - } \to \frac{1}{2}{{\text{H}}_{\text{2}}}(g) + {\text{O}}{{\text{H}}^ - }(aq)$
At the anode, the reaction goes this way,
${\text{C}}{{\text{l}}^ - }(aq) \to \frac{1}{2}{\text{C}}{{\text{l}}_{\text{2}}}(g) + {e^ - }$
The pH of the solution will increase as sodium hydroxide is being formed.
40. Unlike dry cells, the mercury cell has a constant cell potential throughout its useful life. Why?
Ans: The number of ions present in the cell decides the life-time of any cell. In mercury cells, ions are not involved so the mercury cell has a constant cell potential throughout its useful life.
41. Solutions of two electrolytes ‘A’ and ‘B’ are diluted. The ${\Lambda _m}$of ‘B’ increases 1.5 times while that of A increases 25 times. Which of the two is a strong electrolyte? Justify your answer.
Ans: Stronger electrolytes undergo cent percent dissociation. Electrolyte B will be the stronger electrolyte since the concentration of ions remains the same upon dilution.
42. When acidulated water (dil.${H_2}S{O_4}$solution) is electrolyzed, will the pH of the solution be affected? Justify your answer.
Ans: pH of the substance depends on the concentration of hydronium ions present. Here the concentration of hydronium ions remains constant so pH remains constant.
The electrolysis reaction is as follows;
At anode: $2{{\text{H}}_{\text{2}}}{\text{O}}(l) \to {{\text{O}}_{\text{2}}}(g) + 4{{\text{H}}^ + } + 4{e^ - }$
At cathode:$4{{\text{H}}^ + } + 4{e^ - } \to 2{{\text{H}}_{\text{2}}}$
43. In an aqueous solution how does the specific conductivity of electrolytes change with the addition of water?
Ans: Conductivity due to the total number of ions present in the solution is termed as specific conductivity. An increase in the number of ions per unit volume increases. In addition to water, the number of ions per unit volume decreases therefore conductivity decreases.
44. Which reference electrode is used to measure the electrode potential of other electrodes?
Ans: Standard hydrogen electrode(SHE) is used as a reference electrode and its electrode potential is assumed to be zero. The electrode potential of other electrodes is measured concerning standard hydrogen electrodes.
45. Consider a cell given below
$Cu|C{u^{2 + }}||C{l^ - }|C{l_2},Pt$
Write the reactions that occur at anode and cathode
Ans: The given cell reaction consists of two half cell reactions represented below as follows;
At anode: ${\text{Cu}} \to {\text{C}}{{\text{u}}^{2 + }} + 2{e^ - }$
At cathode: ${\text{C}}{{\text{l}}_{\text{2}}} + 2{e^ - } \to 2{\text{C}}{{\text{l}}^ - }$
Here oxidation of copper and reduction of chlorine is taking place
46. Write the Nernst equation for the cell reaction in the Daniel cell. How will the${E_{cell}}$ be affected when the concentration of $Z{n^{2 + }}$ ions are increased?
Ans: The reactions taking place in the Daniel cell is as follows;
${\text{Zn}} + {\text{C}}{{\text{u}}^{2 + }} \to {\text{Z}}{{\text{n}}^{2 + }} + {\text{Cu}}$
The number of electrons involved in two. The Nernst equation is as follows;
${E_{cell}} = {E^0}_{cell} - \frac{{0.059}}{2}\log \frac{{[{\text{Z}}{{\text{n}}^{2 + }}]}}{{[{\text{C}}{{\text{u}}^{2 + }}]}}$
From the above equation, we conclude that increasing the concentration of ${\text{Z}}{{\text{n}}^{2 + }}$,${E_{cell}}$ will decrease.
47. What advantage do the fuel cells have over primary and secondary batteries?
Ans: Primary batteries contain a limited amount of reactants and once consumed are discharged. Secondary batteries take a long time to recharge. Fuel cells run continuously as long as reactants are supplied to them and products are consumed
48. Write the cell reaction of a lead storage battery when it is discharged. How does the density of the electrolyte change when the battery is discharged?
Ans: The cell reaction of a lead storage battery is as follows;
${\text{Pb}} + {\text{Pb}}{{\text{O}}_{\text{2}}} + {\text{2}}{{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}} \to 2{{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}} + 2{{\text{H}}_{\text{2}}}{\text{O}}$
The density of electrolytes changes because water is formed and sulphuric acid is consumed as a product during discharge of the battery
49. Why on dilution the ${\Lambda _m}$of$C{H_3}COOH$ increase drastically, while that of $C{H_3}COONa$ increases gradually?
Ans: Acetic acid is a weak electrolyte and the number of ions on dilution increases due to an increase in the degree of dissociation. In the case of strong electrolytes the number of ions remains the same but interionic attraction decreases.
50. Match the terms given in Column I with the units given in Column II.
Column I | Column II |
(i)${\Lambda _m}$ | (a)$Sc{m^{ - 1}}$ |
(ii)${E_{cell}}$ | (b)${m^{ - 1}}$ |
(iii) $\kappa $ | (c)$Sc{m^2}mo{l^{ - 1}}$ |
(iv)${G^*}$ | (d)${\text{V}}$ |
Ans: (i)The unit of ${\Lambda _m}$is ${\text{S}}{\text{c}}{{\text{m}}^2}{\text{mo}}{{\text{l}}^{ - 1}}$
(ii) The unit of ${E_{cell}}$ is ${{\text{m}}^{ - 1}}$
(iii) The unit of $\kappa $ is ${\text{S}}{\text{c}}{{\text{m}}^{ - 1}}$
(iv)The unit of ${G^*}$ is ${{\text{m}}^{ - 1}}$
Hence,the answer is:
(i)-c
(ii)-d
(iii)-a
(iv)-b
51. Match the terms given in Column I with the units given in Column II.
Column I | Column II |
(i) ${\Lambda _m}$ | (a)intensive property |
(ii)$E_{cell}^0$ | (b)depends on the number of ions/volume |
(iii) $\kappa $ | (c)extensive property |
(iv) ${\Delta _r}{G_{cell}}$ | increases with dilution |
Ans: (i)On dilution, the number of ions per unit volume increases so the molar conductivity increases with dilution.
(ii) $E_{cell}^0$ is an intensive property as it is not dependent on the amount or mass of the substance.
(iii) $\kappa $ is conductivity or be precise specific conductivity which depends on the number of ions
(iv) ${\Delta _r}{G_{cell}}$ is an extensive property as it depends on the amount of substance or number of particles in the solution.
Hence,the answer is:
(i)-d
(ii)-a
(iii)-b
(iv)-c
52. Match the terms given in Column I with the units given in Column II.
Column I | Column II |
(i)Lead storage battery | (a)Maximum efficiency |
(ii)Mercury cell | (b)prevented by galvanization |
(iii)Fuel cell | (c)gives steady potential |
(iv)Rusting | (d)${\text{Pb}}$is the anode,${\text{Pb}}{{\text{O}}_{\text{2}}}$is cathode |
Ans: (i) The cell reaction of a lead storage battery is as follows;
${\text{Pb}} + {\text{Pb}}{{\text{O}}_{\text{2}}} + 2{{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}} \to 2{{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}} + 2{{\text{H}}_{\text{2}}}{\text{O}}$
At cathode:${\text{Pb}}{{\text{O}}_{\text{2}}}(s) + {\text{SO}}_4^{2 - }(aq) + 2{e^ - } \to 2{\text{PbS}}{{\text{O}}_{\text{4}}}(s) + 2{{\text{H}}_{\text{2}}}{\text{O}}(l)$
At anode:${\text{Pb}}(s) + {\text{SO}}_4^{2 - }(aq) \to {\text{PbS}}{{\text{O}}_{\text{4}}}(s) + 2{e^ - }$
Therefore ${\text{Pb}}$ is the anode and ${\text{Pb}}{{\text{O}}_{\text{2}}}$ is cathode
(ii)Mercury cell doesn’t contain ions so it gives steady potential.
(iii)Fuel cells have maximum efficiency as they produce energy due to the combustion reaction of fuel.
(iv)Rusting is prevented by galvanization.
Hence,the answer is:
(i)-d
(ii)-c
(iii)-a
(iv)-b
53. Match the terms given in Column I with the units given in Column II.
Column I | Column II |
(i)$\kappa $ | (a)$I \times t$ |
(ii)${\Lambda _m}$ | (b)$\frac{{{\Lambda _m}}}{{\Lambda _m^0}}$ |
(iii)$\alpha $ | (c)$\frac{\kappa }{c}$ |
(iv)$Q$ | (d)$\frac{G}{R}$ |
Ans: (i)Conductivity $\kappa $ is defined as $\kappa = \frac{G}{R}$
(ii)Molar conductivity is given by ${\Lambda _m} = \frac{\kappa }{c}$
(iii)Degree of dissociation $\alpha $is given by $\alpha = \frac{{{\Lambda _m}}}{{\Lambda _m^0}}$
(iv)Charge $Q$ is the product of current and time $Q = I \times t$
Hence,the answer is:
(i)-d
(ii)-c
(iii)-b
(iv)-a
54. Match the items of column I and column II
Column I | Column II |
(i)Lechlanche cell | (a)cell reaction |
(ii)${\text{Ni}} - {\text{Cd}}$ | (b) does not involve any ion in solution and is used in hearing aids. |
(iii)Fuel cell | (c)rechargeable |
(iv)Mercury cell | (d)reaction at anode |
(e) converts energy of combustion into electrical energy |
Ans: (i)The reaction occurring at Lechlanche cells are
At anode: ${\text{Zn}} \to {\text{Z}}{{\text{n}}^{2 + }} + 2{e^ - }$
At cathode: ${\text{Mn}}{{\text{O}}_{\text{2}}} + {\text{N}}{{\text{H}}_{\text{4}}}^ + + {e^ - } \to {\text{MnO(OH)}} + {\text{N}}{{\text{H}}_{\text{3}}}$
(ii)${\text{Ni}} - {\text{Cd}}$is rechargeable. So it has a longer lifetime.
(iii) Energy in the fuel cell is due to the combustion process. It converts combustion energy into electrical energy $2{{\text{H}}_{\text{2}}} + {{\text{O}}_{\text{2}}} \to 2{{\text{H}}_{\text{2}}}{\text{O}}$
(iv) Mercury cells don’t involve any solution and are used in hearing aids.
Hence,the answer is:
(i)-d
(ii)-c
(iii)-a,e
(iv)-b
55. Match the items of Column I and Column II based on data given below:
Column I | Column II |
(i)${F_2}$ | (a)metal is the strongest reducing agent |
(ii)$Li$ | (b)metal ion which is the weakest oxidizing agent |
(iii)$A{u^{3 + }}$ | (c)non-metal which is the best oxidizing agent |
(iv)$B{r^ - }$ | (d)unreactive metal |
(v)$Au$ | (e)anion that can be oxidized by $A{u^{3 + }}$ |
(vi)$L{i^ + }$ | (f)anion which is the weakest reducing agent |
(vii)${F^ - }$ | (g)metal which is an oxidising agent |
${E^0}_{{F_2}/{F^ - }} = 2.87V$${E^0}_{L{i^ + }/Li} = - 3.5V$$E_{A{u^{3 + }}/Au}^0 = 1.4V$$E_{B{r_2}/B{r^ - }}^0 = 1.09V$
Ans: (i) ${{\text{F}}_{\text{2}}}$is a non-metal which is the best oxidizing agent since the standard reduction potential of ${{\text{F}}_{\text{2}}}$ is $2.87{\text{V}}$.
(ii)${\text{Li}}$is a metal and strongest reducing agent because the standard reduction potential of ${\text{Li}}$ is $ - 3.05{\text{V}}{\text{.}}$
(iii)${\text{A}}{{\text{u}}^{3 + }}$is a metal ion which is an oxidizing agent as standard reduction potential of ${\text{A}}{{\text{u}}^{3 + }}$ is $1.40{\text{V}}{\text{.}}$
(iv) ${\text{B}}{{\text{r}}^ - }$is an anion that can be oxidized by ${\text{A}}{{\text{u}}^{3 + }}$ as standard reduction potential of ${\text{A}}{{\text{u}}^{3 + }}$is $1.40{\text{V}}$ which is more than that of ${\text{B}}{{\text{r}}^ - }$ which is $E_{{\text{B}}{{\text{r}}_{\text{2}}}/{\text{B}}{{\text{r}}^ - }}^0 = 1.09{\text{V}}$.
(v)${\text{Au}}$is an unreactive metal
(vi)${\text{L}}{{\text{i}}^{\text{ + }}}$is a metal ion having the least value of standard reduction potential of $3.05{\text{V}}$. Hence it is the weakest oxidizing agent
(vii)${{\text{F}}^ - }$is an anion that is the weakest reducing agent as it has less oxidation potential.
Hence,the answer is:
(i)-c
(ii)-a
(iii)-g
(iv)-e
(v)-d
(vi)-b
(vii)-f
56. Assertion: $Cu$ is less reactive than hydrogen.
Reason:${E^0}_{C{u^{2 + }}/Cu}$ is negative.
Ans: (i)The electrode potential of ${\text{C}}{{\text{u}}^{2 + }}/{\text{Cu}}$ is negative. The electrode potential of $2{{\text{H}}^ + }/{{\text{H}}_{\text{2}}}$is $0.00{\text{V}}$. Therefore, the assertion is correct and the reason is incorrect and the reason is not a correct explanation of assertion. Therefore, this option is incorrect
(ii) ${E^0}_{{\text{C}}{{\text{u}}^{2 + }}/{\text{Cu}}}$ is not negative so the reason is incorrect. Therefore, this option is incorrect
(iii) The electrode potential of ${\text{C}}{{\text{u}}^{2 + }}/{\text{Cu}}$ is negative. The electrode potential of $2{{\text{H}}^ + }/{{\text{H}}_{\text{2}}}$ is $0.00V$. Therefore, the assertion is correct and the reason is incorrect. Therefore, this option is correct
(iv) Assertion is true and the reason is false therefore this option is incorrect
(v)Assertion is true therefore this option is incorrect.
Correct Option: (iii)
57. Assertion: ${E_{cell}}$ should have a positive value for the cell to function
Reason: ${E_{cathode}} < {E_{anode}}$
Ans: (i)A chemical reaction is spontaneous when the Gibbs free energy is negative. This is possible when ${E_{cell}}$ is positive. ${E_{cathode}} > {E_{anode}}$. Therefore, the assertion is correct and the reason is incorrect and the reason is not a correct explanation of assertion. Therefore, this option is incorrect
(ii) ${E_{cathode}} > {E_{anode}}$so the reason is incorrect. Therefore, this option is incorrect
(iii) A chemical reaction is spontaneous when the Gibbs free energy is negative. This is possible when ${E_{cell}}$ is positive. ${E_{cathode}} > {E_{anode}}$. Therefore, the assertion is correct and the reason is incorrect. Therefore, this option is correct
(iv) Assertion is true and the reason is false therefore this option is incorrect
(v) Assertion is true therefore this option is incorrect.
Correct Option: (iii)
58. Assertion: Conductivity of all electrolytes decreases on dilution
Reason: On dilution the number of ions per unit volume decreases.
Ans: (i) Conductivity is due to the movement of ions. The number of ions present per unit volume decides the conductivity. It decreases on dilution. The number of ions per unit volume decreases on dilution. So, the assertion is correct, the reason is correct and the reason is the correct explanation of the assertion. Therefore, this option is correct
(ii) Assertion is correct, the reason is correct and the reason is the correct explanation of assertion. Therefore, this option is incorrect
(iii) Assertion and true both are correct therefore this option is incorrect
(iv) Assertion is true and the reason is false therefore this option is incorrect
(v) Assertion is true therefore this option is incorrect.
Correct Option: (i)
59. Assertion:${\Lambda _m}$ Weak electrolytes show a sharp increase when the electrolytic solution is diluted.
Reason: For weak electrolytes degree of dissociation increases with the dilution of solution
Ans: (i) Molar conductivity of weak electrolytes increases on dilution. In addition to solution the degree of dissociation increases leading to an increase in the number of ions produced. Therefore, ${\Lambda _m}$ shows a sharp increase.
Therefore, the assertion is correct, the reason is correct and the reason is a correct explanation for assertion.
(ii) Assertion is correct, the reason is correct and the reason is the correct explanation of assertion. Therefore, this option is incorrect
(iii)Assertion and true both are correct therefore this option is incorrect
(iv) Assertion is true and the reason is false therefore this option is incorrect
(v)Assertion is true therefore this option is incorrect.
Correct Option: (i)
60. Assertion: Mercury cell does not give steady potential.
Reason: In the cell reaction, ions are not involved in the solution.
Ans: (i)Mercury cells give a steady potential. The reason is that the ions are not involved in this reaction. The assertion is incorrect and the reason is correct so this option is incorrect
(ii)Assertion is correct and the reason is incorrect so this option is incorrect
(iii)Assertion and true both are correct therefore this option is incorrect
(iv) Assertion is true and the reason is false therefore this option is incorrect
(v) Mercury cells give steady potential. The reason is that the ions are not involved in this reaction. The assertion is incorrect and the reason is correct so this option is correct
Correct Option: (i)
61. Assertion: Electrolysis of NaCl solution gives chlorine at anode instead of ${O_2}$ .
Reason: Formation of oxygen at anode requires overvoltage
Ans: (i)Electrolysis of sodium chloride is represented by the following equations
${{\text{H}}^ + }(aq) + {e^ - } \to \frac{1}{2}{{\text{H}}_{\text{2}}}(g)$
At the anode, the reaction goes this way,
${\text{C}}{{\text{l}}^ - }(aq) \to \frac{1}{2}{\text{C}}{{\text{l}}_{\text{2}}}(g) + {e^ - }$; $E_{cell}^0 = 1.36{\text{V}}$
$2{{\text{H}}_{\text{2}}}{\text{O}}(aq) \to {{\text{O}}_{\text{2}}}(g) + 4{{\text{H}}^ + }(aq) + 4{e^ - }$; $E_{cell}^0 = 1.23{\text{V}}$
The reaction with the lower value $E_{cell}^0$ is preferred but the formation of oxygen requires overpotential
Therefore, the assertion is correct, the reason is correct and the reason is the correct explanation for assertion. Therefore, this option is correct
(ii) Assertion is correct, the reason is correct and the reason is the correct explanation for assertion. Therefore, this option is incorrect
(iii)Both assertion and reason are true so this is incorrect
(iv) Both assertion and reason are true so this is incorrect
(v) Both assertion and reason are true so this is incorrect
Correct Option: (i)
62. Assertion: For measuring the resistance of an ionic solution an AC source is used.
Reason: Concentration of ionic solution will change if DC source is used
Ans: (i) The resistance of an ionic solution is measured by alternating current. The concentration of DC changes with electrolysis. Therefore, the assertion is correct, the reason is correct and the reason is a correct explanation of the assertion.
(ii) Assertion is correct, the reason is correct and the reason is the correct explanation for assertion. Therefore, this option is incorrect
(iii)This option is incorrect since both assertion and reason are true
(iv) This option is incorrect since both assertion and reason are true
(v) This is incorrect since assertion and reason are true.
Correct Option: (i)
63. Assertion: Current stops flowing when ${E_{cell}} = 0$.
Reason: Equilibrium of the cell reaction is attained
Ans: (i)At the equilibrium stage ${E_{cell}} = 0$ and current stops flowing in the cell. Therefore, the assertion is correct, the reason is correct and the reason is the correct explanation for assertion. Therefore, this option is correct
(ii) Assertion is correct, the reason is correct and the reason is the correct explanation for assertion. Therefore, this option is incorrect
(iii) This option is incorrect since both assertion and reason are true
(iv) This option is incorrect since both assertion and reason are true
(v) This option is incorrect since both assertion and reason are true
Correct Option: (i)
64. Assertion: ${E_{A{g^ + }/Ag}}$ increases with increase in the concentration of $A{g^ + }$ ions.
Reason: ${E_{A{g^ + }/Ag}}$ has a positive value
Ans: (i)The Nernst equation can be written as follows
${E_{cell}} = {E^0}_{cell} - \frac{{0.059}}{1}\log \frac{1}{{[{\text{A}}{{\text{g}}^ + }]}}$
${E_{cell}} = {E^0}_{cell} + 0.059\log [{\text{A}}{{\text{g}}^ + }]$
Here we observe that ${E_{{\text{A}}{{\text{g}}^ + }/{\text{Ag}}}}$ increases with increase in the concentration of ${\text{A}}{{\text{g}}^ + }$ ions. It has a positive value.
Here both assertion and reason are correct .The reason is not a correct explanation of assertion. So this option is incorrect
(ii) The Nernst equation can be written as follows
${E_{cell}} = {E^0}_{cell} - \frac{{0.059}}{1}\log \frac{1}{{[A{g^ + }]}}$
${E_{cell}} = {E^0}_{cell} + 0.059\log [A{g^ + }]$
Here we observe that ${E_{{\text{A}}{{\text{g}}^ + }/{\text{Ag}}}}$ increases with an increase in the concentration of ${\text{A}}{{\text{g}}^ + }$ ions. It has a positive value.
Here both assertion and reason are correct. The correct explanation of assertion is not the reason given. So, this option is correct
(iii) This option is incorrect since both assertion and reason are true
(iv) This option is incorrect since both assertion and reason are true
(v) This option is incorrect since both assertion and reason are true
Correct Option: (ii)
65. Assertion: Copper sulfate can be stored in a zinc vessel.
Reason: Zinc is less reactive than copper
Ans: (i)Copper sulfate can’t be stored in zinc vessels due to less reactive nature than zinc due to the negative standard reduction value of zinc.
So both assertion and reason are false. Therefore, this option is incorrect.
(ii) Both assertion and reason are wrong so this is incorrect
(iv) Both assertion and reason are wrong so this is incorrect
(v) Both assertion and reason are wrong so this is incorrect
Correct Option: (iv)
66. Consider Fig. 3.2 and answer the following questions.
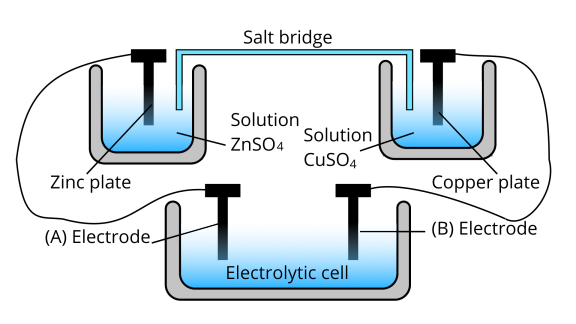
(i) Cell ‘A’ has ${E_{cell}} = 2V$ and Cell ‘B’ has ${E_{cell}} = 1.1V$which of the two cells ‘A’ or ‘B’ will act as an electrolytic cell. Which electrode reactions will occur in this cell?
Ans: Cell ‘B’ will act as an electrolytic cell because to less value of ${E_{cell}}$The reactions occurring in the cell are as follows;
At anode: ${\text{Z}}{{\text{n}}^{2 + }} + 2{e^ - } \to {\text{Zn}}$
At cathode: ${\text{Cu}}(s) \to {\text{C}}{{\text{u}}^{2 + }} + 2{e^ - }$
(ii) If cell ‘A’ has ${E_{cell}} = 0.5V$ and cell ‘B’ has ${E_{cell}} = 1.1V$Then what will be the reactions at anode and cathode?
Ans:Cell ‘B’ has a higher emf so it acts as a galvanic cell. The reactions are as follows;
At anode: ${\text{Zn}} \to {\text{Z}}{{\text{n}}^{2 + }} + 2{e^ - }$
At cathode: ${\text{C}}{{\text{u}}^{2 + }} + 2{e^ - } \to {\text{Cu}}$
67. Consider Fig. 3.2 and answer the questions (i) to (vi) given below.
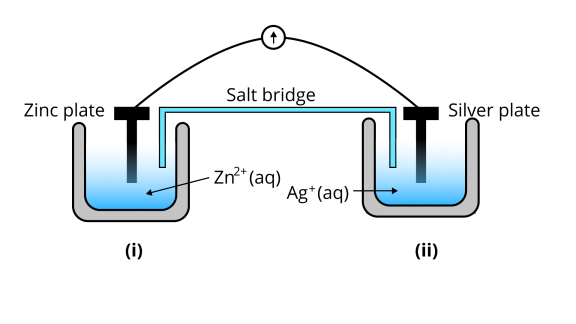
(i) Redraw the diagram to show the direction of electron flow.
Ans: The diagram is as follows;
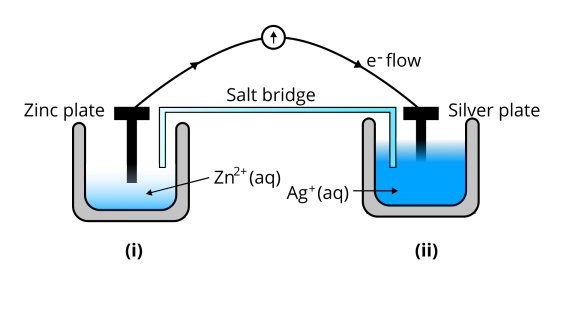
(ii)Is silver plate the anode or cathode?
Ans: ${\text{Ag}}$is cathode where the reduction process is taking place where ${\text{A}}{{\text{g}}^ + }$ takes electrons and deposits them at the cathode
(iii)What will happen if the salt bridge is removed?
Ans: Potential is zero when the salt bridge is suddenly removed.
(iv)When will the cell stop functioning?
Ans: Cell will stop functioning at discharging position when the cell potential is zero
(v)How will the concentration of $Z{n^{2 + }}$ions and $A{g^ + }$ions be affected when the cell functions?
Ans: The concentration of ${\text{Z}}{{\text{n}}^{2 + }}$ ions will increase and the concentration of ${\text{A}}{{\text{g}}^ + }$ ions will decrease due to conversion in oxidized and reduced forms.
(vi)How will the concentration of $Z{n^{2 + }}$ions and$A{g^ + }$ ions be affected after the cell becomes ‘dead?
Ans: When the cell is dead, the potential is zero and at equilibrium condition. Thus, the concentration of ${\text{Z}}{{\text{n}}^{2 + }}$ and ${\text{A}}{{\text{g}}^ + }$ will not change.
68. What is the relationship between Gibbs free energy of the cell reaction in a galvanic cell and the emf of the cell? When will the maximum work be obtained from a galvanic cell?
Ans: The relation between Gibbs free energy and the emf of the cell is as follows;
$\Delta G = - nF{E_{cell}}$
$E$is the cell potential
$E_{cell}^0$ is the standard emf of the cell
Maximum work obtained from the galvanic cell is $nF{E^0}$
Class 12 Chemistry Electrochemistry Vedantu’s NCERT Exemplar Solutions
In Chemistry Chapter 3 Electrochemistry, students learn about an Electrochemical cell, the galvanic and Electrolytic cells, application of the Nernst equation, emf of a galvanic cell, the standard potential of the cell, Gibbs energy of cell reaction and equilibrium constant, resistivity, conductivity, and molar conductivity of ionic solutions, the difference between ionic and Electronic conductivity, calculation of molar conductivity, define molar conductivity at zero concentration/infinite dilution, Kohlrausch law and its applications, quantitative aspects of Electrolysis, construction of some primary and secondary batteries, corrosion as an Electrochemical process.
The study of Electrochemistry is important for both theoretical and practical considerations. It is a very vast and interdisciplinary subject.
The NCERT solutions provided by Vedantu’s subject experts help students to understand all the concepts covered in Chemistry Chapter 3. The solutions are very extremely easy to grasp. This Chapter includes various crucial topics for students important for the board examinations and also for competitive exams like JEE Mains and NEET. Students are advised to solve the NCERT exemplar problems to score great marks in all Chemistry exams. NCERT exemplar problems should be solved thoroughly along with the previous year’s Chemistry question papers and sample papers in order to manage the time effectively in final exams.
FAQs on NCERT Exemplar for Class 12 Chemistry Chapter-3 (Book Solutions)
1. Do I need to practice all the questions provided in NCERT Exemplar for Class 12 Chemistry Chapter 3?
Solving NCERT exemplar problems for Class 12 is an effective way to score good marks in the final examinations. Students can get their doubts cleared and solving all the NCERT exemplar problems helps them in understanding the concepts better. It is beneficial to practice all the questions and refer to the solutions provided by Vedantu experts (as per CBSE guidelines). The solutions can be understood and used by the students easily. Class 12 students are strictly advised to study all the Chemistry Chapters thoroughly.
2. What are the important topics of Chapter 3 Electrochemistry?
Let's take a closer look at the important topics covered in NCERT Chapter 3 Chemistry:
Electrochemical Cells
Galvanic Cells
Measurement of Electrode potential
Nernst Equation
The equilibrium constant from Nernst Equation
Electrochemical cells and Gibbs energy
Conductance of Electrolytic solutions
Measurement of conductivity of Ionic solutions
Variation of conductivity with concentration
Electrolytic cells and Electrolysis
Products of Electrolysis
Batteries - Primary batteries and Secondary batteries
Fuel Cells
Corrosion
3. Where can I get the free PDF of NCERT Solutions of Class 12 Chemistry Chapter 3 - Electrochemistry?
Students can download the free PDF of NCERT Exemplar Solutions of Class 12 Chemistry Chapter 3 on Vedantu’s website which provides free PDF on all the topics of Chemistry. The free PDF by Vedantu contains all the NCERT Exemplar solutions for Chemistry Chapter 3 - Electrochemistry. The detailed explanations help students in revising all the concepts thoroughly. The solutions are designed by Chemistry experts that follow the latest CBSE guidelines. The solutions are very effective for Class 12 students in order to ace their Chemistry board exams.
4. How is Chapter 3 Electrochemistry important for Board Exams?
Chapter 3 plays a vital role in CBSE Class 12 Board Exams and carries important questions needed to score good marks in the final Chemistry examination. Students are encouraged to solve the NCERT exemplar problems as they help them in understanding all concepts covered in Chapter 3 properly. This Chapter also has some important derivations like the relation between the standard potential of the cell, Gibbs energy of cell reaction, and its equilibrium constant. Vedantu provides a comprehensive free PDF of NCERT solutions designed by the best subject experts that can be downloaded by students to learn, practice, and revise the Chemistry Chapters.
5. How should I practice the NCERT problems for Class 12 Chemistry Chapter - 3 Electrochemistry?
Vedantu’s NCERT exemplar solutions have been crafted by Chemistry experts to benefit Class 12 students. All the concepts are covered in accordance with the CBSE guidelines. Solutions are explained in an elaborative way by using very simple language and students are advised to revise the NCERT exemplar solutions regularly. The explanations provided for each answer help in boosting students’ confidence in order for them to nail the examination. After completing the NCERT textbook questions, students can refer to the solutions provided by Vedantu’s expert faculty and understand the method of answering each question. The solutions by Vedantu have detailed explanations and are designed from an exam point of view.

























