
Chemistry Notes for Chapter 5 Coordination Compounds Class 12 - FREE PDF Download
Vedantu provides Class 12 Chemistry Chapter 5 Coordination Compounds Notes, offering a clear and concise understanding of the topic. This chapter covers important concepts such as coordination numbers, ligands, types of coordination compounds, and their applications. The notes explain the bonding, structures, and nomenclature of these compounds in a simplified manner. These notes are ideal for students preparing for exams as they provide an easy-to-follow guide. Download the FREE PDF of these notes from Vedantu for thorough and efficient revision.
 Table of Content
Table of ContentClass 12 Chapter 5 Coordination Compounds notes let you quickly access and review the chapter content. For a comprehensive study experience, check out the Class 12 Chemistry Revision Notes FREE PDF here and refer to the CBSE Class 12 Chemistry syllabus for detailed coverage. Vedantu's notes offer a focused, student-friendly approach, setting them apart from other resources and providing you with the best tools for success.
Access Class 12 Chemistry Chapter 5 Coordination Compounds Notes
1. Introduction
Coordination compounds are extremely important. It is important to recognize that life would not have been possible without the presence of chlorophyll (Mg - complex) in plants and haemoglobin (Fe- complex) in human blood. The study of these compounds will broaden our understanding of chemical bonding and the physical properties of coordination compounds such as magnetic properties.
2. Molecular or Addition Compounds
When a solution containing two or more simple stable compounds in molecular proportions is allowed to evaporate, it produces crystals of new substances known as molecular or addition compounds.
Example:
${\text{KCl + MgC}}{{\text{l}}_{\text{2}}}{\text{ + 6}}{{\text{H}}_{\text{2}}}{\text{O }} \to {\text{ }}\mathop {{\text{KCl}}{\text{.MgC}}{{\text{l}}_{\text{2}}}.6{{\text{H}}_{\text{2}}}{\text{O}}}\limits_{\left( {{\text{Camallite}}} \right)}$
${\text{CuS}}{{\text{O}}_{\text{4}}}{\text{ + 4N}}{{\text{H}}_{\text{3}}}{\text{ }} \to {\text{ }}\mathop {\left[ {{\text{Cu}}{{\left( {{\text{N}}{{\text{H}}_3}} \right)}_4}} \right]{\text{S}}{{\text{O}}_4}}\limits_{\left( {{\text{Tetrammine copper }}\left( {{\text{II}}} \right){\text{ sulphate}}} \right)}$
2.1 Types of Molecular Compounds
2.1.1 Double Salt
A double salt is a substance formed by combining two different salts that crystallize as a single substance but ionize as two distinct salts when dissolved in water. These salts lose their identity in solution, which means that when dissolved in water, they test positive for all of the ions present in the salt. eg. Mohr's salt, potash alum.
Example:
$\mathop {{\text{FeS}}{{\text{O}}_{\text{4}}}{\text{.}}{{\left( {{\text{N}}{{\text{H}}_{\text{4}}}} \right)}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}{\text{.6}}{{\text{H}}_{\text{2}}}{\text{O }}}\limits_{{\text{Mohr's salt }}} \to {\text{ F}}{{\text{e}}^{{\text{2 + }}}}_{\left( {aq} \right)}{\text{ + 6}}{{\text{H}}_{\text{2}}}{\text{O + 2N}}{{\text{H}}_{\text{4}}}{^{\text{ + }}_{\left( {aq} \right)}}{\text{ + 2S}}{{\text{O}}_{\text{4}}}{^{{\text{2--}}}_{\left( {aq} \right)}}$
2.2 Coordination Compounds
A coordination compound is a molecular compound formed by the combination of two or more simple molecular compounds that retains its identity both solid and dissolved.
Example:
$\left[ {{\text{Cu}}{{\left( {{\text{N}}{{\text{H}}_{\text{3}}}} \right)}_{\text{4}}}} \right]{\text{S}}{{\text{O}}_{\text{4}}} \rightleftharpoons {\left[ {{\text{Cu}}{{\left( {{\text{N}}{{\text{H}}_{\text{3}}}} \right)}_{\text{4}}}} \right]^{2 + }}{\text{ + S}}{{\text{O}}_{\text{4}}}^{2 - }$
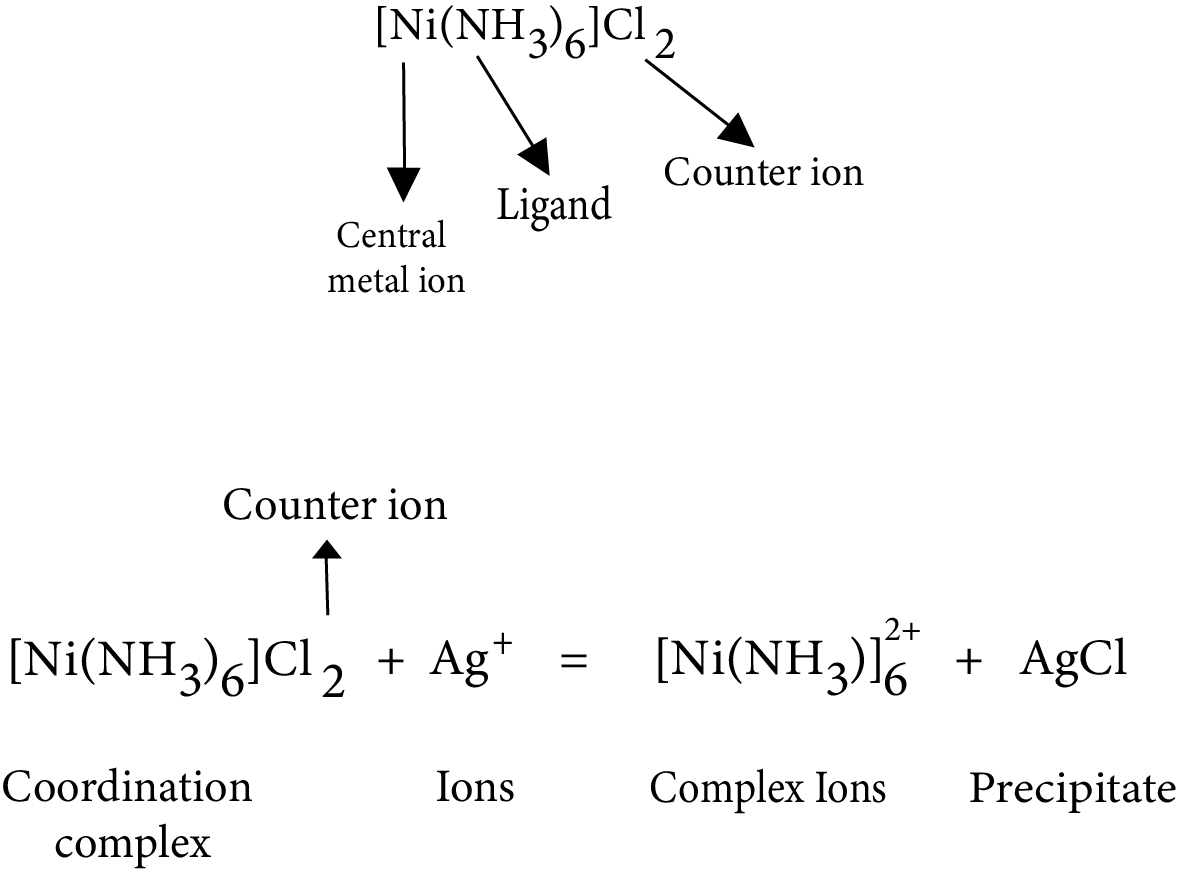
3. Coordination Compounds
A ligand, a central atom, a complex ion, a cation, or an anion make up a coordination compound. In general, the complex ion is written in a square box, and the ion (cation or anion) is written outside the complex ion.
eg:
$\left[ {{\text{Co}}{{\left( {{\text{N}}{{\text{H}}_{\text{3}}}} \right)}_{\text{6}}}{\text{ }}} \right]{\text{ C}}{{\text{l}}_{\text{3}}}{\text{ }}$
$\left[ {{\text{Complex ion}}} \right]{\text{ anion}}$
General Formula: ${{\text{A}}_{\text{x}}}\left[ {{\text{M}}{{\text{L}}_{\text{n}}}} \right]{\text{/}}\left[ {{\text{M}}{{\text{L}}_{\text{n}}}} \right]{{\text{B}}_{\text{y}}}$ where M is the central metal atom/ion, L is the ligand, A is the cation and B is the anion.
Some Important Terms Pertaining to Coordination Compounds
3.1 Coordination Entity
It is the fixed central metal atom or ion that is bonded to a specific number of ions or molecules. Six ammonia molecules, for example, are surrounded by three chloride ions in $\left[ {{\text{Co}}{{\left( {{\text{N}}{{\text{H}}_{\text{3}}}} \right)}_{\text{6}}}} \right]{\text{C}}{{\text{l}}_{\text{3}}}{\text{,}}$ a coordination entity.
3.2 Central Atom/Ion
In a specific geometrical arrangement, it is the central cation that is surrounded and coordinately bonded to one or more neutral molecules or negatively charged ions. In the complex $\left[ {{\text{Co}}{{\left( {{\text{N}}{{\text{H}}_{\text{3}}}} \right)}_{\text{6}}}} \right]{\text{C}}{{\text{l}}_{\text{3}}}{\text{,}}$ for example, ${\text{C}}{{\text{o}}^{{\text{3 + }}}}$ is the central metal ion that is positively charged and is coordinately bonded to six neutral NH3 molecules within the coordination sphere. The central metal/ion is also known as Lewis acid.
3.3 Ligands
Ligands are ions or molecules that are bound to the coordination entity's central atom/ion. These can be simple ions like ${\text{C}}{{\text{l}}^{\text{--}}}{\text{,}}$ small molecules like ${{\text{H}}_{\text{2}}}{\text{O or N}}{{\text{H}}_{\text{3}}}{\text{,}}$ or larger molecules like ${{\text{H}}_{\text{2}}}{\text{NC}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{N}}{{\text{H}}_{\text{2}}}{\text{.}}$
3.4 Coordination Number (C.N)
The number of atoms in the ligands that are directly bound to the central metal atom or ion by coordinate bonds is known as the metal atoms or ion's coordination number. It is also the same as secondary valency.
${{\text{Complex }}} and {{\text{ Coordination number}}}$
${{{\text{K}}_{\text{4}}}\left[ {{\text{Fe}}{{\left( {{\text{CN}}} \right)}_{\text{6 }}}} \right]} and {\text{ 6}}$
${{{\left[ {{\text{Ag}}{{\left( {{\text{CN}}} \right)}_{\text{2}}}} \right]}^ - }}{\text{2}}$
${\left[ {{\text{Pt}}{{\left( {{\text{N}}{{\text{H}}_{\text{3}}}} \right)}_{\text{2}}}{\text{C}}{{\text{l}}_{\text{2}}}} \right]}{\text{4}}$
${{{\left[ {{\text{Ca}}\left( {{\text{EDTA}}} \right)} \right]}^{2 - }}}{\text{6}}$
3.5 Coordination Sphere
A square bracket surrounds the central metal atom or ion and the ligands that are directly attached to it. This was known as the coordination sphere or the first sphere of attraction. Because the metal ion tightly holds the ligands in the coordination sphere, it behaves as a single unit.
Coordination sphere
3.6 Coordination Polyhedron
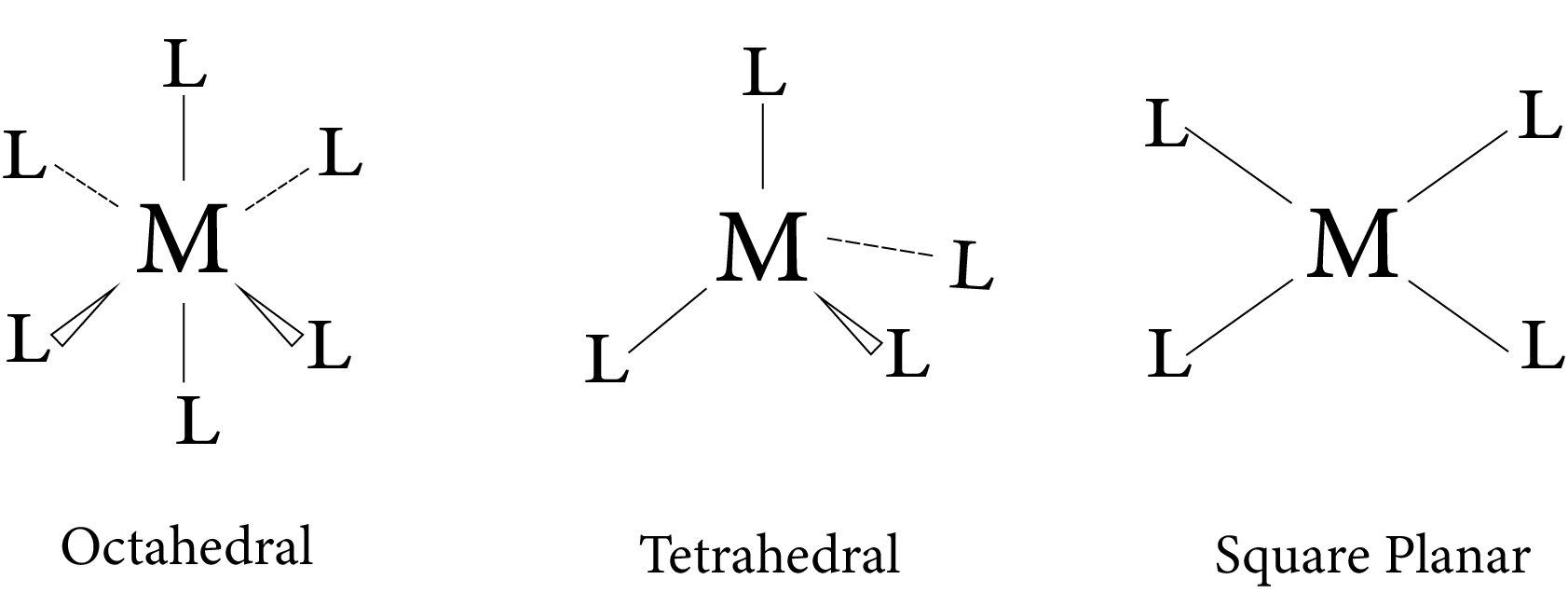
The spatial arrangement of the ligand atoms that are directly attached to the central atom/ion is referred to as a coordination polyhedron. ${\left[ {{\text{Co}}{{\left( {{\text{N}}{{\text{H}}_{\text{3}}}} \right)}_{\text{6}}}} \right]^{{\text{3 + }}}}{\text{,}}$ for example, is octahedral, $\left[ {{\text{Ni}}{{\left( {{\text{CO}}} \right)}_{\text{4}}}} \right]$ is tetrahedral, and ${\left[ {{\text{PtC}}{{\text{l}}_{\text{4}}}} \right]_{\text{2}}}$ is square planar.
3.7 Oxidation Number of Central Metal Atom
It is defined as the charge that the central metal ion would have if all ligands and electron pairs were removed. It is computed as follows:
Example:
${{\text{K}}_{\text{4}}}\left[ {{\text{Fe}}{{\left( {{\text{CN}}} \right)}_{\text{6}}}} \right] \to 4{{\text{K}}^ + } + {\left[ {{\text{Fe}}{{\left( {{\text{CN}}} \right)}_{\text{6}}}} \right]^{4 - }}$
Charge on the complex ion is -4.
Let charge on Fe be x.
Now, the charge on cyanide ions is -1.
$\Rightarrow x + 6 \times \left( { - 1} \right) = - 4$
$\Rightarrow x = + 2 $
Hence, the oxidation number of Fe is +2 (II).
3.8 Homoleptic and Heteroleptic Complexes
Homoleptic complexes are those in which the central atom is coordinated with only one type of ligand, such as ${\left[ {{\text{Co}}{{\left( {{\text{N}}{{\text{H}}_{\text{3}}}} \right)}_6}} \right]^{{\text{3 + }}}}{\text{.}}$ Hetroleptic complexes are those in which the central atom is coordinated with more than one type of ligand, such as ${\left[ {{\text{Co}}{{\left( {{\text{N}}{{\text{H}}_{\text{3}}}} \right)}_4}{\text{C}}{{\text{l}}_2}} \right]^{\text{ + }}}{\text{.}}$
4. Nomenclature of Coordination Compounds
4.1 Nomenclature
The Following Rules are Followed When Naming a Complex Ion:
Cations are named first, followed by anions.
The central metal ion's oxidation state (O.S.) is denoted by a Roman numeral.
The ligand names are listed first, followed by the name of the central metal ion.
Anion ligand names that end in 'ide' are changed to 'o', 'ite' are changed to 'ito' and 'ate' are changed to 'ato'
The unmodified name is used for many ligands that are molecules.
Positive groups are terminated by –ium. For example: $\mathop {\text{N}}\limits^{ \cdot \cdot } {{\text{H}}_2} - {\text{N}}{{\text{H}}_3}^ + $ hydrazinium.
When there are multiple ligands of the same type, the prefixes di, tri, tetra, penta, and hexa are used to indicate the number of ligands of that type. An exception occurs when the name of the ligand contains a number, as in ethylenediamine (en). To avoid confusion, bis, tris, and tetrakis are used instead of di, tri, and tetra, and the ligand name is enclosed in brackets. as in bis (ethylenediamine)
If anion is a complex, metal is followed by 'ate'.
${\left[ {{\text{Ni}}{{\left( {{\text{CN}}} \right)}_4}} \right]^{2 - }}$: tetracyanonickelate (II) ion
Lead – plumbate
Gold – aurate
Zinc – zincate
Tin – stannate
Silver – argentate
Cobalt – cobaltate
Iron – ferrate
Aluminium – aluminate
Manganese – manganate
Copper – cuprate
Chromium – chromate
Platinum – platinate
A complex is said to be polynuclear if it contains two or more metal atoms. The prefix – $\mu $ denotes the bridging ligands that connect the two metal atoms.
Ambidentate ligands can be connected via different atoms.–
${\text{M}} \leftarrow {\text{N}}{{\text{O}}_{\text{2}}}$
${\text{M}} \leftarrow {\text{ONO}}$
When writing (not naming) the complex formula:
Complex ion should be enclosed by square brackets and
Ligands are placed alphabetically after metal, but first negative ligands, then neutral, then positive.
5. Werner’s Theory
Werner explained the nature of bonding in complexes and came to the conclusion that the metal in complexes has two different types of valency.
5.1 Primary Valency
The oxidation state of the central metal atom or ion determines the primary valency. These are asymmetrical.
Example: What are the primary valency of ${{\text{K}}_4}\left[ {{\text{Fe}}{{\left( {{\text{CN}}} \right)}_{\text{6}}}} \right]{\text{ and }}\left[ {{\text{Cu}}{{\left( {{\text{N}}{{\text{H}}_{\text{3}}}} \right)}_{\text{4}}}} \right]{\text{S}}{{\text{O}}_{\text{4}}}$?
Sol.
The primary valency of ${{\text{K}}_4}\left[ {{\text{Fe}}{{\left( {{\text{CN}}} \right)}_{\text{6}}}} \right]{\text{ and }}\left[ {{\text{Cu}}{{\left( {{\text{N}}{{\text{H}}_{\text{3}}}} \right)}_{\text{4}}}} \right]{\text{S}}{{\text{O}}_{\text{4}}}$ is 2.
5.2 Secondary Valency
Secondary valency refers to the number of ligand atoms that are co-ordinated to the central metal atom. Because these are directional, a complex ion has a specific shape.
Example: What are the secondary valency of $\left[ {{\text{Co}}{{\left( {{\text{N}}{{\text{H}}_{\text{3}}}} \right)}_{\text{6}}}} \right]{\text{C}}{{\text{l}}_{\text{3}}}{\text{ and }}{{\text{K}}_{\text{4}}}\left[ {{\text{Fe}}{{\left( {{\text{CN}}} \right)}_{\text{6}}}} \right]$ ?
Sol. The secondary valency in $\left[ {{\text{Co}}{{\left( {{\text{N}}{{\text{H}}_{\text{3}}}} \right)}_{\text{6}}}} \right]{\text{C}}{{\text{l}}_{\text{3}}}$ is 6.
${{\text{K}}_{\text{4}}}\left[ {{\text{Fe}}{{\left( {{\text{CN}}} \right)}_{\text{6}}}} \right]$: six ligands are coordinated to Fe. As a result, the secondary valency is 6. Ions attached to complex ions satisfy the primary valency. It is represented by dotted lines. Ionisable valency is another name for primary valency. The ligands satisfy the secondary valency; they are non-ionisable and are represented by a solid line $\left[ {{\text{Co}}{{\left( {{\text{N}}{{\text{H}}_{\text{3}}}} \right)}_{\text{6}}}} \right]{\text{C}}{{\text{l}}_{\text{3}}}$
An anion found in the co-ordination and ionization sphere is represented by ……..
Every element is capable of satisfying both its primary and secondary valencies. When a negative ion is present in the coordination sphere, it exhibits dual behavior. It has the potential to satisfy both primary and secondary valencies.
The ligands that satisfy the secondary valencies are aimed at specific locations in space. The coordination number determines the geometry of the complex ion. If the metal has coordination number 6, the complex is octahedral, which means that six donor atoms of the ligands occupy six positions around the metal octahedrally. If, on the other hand, the coordination number is 4, the complex's geometry can be tetrahedral or square planar. This postulate predicted that different types of isomerism would exist in coordination compounds.
Three dimensional arrangement of ligand in octahedral, tetrahedral and square planar complex
Examples:
Octahedral
$(\mathrm{C} \cdot \mathrm{N}=6)$
${\left[\mathrm{Cr}\left(\mathrm{CH}_{3}\right)_{6}\right]^{3+}}$
${\left[\mathrm{Co}\left(\mathrm{NH}_{3}\right)_{6}\right]^{3+} ;\left[\mathrm{Cr}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right]^{3+}}$
${\left[\mathrm{Fe}(\mathrm{CN})_{6}\right]^{2-} ;\left[\mathrm{Fe}\left(\mathrm{F}_{6}\right)\right]^{3-}}$
${\left[\mathrm{Pt}\left(\mathrm{NH}_{3}\right)_{6}\right]^{4+} ;\left[\mathrm{PtCl}_{6}\right]^{2-}}$
Square planar
$($ C. $N=4)$
$\left[\mathrm{Ni}(\mathrm{CN})_{4}\right]^{2-}$
$\left[\mathrm{Ni}\left(\mathrm{NH}_{3}\right)_{4}\right]^{2+}$
$\left[\mathrm{Cu}\left(\mathrm{NH}_{3}\right)_{4}\right]^{2+}$
$\mathrm{X}=\mathrm{Cl}^{-}, \mathrm{Br}, \mathrm{I}^{-}$
Familiar C.N.’s of Some Common Metal Ions.
Univalent | C.N | Divalent | C.N. |
$\mathrm{Ag}^{+}$ | 2 | $\mathrm{V}^{2+}$ | 6 |
$\mathrm{Au}^{+}$ | 2, 4 | $\mathrm{Fe}^{2+}$ | 6 |
$\mathrm{Ti}^{+}$ | 2 | $\mathrm{Co}^{2+}$ | 4, 6 |
$\mathrm{Cu}^{+}$ | 2, 4 | $\mathrm{Ni}^{2+}$ | 4, 6 |
$\mathrm{Cu}^{2+}$ | 4, 6 | ||
$\mathrm{Zn}^{2+}$ | 4 | ||
$\mathrm{Pd}^{2+}$ | 4 | ||
$\mathrm{Pt}^{2+}$ | 4 | ||
$\mathrm{Ag}^{2+}$ | 4 |
Trivalent | C.N. | Tetravalent | C.N. |
$\mathrm{Sc}^{3+}$ | 6 | $\mathrm{Pt}^{4+}$ | 6 |
$\mathrm{Cr}^{3+}$ | 6 | $\mathrm{Pd}^{4+}$ | 6 |
$\mathrm{Fe}^{3+}$ | 6 | ||
$\mathrm{Co}^{3+}$ | 6 | ||
$\mathrm{Os}^{3+}$ | 6 | ||
$\mathrm{Ir}^{3+}$ | 6 | ||
$\mathrm{Au}^{3+}$ | 4 |
6. Effective Atomic Number (Ean)
Sidgwick proposed effective atomic number (EAN), which is defined as the number of electrons gained by the metal atom or ion after gaining electrons from the donor atoms of the ligands. In some cases, the effective atomic number (EAN) coincides with the atomic number of the next inert gas. The following relationship is used to calculate EAN:
EAN = Atomic number of the metal – number of electrons lost in ion formation + number of electrons gained from the donor atoms of the ligands. (2 × CN)
The EAN Values of Various Metals in Their Complexes Are Listed Below:
Complex | Metal (Oxidation state) | Atomic Number of Metal | Coordination Number | Effective Atomic Number |
${{\text{K}}_{\text{4}}}\left[ {{\text{Fe}}{{\left( {{\text{CN}}} \right)}_{\text{6}}}} \right]$ | +2 | 26 | 6 | $\left( {26 - 2} \right) + \left( {6 \times 2} \right) = 36$ |
$\left[ {{\text{Cu}}{{\left( {{\text{N}}{{\text{H}}_{\text{3}}}} \right)}_{\text{4}}}} \right]{\text{S}}{{\text{O}}_{\text{4}}}$ | +2 | 29 | 4 | $\left( {29 - 2} \right) + \left( {4 \times 2} \right) = 35$ |
$\left[ {{\text{Co}}{{\left( {{\text{N}}{{\text{H}}_{\text{3}}}} \right)}_{\text{6}}}} \right]{\text{C}}{{\text{l}}_{\text{4}}}$ | +3 | 27 | 6 | $\left( {27 - 3} \right) + \left( {6 \times 2} \right) = 36$ |
${\text{Ni}}{\left( {{\text{CO}}} \right)_{\text{4}}}$ | 0 | 28 | 4 | $\left( {28 - 0} \right) + \left( {4 \times 2} \right) = 36$ |
${{\text{K}}_{\text{2}}}\left[ {{\text{Ni}}{{\left( {{\text{CN}}} \right)}_{\text{4}}}} \right]$ | +2 | 28 | 4 | $\left( {28 - 2} \right) + \left( {4 \times 2} \right) = 34$ |
7. Valence Bond Theory
Valence Bond Theory (VBT) can explain the bonding in coordination compounds because the d orbitals of the majority of transition metal complexes are incomplete. Valence bond considers orbital hybridization because penultimate d-orbitals are close in energy to s and p-orbitals of the outermost shell, allowing for various types of hybridization.
The Following Assumption is Made by VBT:
The central metal ion has a number of empty orbitals that can accept electrons donated by the ligands. The coordination number of the metal ion for the specific complex is equal to the number of empty d-orbitals.
Strong bonds are formed when the metal orbitals and ligand orbitals overlap. The greater the extent of overlapping, the more stable the complex. Different orbitals (s, p, or d) hybridize to form a set of equivalent hybridized orbitals that participate in ligand bonding.
Each ligand contributes two electrons to the central metal ion/atom.
The inner orbitals contain non-bonding metal electrons that do not participate in chemical bonding.
A complex is paramagnetic if it contains unpaired electrons. The complex is diamagnetic if it does not contain an unpaired electron.
Under the influence of a strong ligand (CN, CO), electrons can be forced to pair up, thereby violating Hund's rule of multiplicity.
Common Types of hybridization
Coordination Number | Hybridization | Shape | Geometry |
2 | sp | Linear | X-A-X |
4 | sp³ | Tetrahedron | 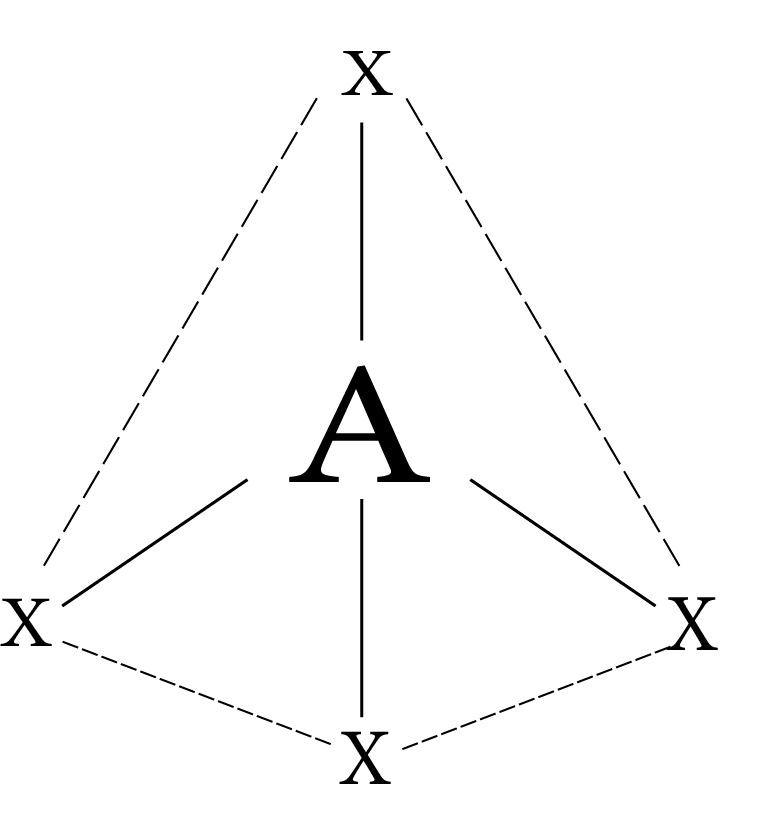
|
4 | dsp² | Square Planar | 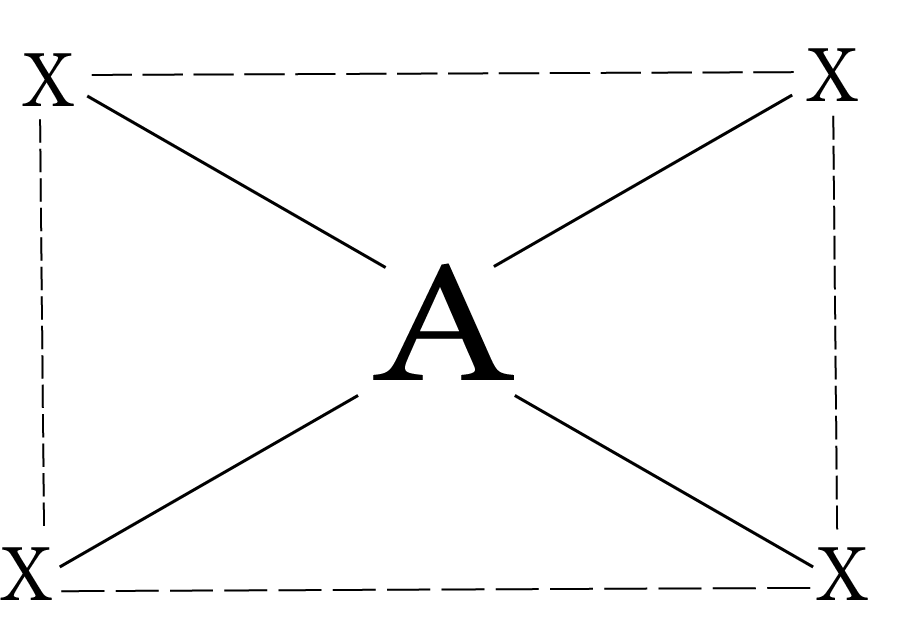
|
5 | sp³d or dsp³ | Trigonal Bipyramidal | 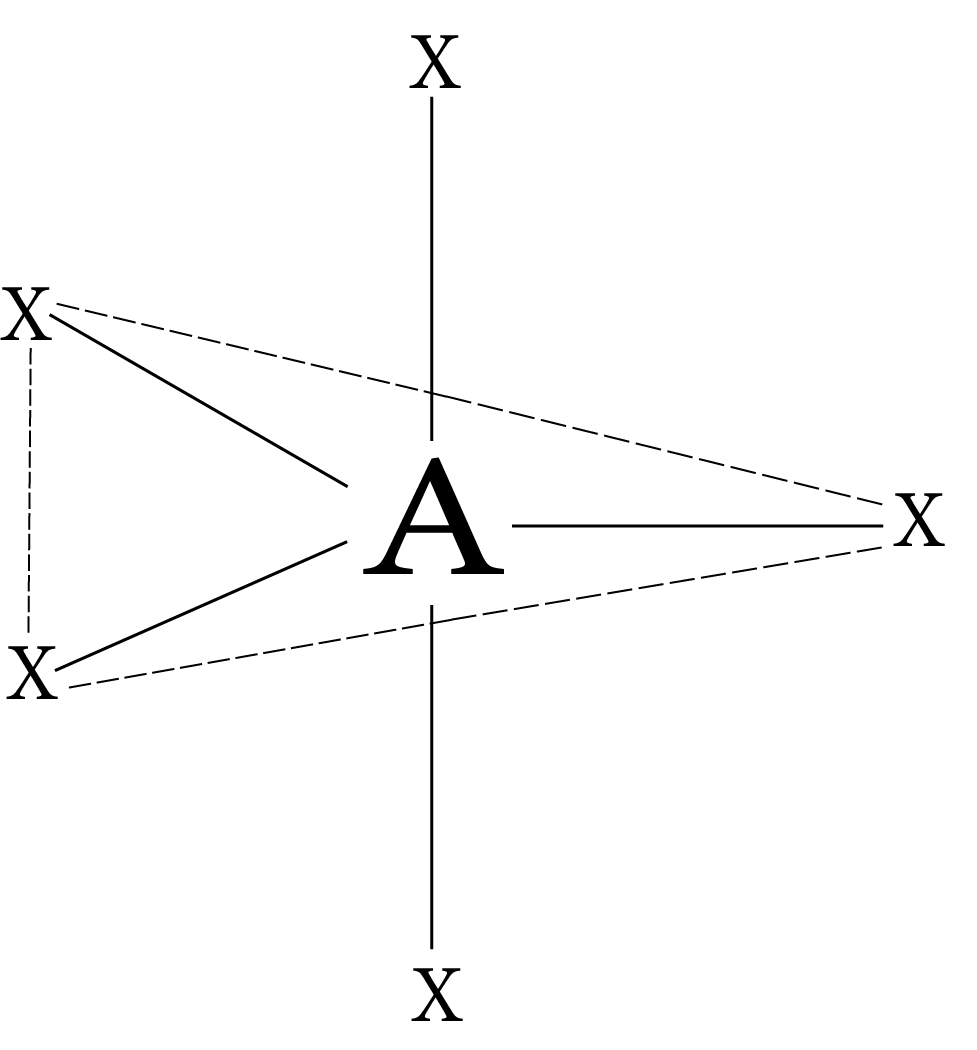
|
6 | d²sp³ or sp³d² | Octahedral | 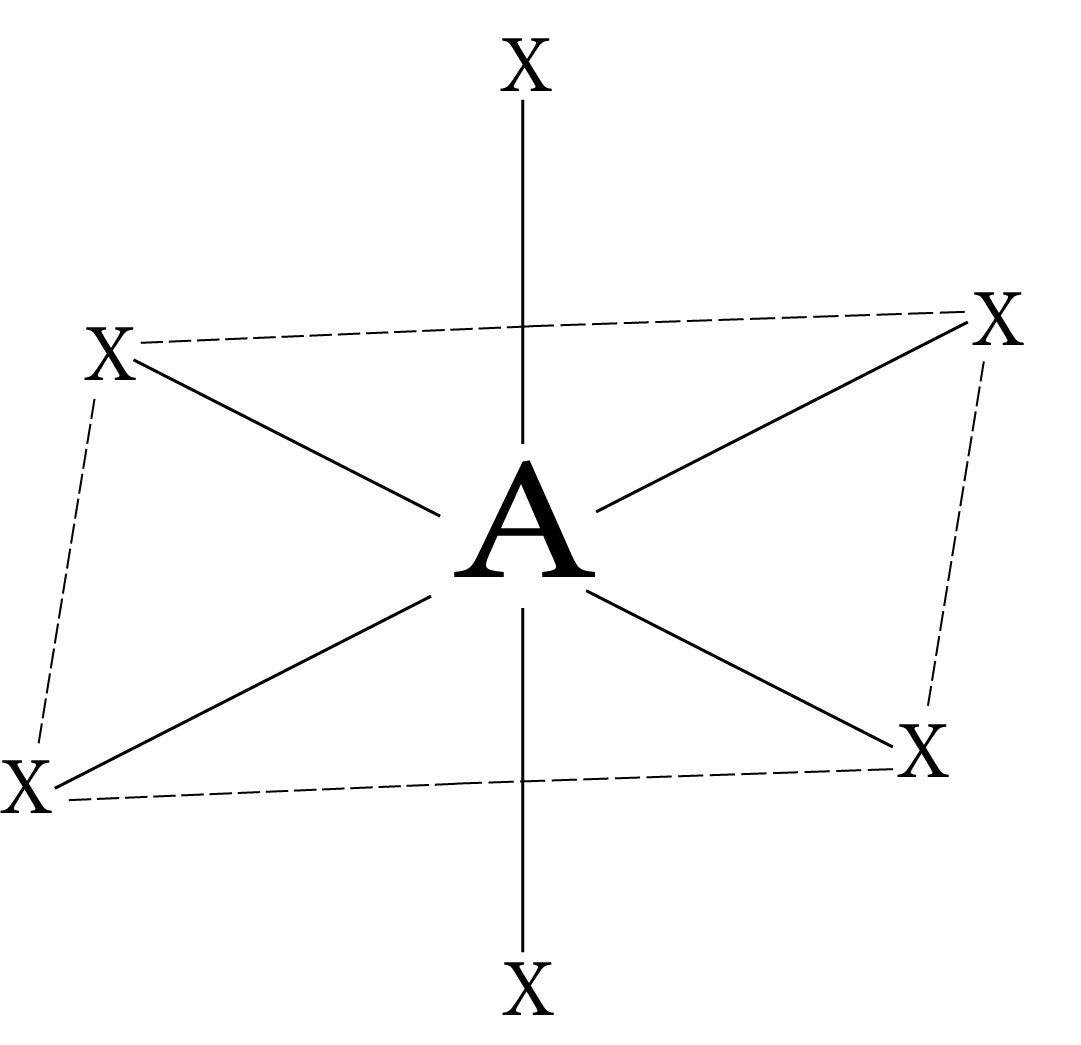
|
Note: Inner d-orbitals (3d orbital) have been used for bonding in $\mathrm{d}^{2} \mathrm{sp}^{3}$ hybridisation; such complexes are known as inner orbital complexes or low spin complexes. The outer d-orbitals (4d orbital) have been used for bonding in $\mathrm{sp}^{3} \mathrm{~d}^{2}$ hybridisation; such complexes are known as outer orbital complexes or high spin complexes. $\sqrt {n\left( {n + 2} \right)} $ where n is the number of unpaired electrons, gives the magnetic moment.
7.1 Limitations of VBT
The change in ligand and metal ion properties could not be explained.
The valence bond theory is silent on why some complexes are more labile than others.
The VBT does not explain the existence of inner and outer orbital complexes satisfactorily.
The VBT was unable to explain the color of complexes.
8. Crystal Field Theory
The valence bond theory is less widely accepted than the Crystal Field Theory. It is assumed that the attraction between a complex's central metal and its ligands is purely electrostatic. The following assumptions are made in the crystal field.
Ligands are considered point charges.
Metal orbitals and ligand orbitals have no interaction.
In the free atom, all of the d orbitals on the metal have the same energy (that is, they are degenerate). However, when a complex is formed, the ligands destroy the degeneracy of these orbitals, resulting in different energies for the orbitals.

Degeneracy of d-orbital
8.1 Octahedral Complexes
The metal is at the center of an octahedral complex, and the ligands are at the six corners. As shown, the directions x, y, and z point to three adjacent corners of the octahedron. The lobes of the ${{\text{e}}_g}{\text{ and }}{{\text{d}}_{{{\text{x}}^{\text{2}}}{\text{ - }}{{\text{y}}^{\text{2}}}}}{\text{, }}{{\text{d}}_{{{\text{z}}^2}}}$ orbitals point along the x, y, and z axes and the lobes of the t2g ${{\text{t}}_{2g}}{\text{ and }}{{\text{d}}_{{\text{xy}}}}{\text{, }}{{\text{d}}_{{\text{xz}}}}{\text{, }}{{\text{d}}_{{\text{yz}}}}$ are located between the axes. The approach of six ligands along the x, y, z, –x, –y, and –z directions increases the energy of the ${{\text{d}}_{{{\text{x}}^{\text{2}}}{\text{ - }}{{\text{y}}^{\text{2}}}}}{\text{ and }}{{\text{d}}_{{{\text{z}}^2}}}$ orbitals (which point along the axes) much more than the energy of the dxy, d xz, and d yz orbitals (which point between the axes). Thus, the d orbitals split into two groups under the influence of an octahedral ligand field.
Weak field ligands are those that cause only a minor amount of crystal field splitting. Strong field ligands are ligands that cause a large splitting. The common ligands can be arranged in ascending crystal field splitting $\Delta .$
Spectrochemical Series
$\mathrm{I}^{-}<\mathrm{Br}^{-}<\mathrm{S}^{2-}<\mathrm{Cl}^{-}<\mathrm{NO}_{3}^{-}<\mathrm{F}^{-}<\mathrm{OH}^{-}<\mathrm{EtOH}<\text { oxalate }<\mathrm{H}_{2} \mathrm{O}$
$\text { (weak field ligands) }<\mathrm{EDTA}<\left(\mathrm{NH}_{3}=\text { pyridine }\right)<\text { ethylenediamine }<\text { dipyridy }<0 \text { - phenanthroline }<\mathrm{NO}_{2}<\mathrm{CN}^{-}$
$<\mathrm{CO} \text { (strong field ligands) }$
A pattern of increasing donation is followed:
$\text { Halide donors }<\mathrm{O} \text { donors }<\mathrm{N} \text { donors }<\mathrm{C} \text { donors }$
The total crystal field stabilization energy is given by
${\text{CFS}}{{\text{E}}_{\left( {{\text{octahedral}}} \right)}} = - 0.4{n_{\left( {{t_{2g}}} \right)}} + 0.6{n_{\left( {{e_g}} \right)}}$
where ${n_{{t_{2g}}}}{\text{ and }}{n_{{e_g}}}$ are the number of electrons occupying the$t_{2g}$ and $e_g$ orbitals respectively. The CFSE is zero for ions with $d^0$ and $d^{10}$ configurations in both strong and weak ligand fields. The CFSE is also zero for $d^5$ configurations in a weak field.
Effects of Crystal Field Splitting
CFSE and electronic arrangements in octahedral complexes

Arrangement of electrons weak ligand field and strong ligand field
8.2 Tetrahedral Complexes
A cube is related to a regular tetrahedron. As shown, one atom is in the center of the cube, and four of the cube's eight corners are occupied by ligands.
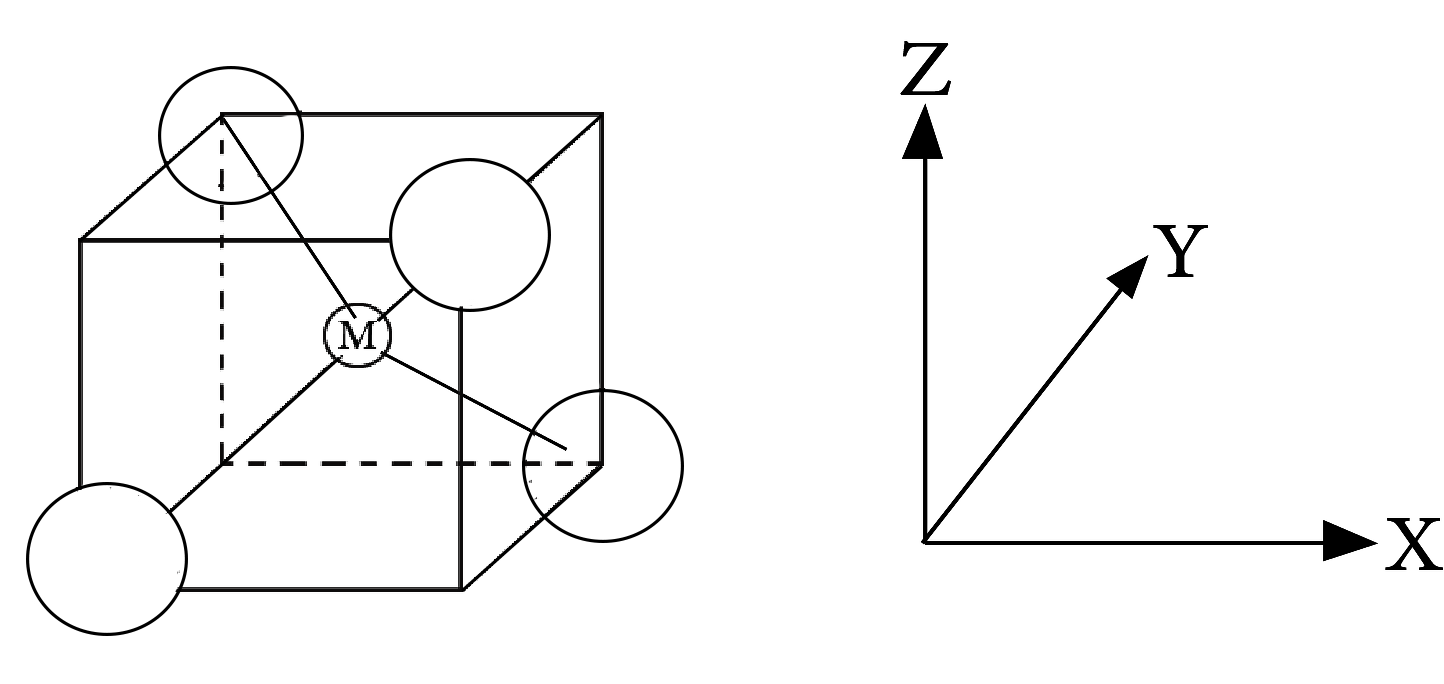
Degeneracy of d-orbital in tetrahedral complex
The directions x, y, and z point to the cube's face centers. The e orbitals are oriented along the x, y, and z axes (that is to the centres of the faces). The t2 orbitals are located between the x, y, and z axes (that is towards the centres of the edges of the cube). The ligands' approach directions do not exactly coincide with the e or t2 orbitals.
As a result, the t2 orbitals are closer to the ligand direction than the e orbitals. The ligands' approach raises the energy of both sets of orbitals. Because they are closest to the ligands, the energy of the t2 orbitals is increased the most. The crystal field splitting in octahedral complexes is the inverse of that in octahedral complexes.
The t2 orbitals are $0.4{\Delta _t}$ higher than the weighted average energy of the two groups (the Bari center), while the e orbitals are $0.6{\Delta _t}$ lower.
In tetrahedral complexes, the magnitude of the crystal field splitting t is much smaller than in octahedral fields. This is due to two factors:
Because there are only four ligands rather than six, the ligand field is only two-thirds the size; consequently, the ligand field splitting is also two-thirds the size.
The orbital direction does not coincide with the ligand direction. This reduces the crystal field splitting by about two-thirds.
Thus the tetrahedral crystal field splitting ${\Delta _t}$ is roughly 2/3 × 2/3 = 4/9 of the octahedral crystal field splitting ${\Delta _t}.$
9. Organometallic Compounds
Organometallic compounds are those that contain at least one carbon-metal bond. The Grignard reagent, RMgX, is a well-known example of an organometallic compound in which $\mathrm{R}$ is an alkyl group. Organometallic compounds include diethyl zinc $\left[\mathrm{Zn}\left(\mathrm{C}_{2} \mathrm{H}_{5}\right)_{2}\right]$, lead tetraethyl $\left[\mathrm{Pb}\left(\mathrm{C}_{2} \mathrm{H}_{5}\right)_{4}\right]$, ferrocene $\left[\mathrm{Fe}\left(\mathrm{C}_{5} \mathrm{H}_{5}\right)_{2}\right]$, dibenzene chromium $\left[\mathrm{Cr}\left(\mathrm{C}_{6} \mathrm{H}_{6}\right)_{2}\right]$, and metal carbonyls. Organometallic compounds are divided into three types:
Complexes with the sigma $\left( \sigma \right)$
Bonded complexes of Pi $\left( \pi \right)$
Complexes with both sigma and pi bonding properties.
9.1 Sigma Bonded Complexes
The metal atom and carbon atom of the ligand are joined together with a sigma bond in these complexes, i.e., the ligand contributes one electron and is thus referred to as a one electron donor.
Grignard reagent, $R-M g-X$, where $R$ is an alkyl or aryl group and $X$ is halogen.
Zinc compounds with the formula $\mathrm{R}_{2} \mathrm{Zn}$, for example, $\left(\mathrm{C}_{2} \mathrm{H}_{5}\right)_{2} \mathrm{Zn}$. Frankland was the first to isolate this in 1849 . Other comparable compounds include $\left(\mathrm{CH}_{3}\right)_{4} \mathrm{Sn},\left(\mathrm{C}_{2} \mathrm{H}_{5}\right)_{4} \mathrm{~Pb}$, $\mathrm{Al}_{2}\left(\mathrm{CH}_{3}\right)_{6}, \mathrm{Al}_{2}\left(\mathrm{C}_{2} \mathrm{H}_{5}\right)_{6}$, and $\mathrm{Pb}\left(\mathrm{CH}_{3}\right)_{4}$
9.2 Pi Bonded Organometallic Compounds
These are the compounds of metals that are combined with alkenes, alkynes, benzene, and other ring compounds. In these complexes, the metal and ligand form a bond that involves the pi electrons of the ligand. Three common examples are Zeise’s salt, ferrocene and dibenzene chromium. These are shown here :
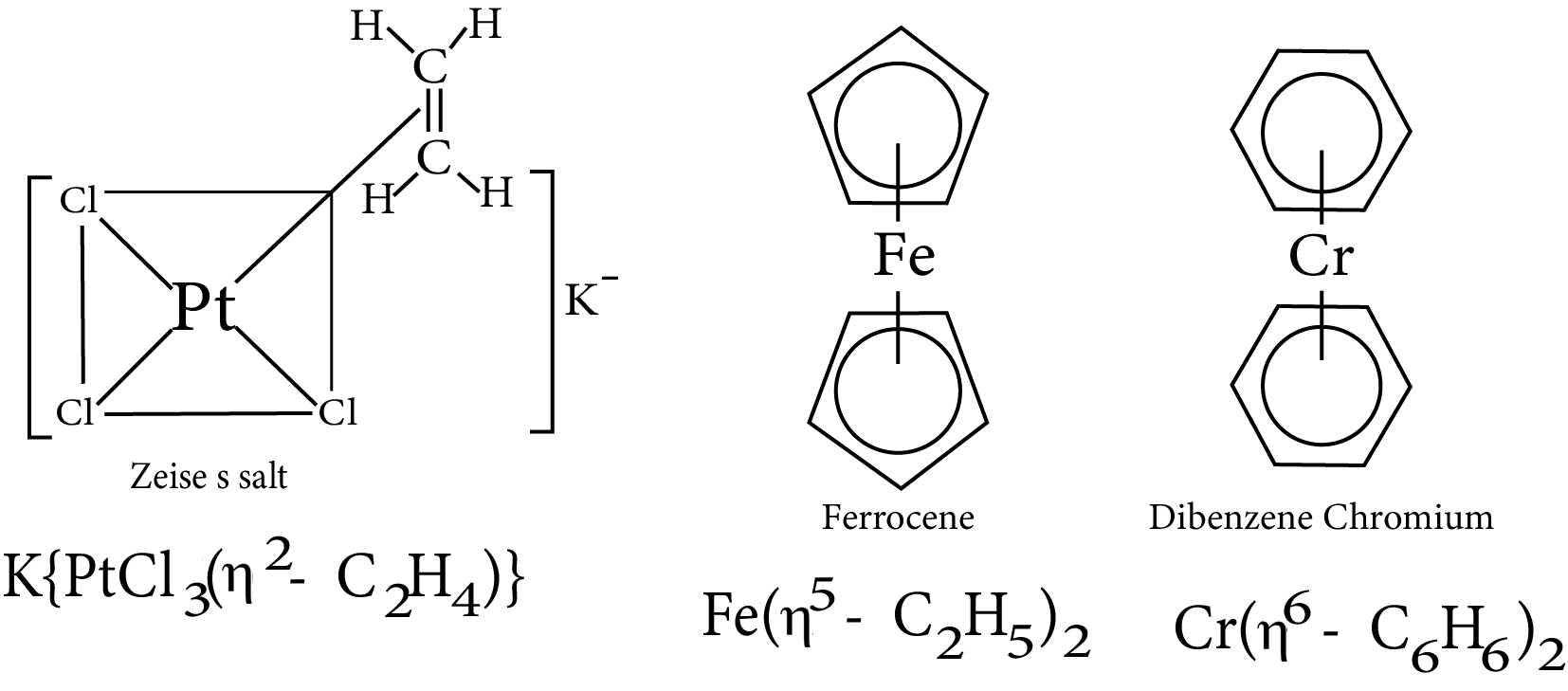
Pi bonded organometallic compounds
9.3 Sigma– and Pi–Bonded Organometallic Compounds
This class includes metal carbonyls, which are compounds formed by combining metal and carbon monoxide. These compounds have sigma and pi bonding. Metal atoms in these compounds have no oxidation state. Carbonyls can be monomeric, bridged, or polynuclear in nature.

Sigma– and pi–bonded carbonyl compounds
The metal–carbon bond in a metal carbonyl has both the sigma– and pi–character. When a vacant hybrid orbital of the metal atom overlaps with an orbital on the C atom of carbon monoxide containing a lone pair of electrons, a sigma bond is formed.
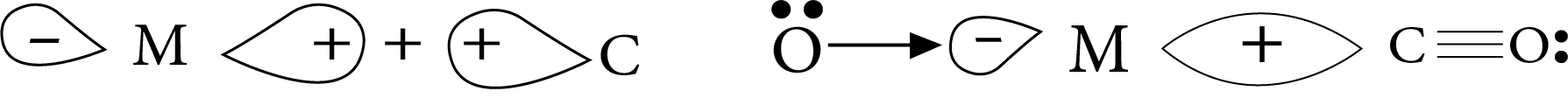
Sigma– overlap in carbonyl compounds
When a filled orbital of a metal atom overlaps with a vacant antibonding pi* orbital of a carbon monoxide atom, a pi–bond is formed. This overlap is also known as metal atom back donation of electrons to carbon. As an example, consider the following:
pi– overlap in carbonyl compounds
The pi–overlap is perpendicular to the sigma–bond nodal plane.
In olefinic complexes, bonding pi–orbital electrons are donated to the metal atoms' empty orbital while back bonding occurs from the metal atoms' filled orbital to the antibonding pi–orbital of the olefin.
10. Isomerism
Isomers are compounds that have the same molecular formula but a different structural formula.
10.1 Structural Isomerism
10.1.1 Ionisation Isomerism :
This isomerism occurs when the coordination compounds produce different ions in solution. For example, the formula has two isomers.
$\underset{violet}{[Co(NH_3)_5Br]SO_4} \rightleftharpoons \underset{Pentaamine Bromide}{[Co(NH_3)_5Br]^{2+}} - cobalt(III)\,\, ion + {So_4}^{2-}$
This isomer produces a white precipitate of $BaSO_4$ in a solution of $BaCl_2$.
$\underset{Red}{[Co(NH_3)_5 SO_4]Br} \rightleftharpoons \underset{Pentaamine Sulphato}{[Co(NH_3)_5SO_4]^{+}} - cobalt(III)\,\, ion + {Br}^{-}$ With $\mathrm{AgNO}_{3}$ solution, the above isomer produces a light yellow precipitate.
10.1.2 Hydrate Isomerism:
When different numbers of water molecules are present inside and outside the coordination sphere, this type of isomerism occurs. This isomerism is best exemplified by the three isomers with the formula ${\text{CrC}}{{\text{l}}_{\text{3}}}{\text{.6}}{{\text{H}}_{\text{2}}}{\text{O}}{\text{.}}$
$\left[ {{\text{Cr}}{{\left( {{{\text{H}}_{\text{2}}}{\text{O}}} \right)}_{\text{6}}}} \right]{\text{C}}{{\text{l}}_{\text{3}}}{\text{ , }}\left[ {{\text{Cr}}{{\left( {{{\text{H}}_{\text{2}}}{\text{O}}} \right)}_{\text{5}}}{\text{Cl}}} \right]{\text{C}}{{\text{l}}_{\text{2}}}{\text{.}}{{\text{H}}_{\text{2}}}{\text{O, and }}\left[ {{\text{Cr}}{{\left( {{{\text{H}}_{\text{2}}}{\text{O}}} \right)}_{\text{4}}}{\text{C}}{{\text{l}}_{\text{2}}}} \right]{\text{Cl}}{\text{.2}}{{\text{H}}_{\text{2}}}{\text{O}}$ are its Hydrate Isomers.
10.1.3 Coordination Isomerism:
This type of isomerism can be found in coordination compounds that contain both cationic and anionic complex ions. To form isomers, the ligands in both the cationic and anionic ions are exchanged. Here are some examples:
$\left[ {{\text{Pt}}{{\left( {{\text{N}}{{\text{H}}_{\text{3}}}} \right)}_{\text{4}}}} \right]\left[ {{\text{CuC}}{{\text{l}}_{\text{4}}}} \right]{\text{ and }}\left[ {{\text{Cu}}{{\left( {{\text{N}}{{\text{H}}_{\text{3}}}} \right)}_{\text{4}}}} \right]\left[ {{\text{PtC}}{{\text{l}}_{\text{4}}}} \right]$
10.1.4 Linkage Isomerism:
This isomerism occurs in complex compounds containing ambidentate ligands such as ${\text{N}}{{\text{O}}_{\text{2}}}{\text{, C}}{{\text{N}}^{\text{ - }}}{\text{, SC}}{{\text{N}}^{\text{ - }}}{\text{, }}{{\text{S}}_{\text{2}}}{\text{O}}_3^{2 - }{\text{, and CO}}{\text{.}}$ For example, $\left[ {{\text{Co}}{{\left( {{\text{N}}{{\text{H}}_{\text{3}}}} \right)}_{\text{5}}}{\text{N}}{{\text{O}}_{\text{2}}}} \right]{\text{C}}{{\text{l}}_{\text{2}}}{\text{ and }}\left[ {{\text{Co}}{{\left( {{\text{N}}{{\text{H}}_{\text{3}}}} \right)}_{\text{5}}}{\text{ONO}}} \right]{\text{C}}{{\text{l}}_{\text{2}}}$ are linkage isomers because ${\text{NO}}_{\text{2}}^{\text{ - }}$ can be linked via N or O.
10.1.5 Ligand Isomerism:
Some ligands can exist as isomers; for example, diamino propane can exist as both 1, 2-diamino propane (pn) and 1, 3-diamino propane, also known as trimethylene diamine (tn).
When these ligands (pn and tn) combine to form complexes, the complexes are isomers of each other. This ligand is found in isomeric complexes such as ${\left[ {{\text{Co}}{{\left( {{\text{pn}}} \right)}_{\text{2}}}{\text{C}}{{\text{l}}_{\text{2}}}} \right]^{\text{ + }}}{\text{ and }}{\left[ {{\text{Co}}{{\left( {{\text{tn}}} \right)}_{\text{2}}}{\text{C}}{{\text{l}}_{\text{2}}}} \right]^{\text{ + }}}$ ions.
10.1.6 Coordination Position Isomerism:
This type of isomerism is exhibited by polynuclear complexes by changing the position of ligands with respect to different metal atoms present in the complex. For example,

Coordination position isomerism
10.2 Stereo Isomerism
Compounds with stereo isomerism have the same number of atoms or groups in the same position, but the atoms or groups are arranged differently around the central atom.
10.2.1 Geometrical Isomerism
Complex compounds with the same ligands in the coordination sphere but different relative positions of the ligands around the central metal atom are referred to as geometrical isomers, and the phenomenon is referred to as geometrical isomerism.
10.2.1.1 Geometrical Isomerism in Square Planar Complexes
A square planar complex with two similar ligands at opposite positions (180o a part) is called a trans-isomer, while a square planar complex with two similar ligands at adjacent positions (90o a part) is called a cis - isomer.
Geometrical isomers (cis and trans) of $\left.\mathrm{Pt}\left[\mathrm{NH}_{3}\right)_{2} \mathrm{Cl}_{2}\right]$
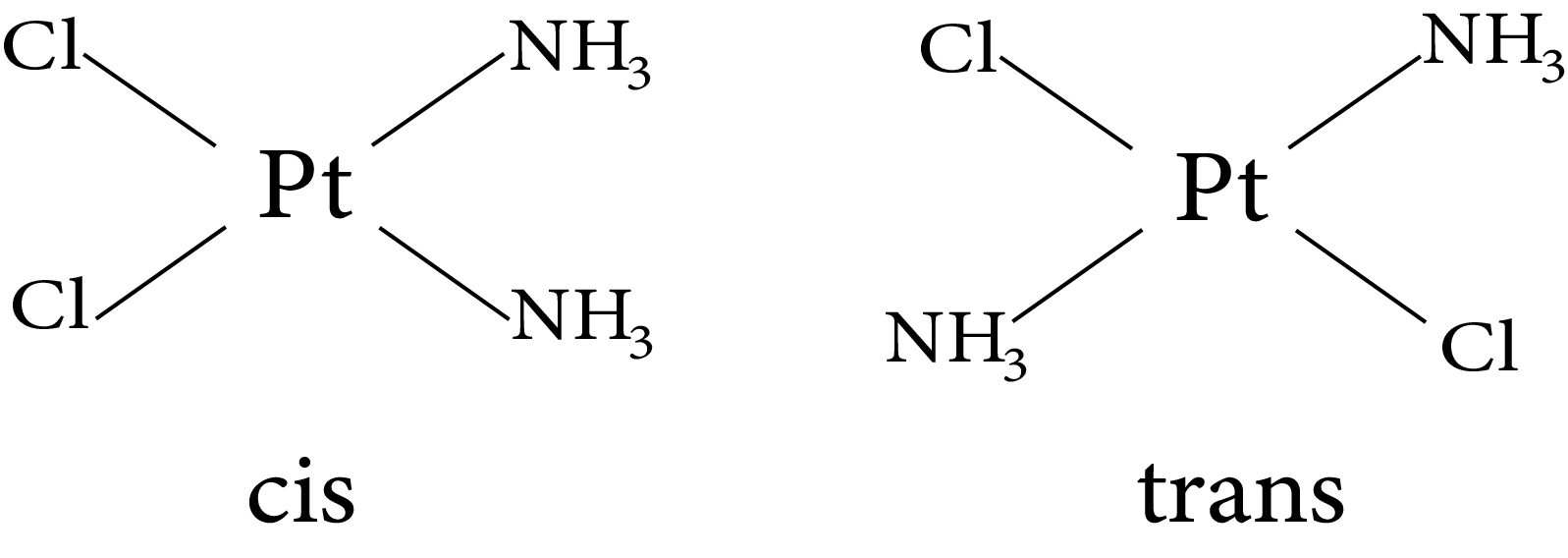
Geometrical isomerism
Geometrical isomers (cis and trans) $o f\left[\mathrm{Co}\left(\mathrm{NH}_{3}\right)_{4} C l_{2}\right]^{+}$
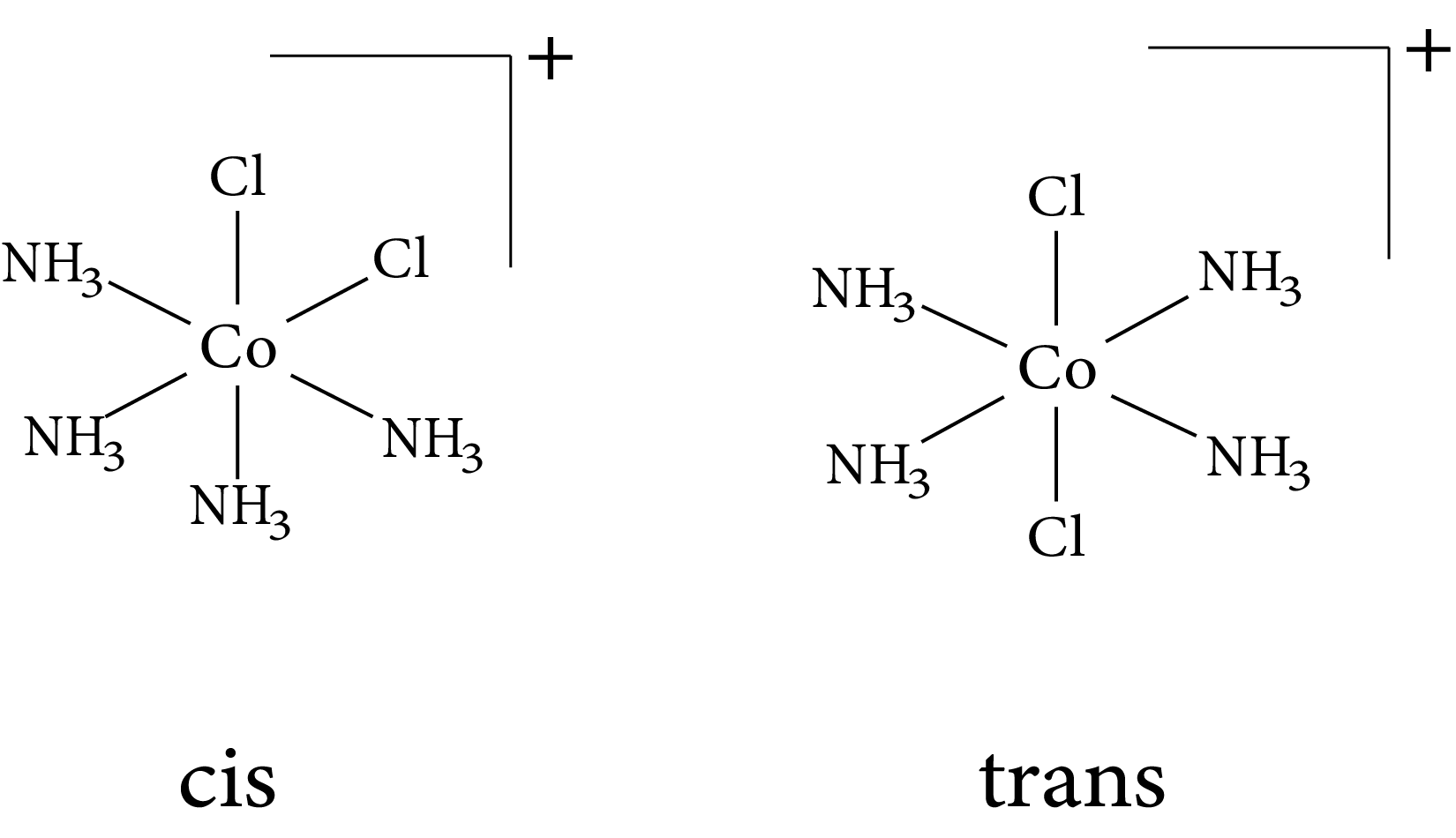
Isomerism in complex
Mabcd
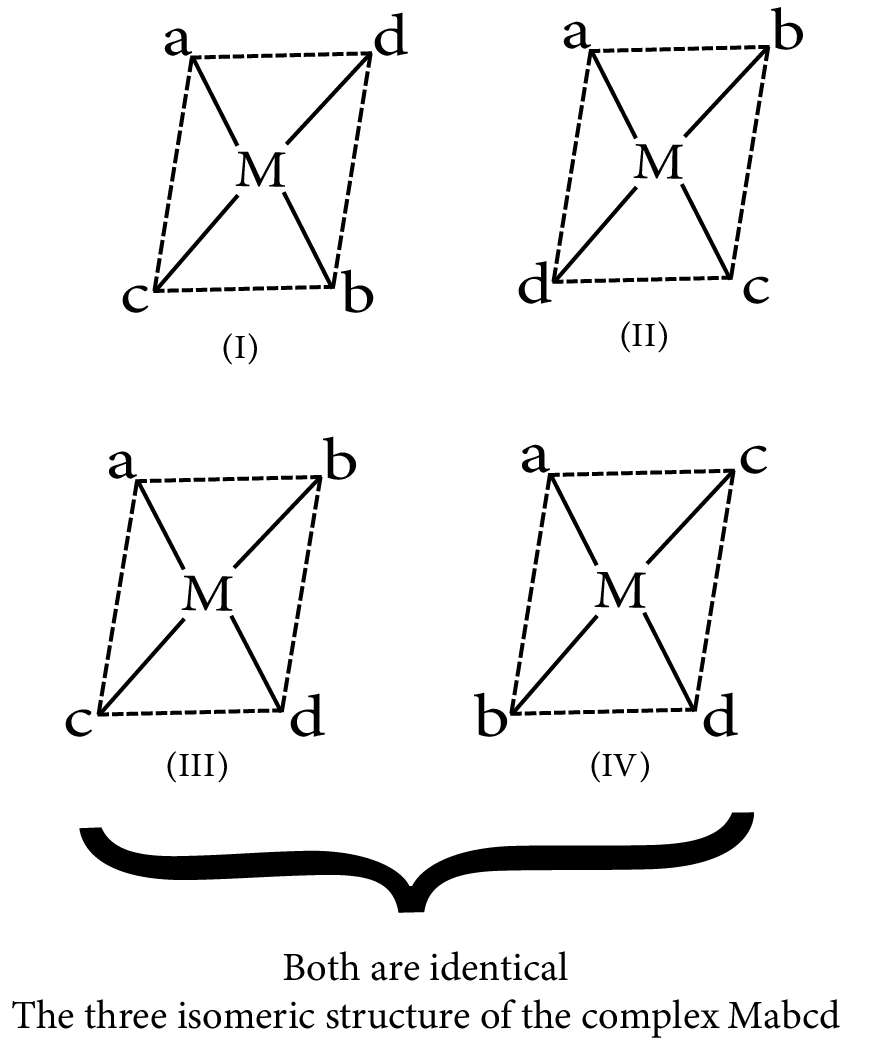
Isomerism in complex
$M(AB)_2$
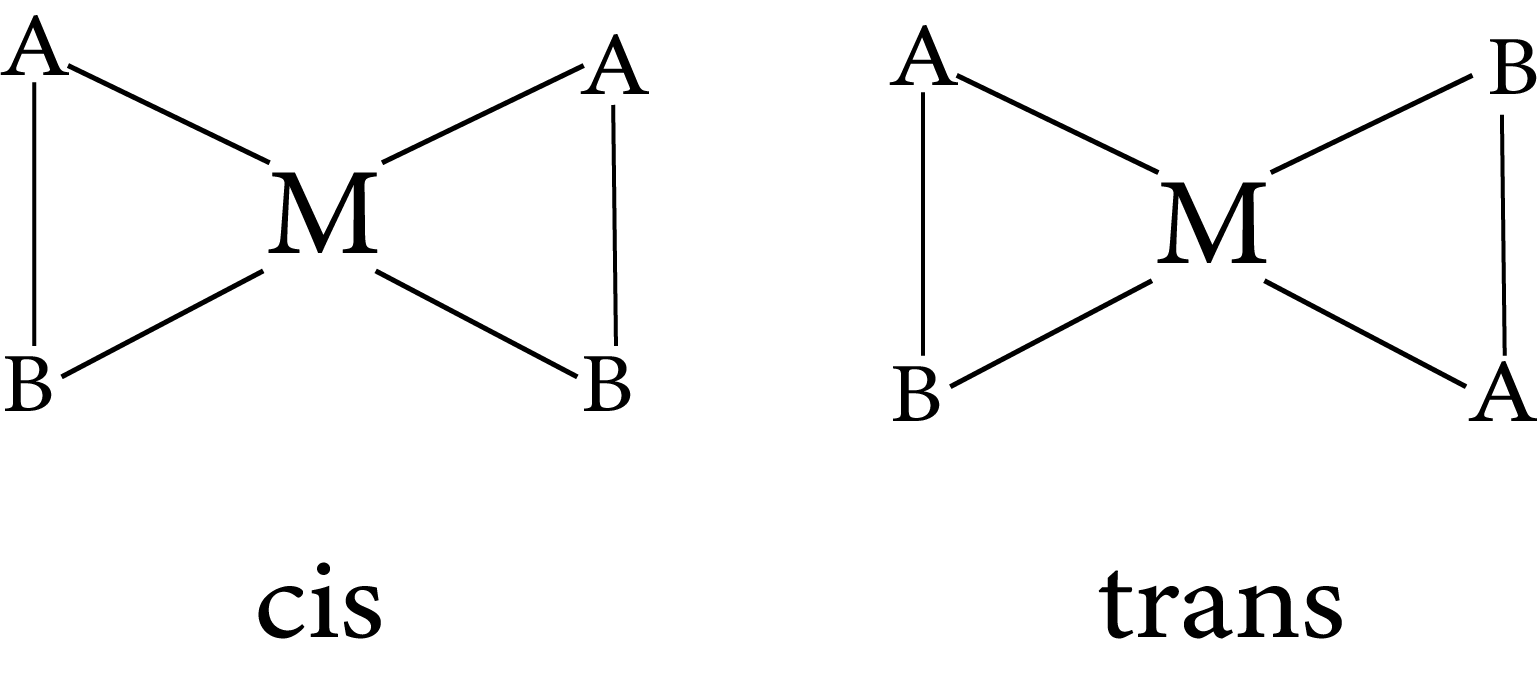
Isomerism in complex
10.2.1.2 Geometrical Isomerism in Octahedral Complexes
$Ma_4b_2$
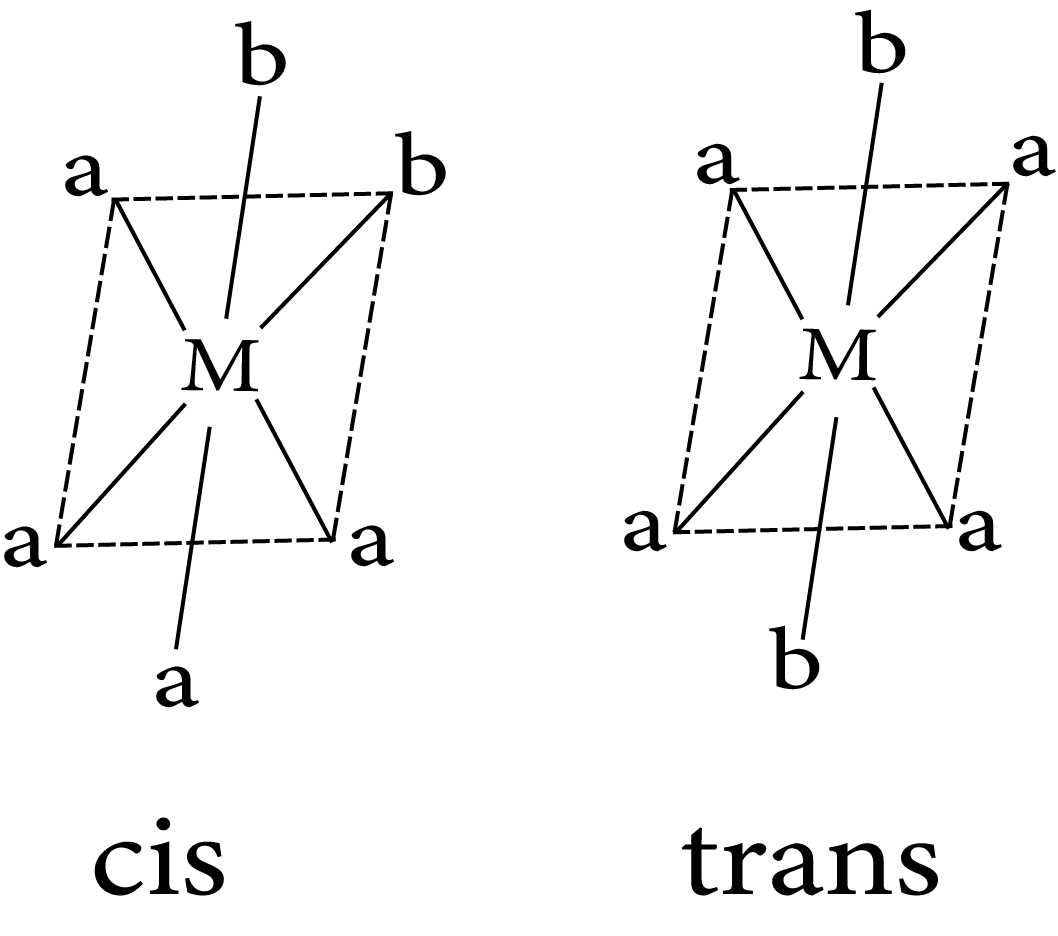
Geometrical isomerism in complex
$Ma_3b_3$
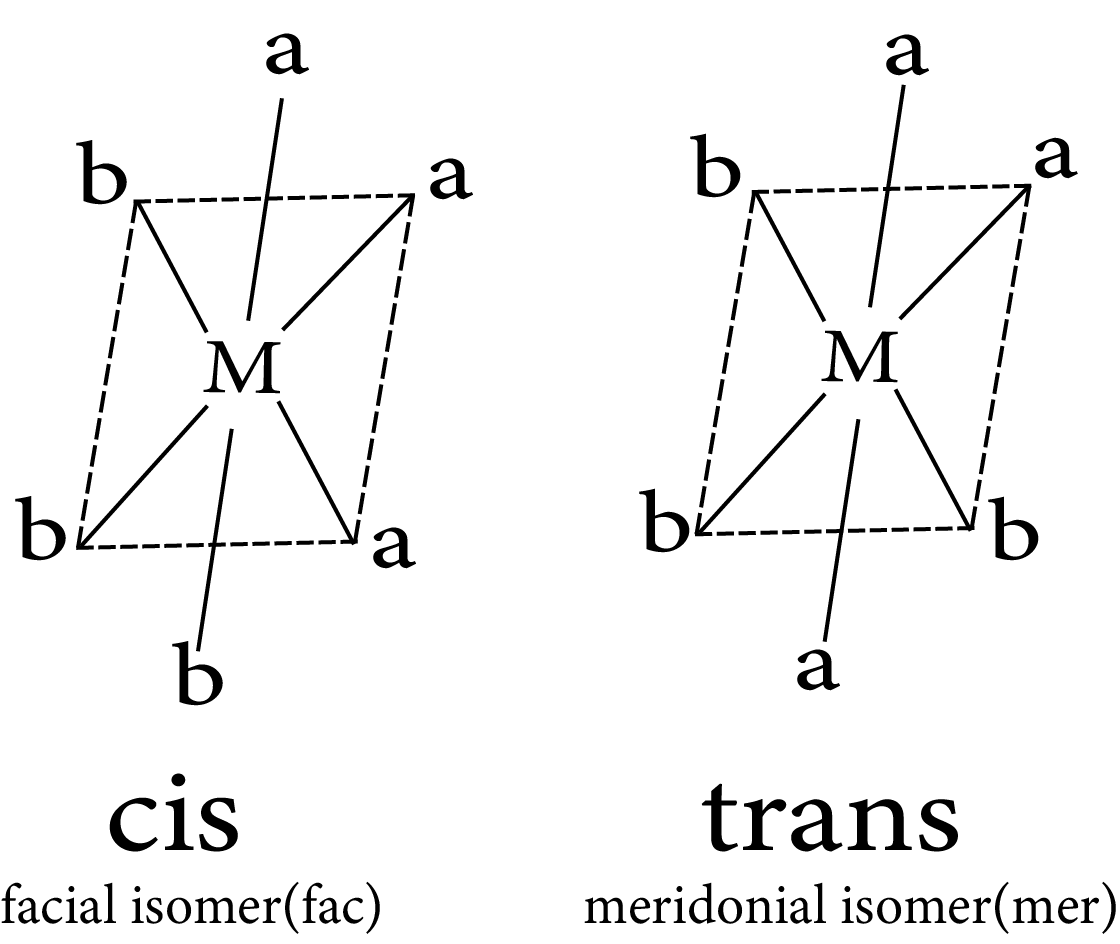
Geometrical isomerism in complex
Mabcdef: They form 15 isomers
$M(AB)_3$
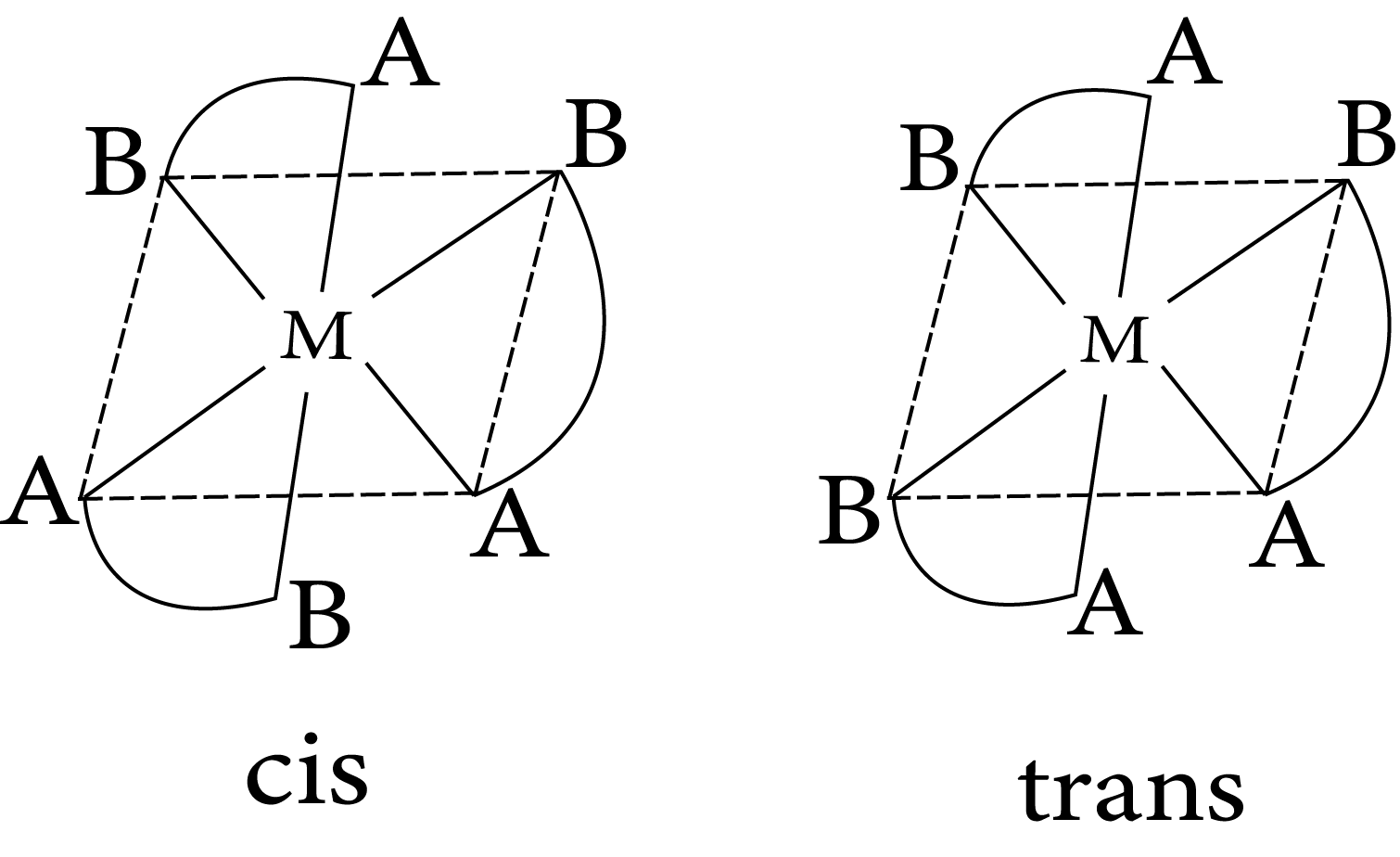
Geometrical isomerism in complex
$M(AA)_2b_2$
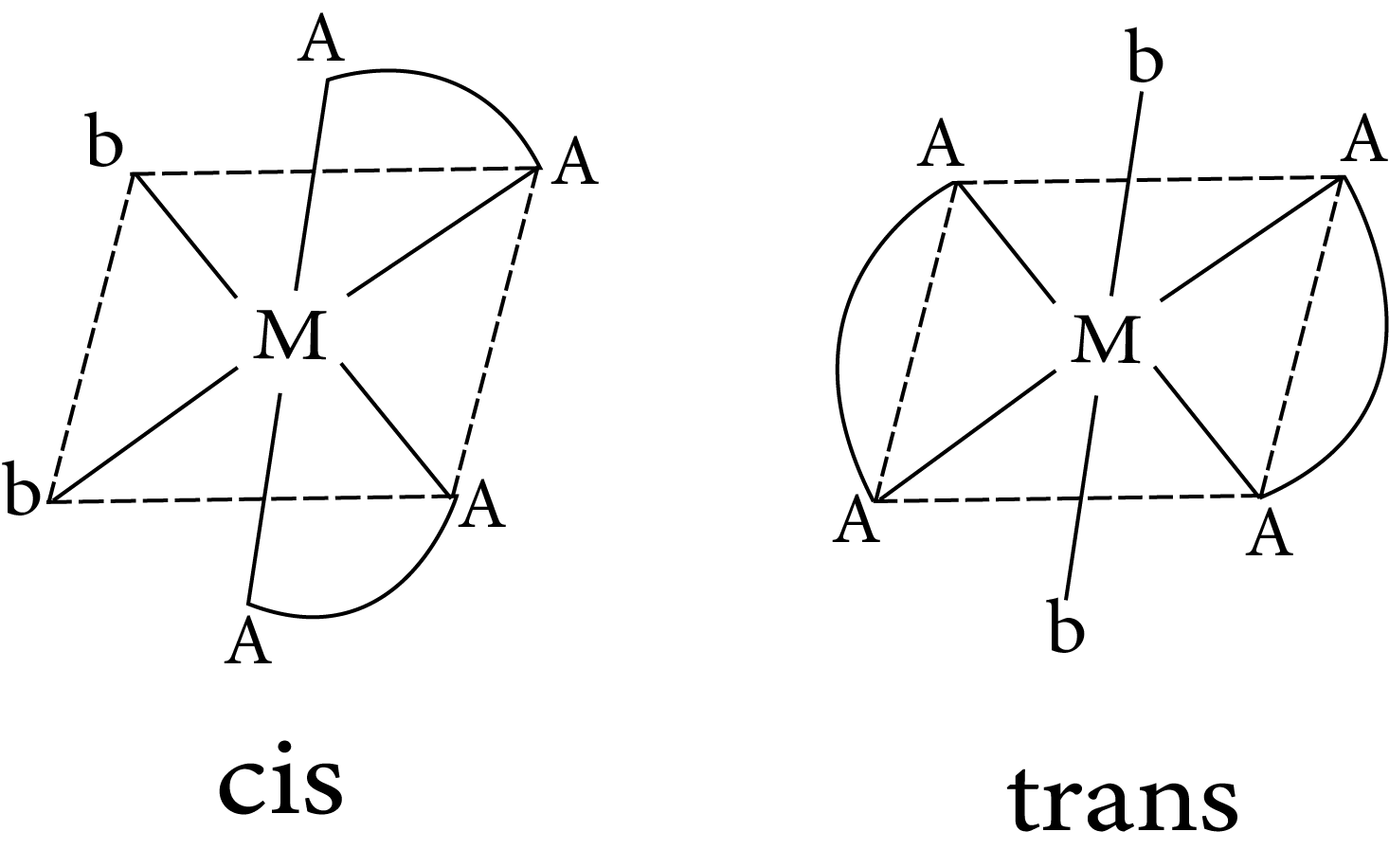
Geometrical isomerism in complex
$M(AA)_2bc$
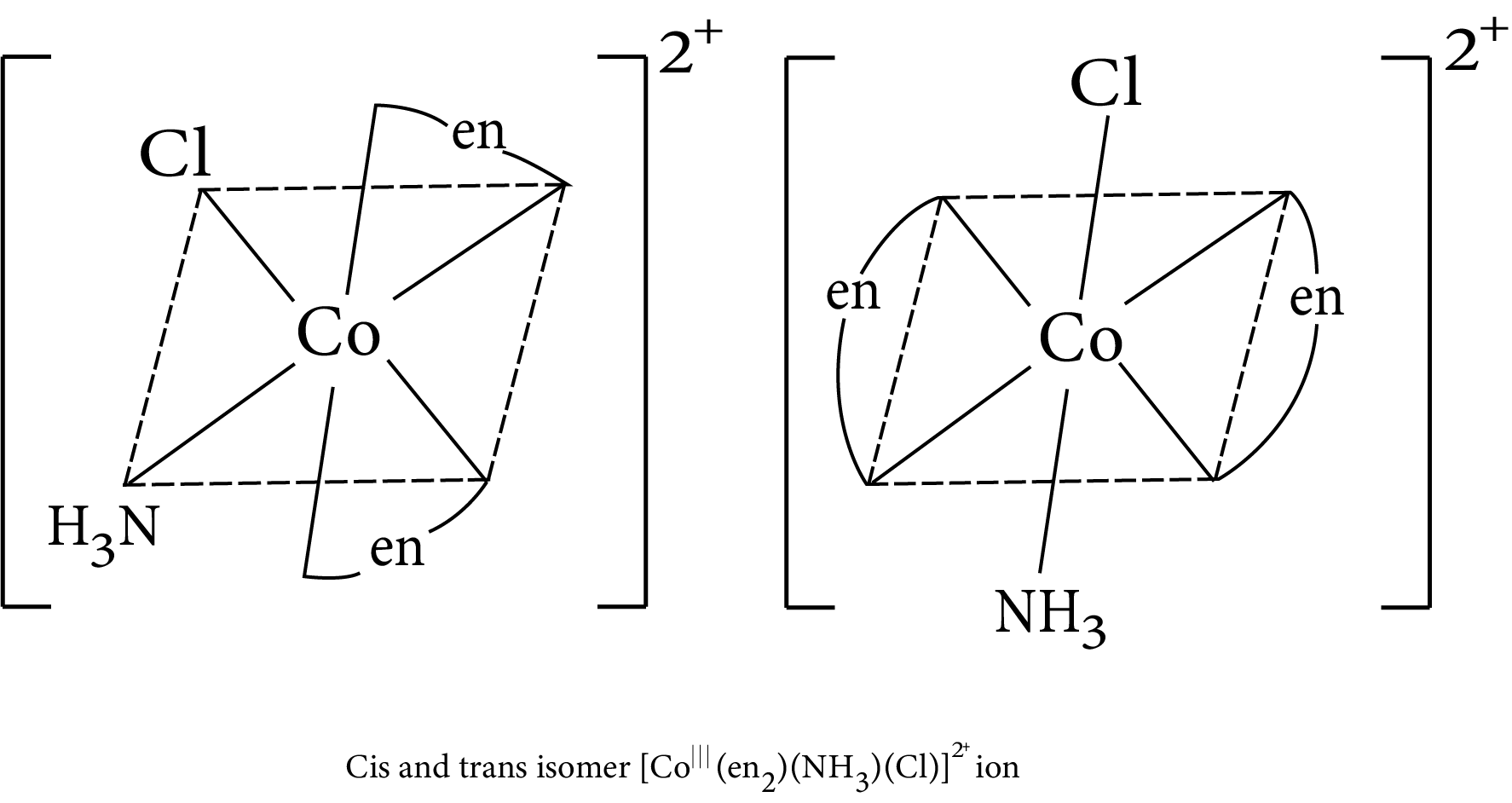
Geometrical isomerism in complex
$M(AA)a_2b_2$
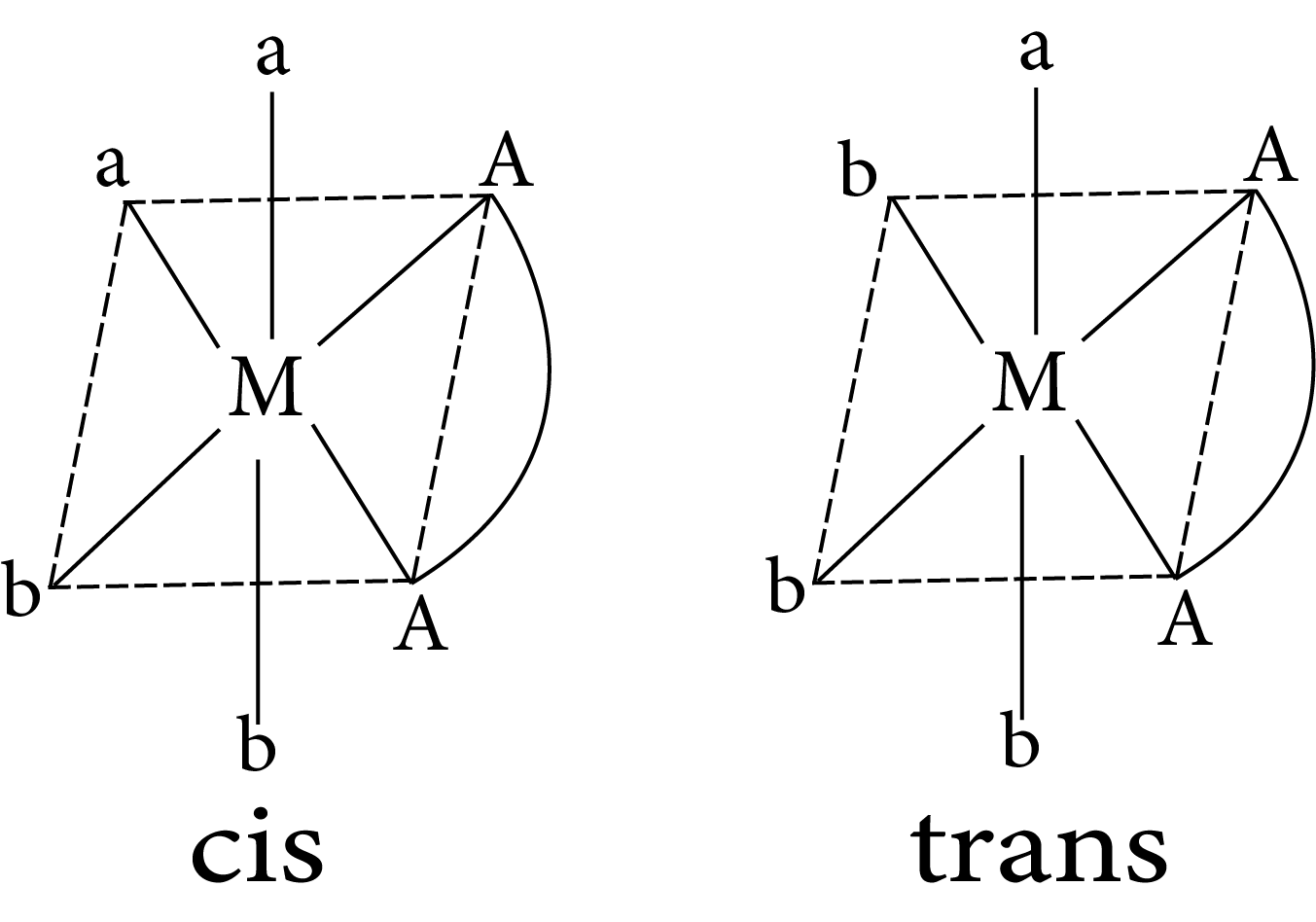
Geometrical isomerism in complex
$Ma_2b_2c_2$
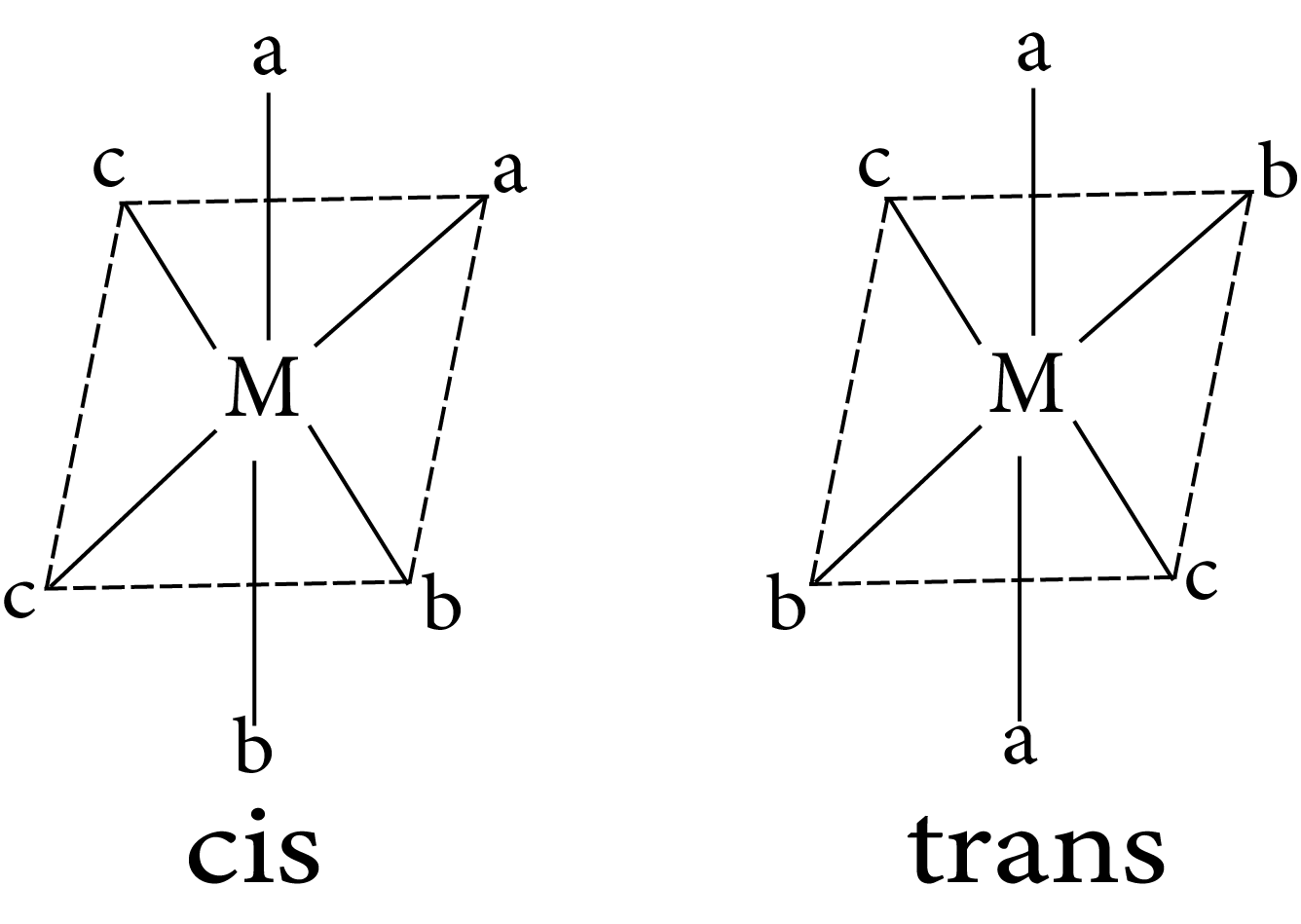
Geometrical isomerism in complex
Optical Isomerism in octahedral complexes
Mabcdef
Example: Draw the optical isomers of $\left[ {{\text{Pt}}\left( {{\text{Cl}}} \right)\left( {{\text{Br}}} \right)\left( {\text{I}} \right)\left( {{\text{py}}} \right)\left( {{\text{N}}{{\text{O}}_{\text{2}}}} \right)\left( {{\text{N}}{{\text{H}}_{\text{3}}}} \right)} \right]$

Optical Isomerism in octahedral complex Mabcdef
$M(AA)_3$
Example: Draw the optical isomers of ${\left[ {{\text{Co}}{{\left( {{\text{en}}} \right)}_{\text{3}}}} \right]^{{\text{3 + }}}}$
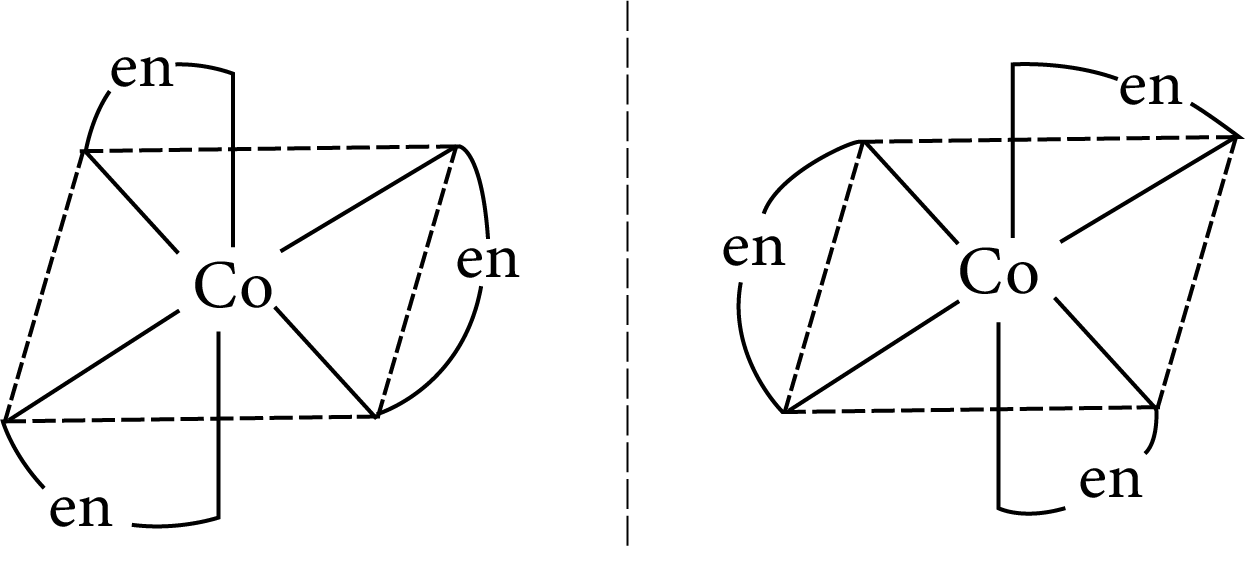
Optical Isomerism in octahedral complex
The two optical isomeric forms of the complex ${\left[ {{\text{Co}}{{\left( {{\text{en}}} \right)}_{\text{3}}}} \right]^{{\text{3 + }}}}$
$M(AB)_3$
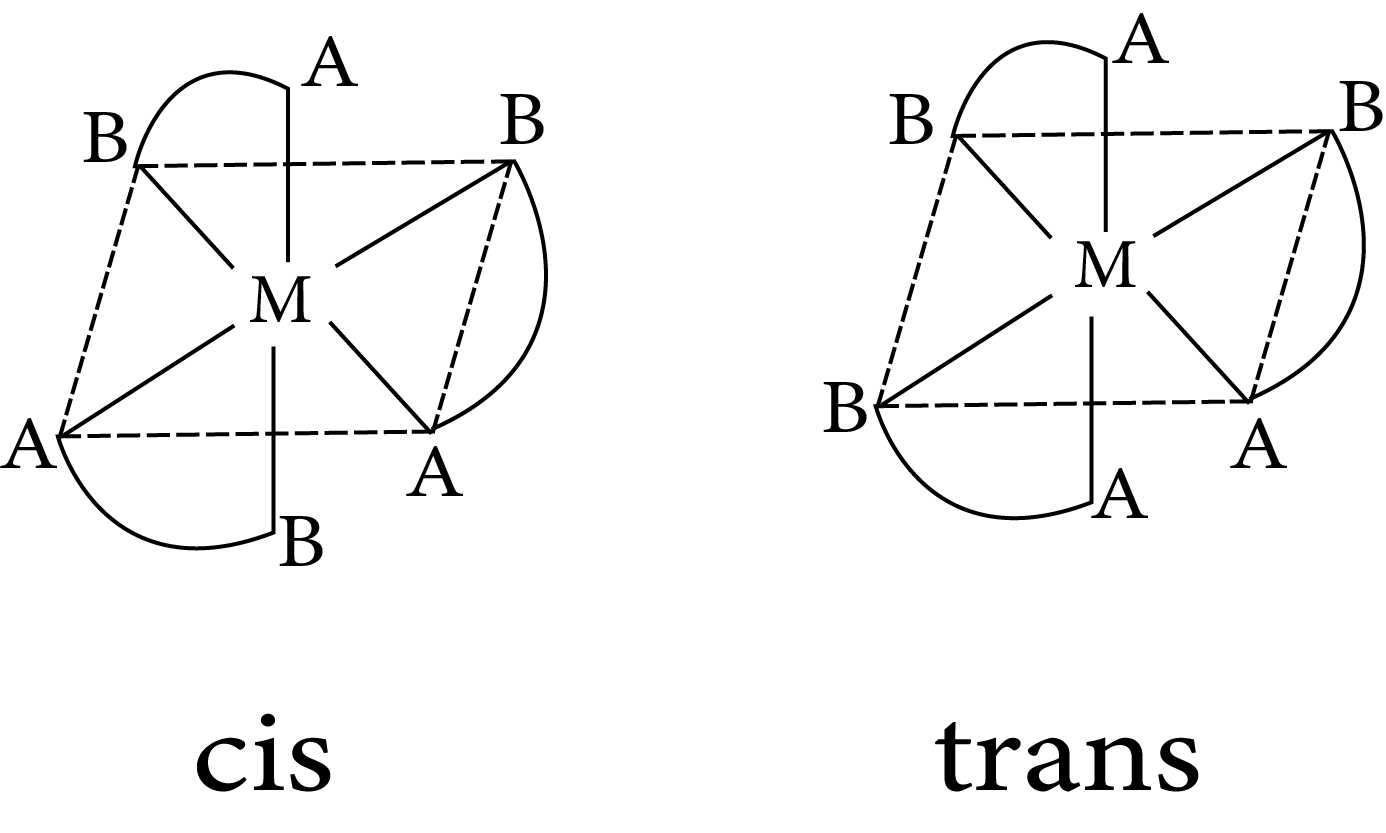
Isomerism in octahedral complexes
cis $M(AA)_2b_2$
Example: Draw the optical isomers of $\left[ {{\text{RhC}}{{\text{l}}_{\text{2}}}{{\left( {{\text{en}}} \right)}_{\text{2}}}} \right]{{\text{ }}^{\text{ + }}}$
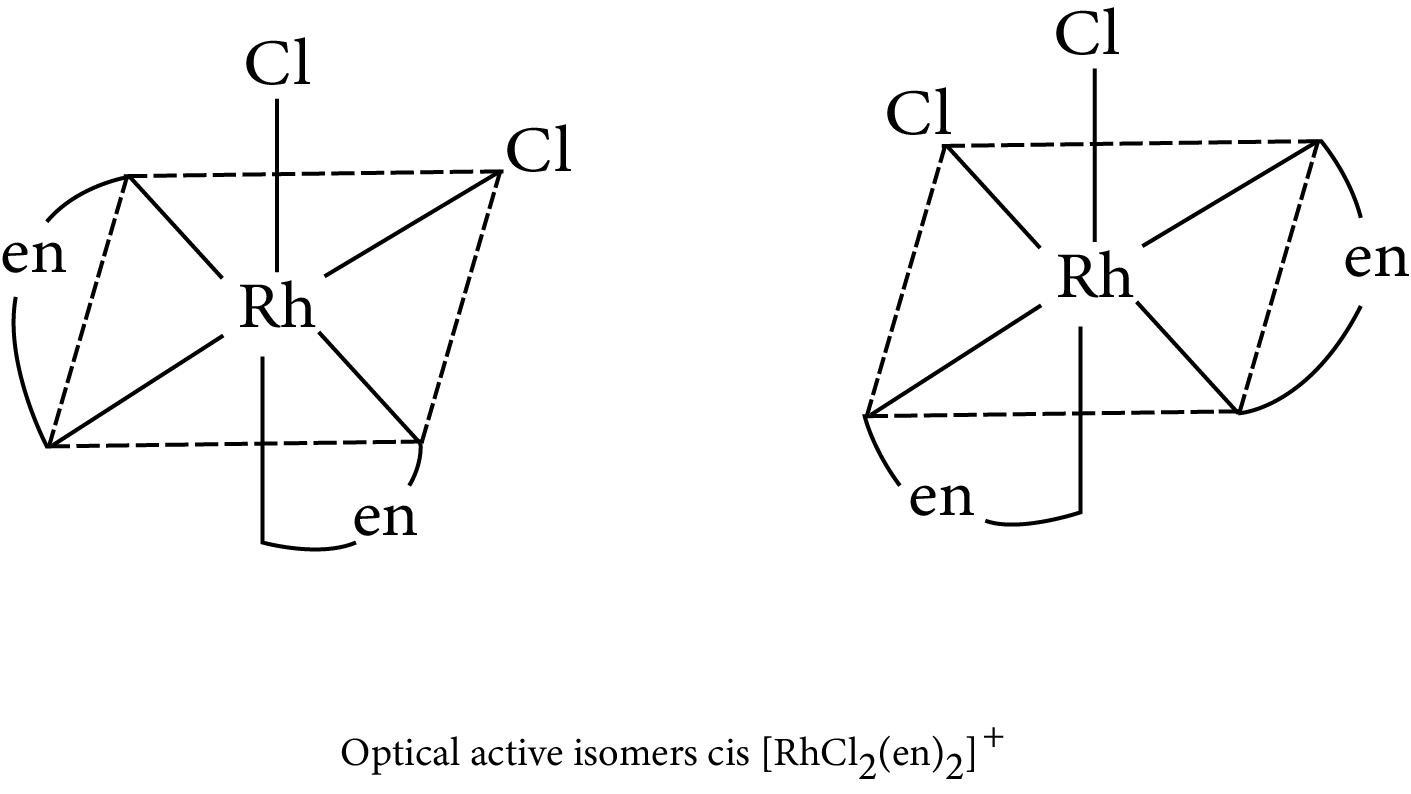
Optical Isomerism in octahedral complex
Cis $Ma_2b_2c_2$
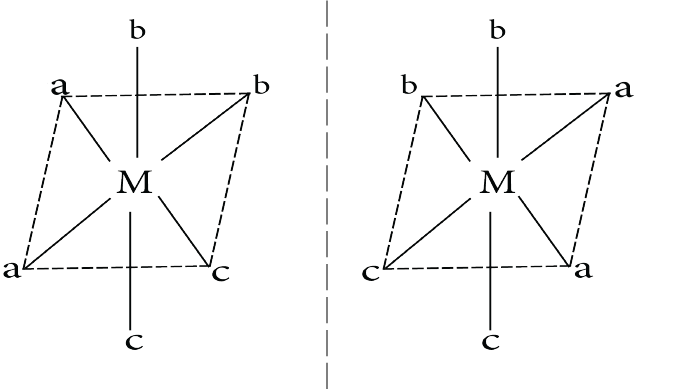
Optical Isomerism in cis
cis $M(AA)b_2c_2$
Example: Draw the optical isomers of ${\left[ {{\text{CoC}}{{\text{l}}_{\text{2}}}\left( {{\text{en}}} \right){{\left( {{\text{N}}{{\text{H}}_{\text{3}}}} \right)}_{\text{2}}}} \right]^{\text{ + }}}$
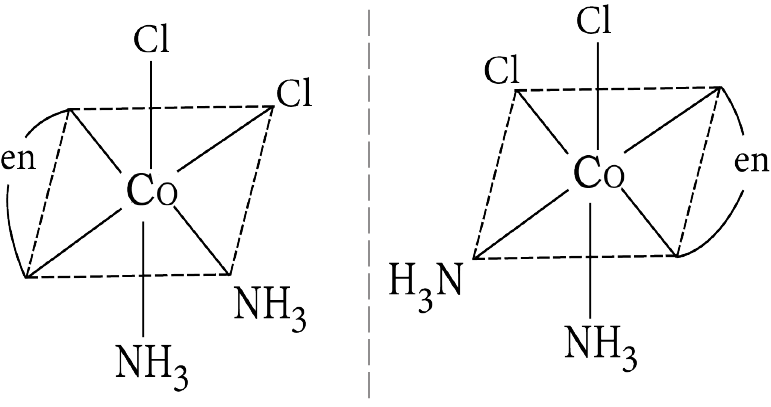
Optical Isomerism in cis
cis $M(AA)_2 bc$
11. Stability Of Coordination Compounds
The degree of association between the two species involved in the state of equilibrium is referred to as the stability of a complex in solution. If we get a reaction like this:${\text{M + 4L\;}} \rightleftharpoons {\text{\;M}}{{\text{L}}_{\text{4}}}$
The greater the stability constant, the greater the proportion of ML4 in solution. Because free metal ions are rare in solution, M is usually surrounded by solvent molecules, which compete with and eventually replace the ligand molecules, L. To keep things simple, we ignore the solvent molecules and write the four stability constants as follows:
${\text{M + L }} \rightleftharpoons {\text{ ML }}{{\text{K}}_{\text{1}}}{\text{ = }}\left[ {{\text{ML}}} \right]{\text{/}}\left[ {\text{M}} \right]\left[ {\text{L}} \right]$
${\text{ML + L }} \rightleftharpoons {\text{ M}}{{\text{L}}_{\text{2}}}{\text{ }}{{\text{K}}_{\text{2}}}{\text{ = }}\left[ {{\text{M}}{{\text{L}}_{\text{2}}}} \right]{\text{/}}\left[ {{\text{ML}}} \right]\left[ {\text{L}} \right]$
${\text{M}}{{\text{L}}_{\text{2}}}{\text{ + L }} \rightleftharpoons {\text{ M}}{{\text{L}}_{\text{3}}}{\text{ }}{{\text{K}}_{\text{3}}}{\text{ = }}\left[ {{\text{M}}{{\text{L}}_{\text{3}}}} \right]{\text{/}}\left[ {{\text{M}}{{\text{L}}_{\text{2}}}} \right]\left[ {\text{L}} \right]$
${\text{M}}{{\text{L}}_{\text{3}}}{\text{ + L }} \rightleftharpoons {\text{ M}}{{\text{L}}_{\text{4}}}{\text{ }}{{\text{K}}_{\text{4}}}{\text{ = }}\left[ {{\text{M}}{{\text{L}}_{\text{4}}}} \right]{\text{/}}\left[ {{\text{M}}{{\text{L}}_{\text{3}}}} \right]\left[ {\text{L}} \right]$
where $\mathrm{K}_{1}, \mathrm{~K}_{2}$, etc are known as stepwise stability constants. Alternatively, we can express the overall stability constant as follows:
${\text{M + 4L }} \rightleftharpoons {\text{ M}}{{\text{L}}_{\text{4}}}{\text{ }}{{\text{\beta }}_4} = \left[ {{\text{M}}{{\text{L}}_{\text{4}}}} \right]/\left[ {\text{M}} \right]{\left[ {\text{L}} \right]^4}$
12. Importance and Applications of Coordination Compounds
Analytical Chemistry:
The analytical applications of coordination chemistry are in
a. Qualitative and Quantitative Analysis :
Metal ions react with a variety of ligands to form colored coordination compounds. These reactions are used to detect metal ions. The formed colored complexes can be used to estimate metals using traditional or instrumental methods such as gravimetry or colorimetry. The following are some examples:
The addition of potassium ferrocyanide solution detects the presence of iron ions ($Fe^{3+}$), resulting in the formation of the Prussian blue complex.
${\text{F}}{{\text{e}}^{{\text{2 + }}}}{\text{ + }}{{\text{K}}_{\text{3}}}{\text{ Fe}}{\left( {{\text{CN}}} \right)_{{\text{6\;}}}} \to {\text{KFe}}\left[ {{\text{Fe}}{{\left( {{\text{CN}}} \right)}_{\text{6}}}} \right]{\text{ + 2}}{{\text{K}}^{\text{ + }}}$
b. Volumetric Analysis:
Titration with EDTA can be used to determine the hardness of water. $\mathrm{Ca}^{2+}$ and $\mathrm{Mg}^{2+}$, the metal ions that cause hardness, form stable complexes with EDTA.
Metal Extraction and Purification:
Metals such as silver and gold are extracted by forming water-soluble cyanide complexes with the ore. By adding zinc to the solution, pure gold can be extracted. Metals can also be purified by forming and then decomposing their coordination compounds. For example, after extraction, impure nickel can be converted to pure nickel by first converting it to nickel carbonyl and then decomposing it.
Catalysis:
Catalysts for coordination compounds are used in critical commercial processes. For example,
In the formation of polyethene, the Ziegler-Natta catalyst ($TiCl_4$ and trialkyl aluminium) is used as a catalyst.
In the hydrogenation of alkenes, the Wilkinson catalyst - $\operatorname{RhCl}\left(\mathrm{PPh}_{3}\right)_{3}$ is used.
Various rhodium complexes, such as $\left[\mathrm{Rh}(\mathrm{CO})_{2} \mathrm{I}_{2}\right],\left[\mathrm{Rh}(\mathrm{Cl})(\mathrm{CO})\left(\mathrm{PPh}_{3}\right)_{2}\right]$, or $\left[\mathrm{Rh}(\mathrm{Cl})(\mathrm{CO})_{2}\right]_{2}$ are used as catalysts in the Monsanto acetic acid process in the presence of $\mathrm{CH}_{3} \mathrm{l}, \mathrm{I}_{2}$, or HI as activator.
Electroplating:
Gold, silver, and copper coordination compounds are used as components in baths used for electroplating articles made of other metals with these metals. $\mathrm{K}\left[\mathrm{Ag}(\mathrm{CN})_{2}\right]$ is used as an electrolyte in silver plating; $\mathrm{K}\left[\mathrm{Au}(\mathrm{CN})_{2}\right]$ is used as an electrolyte in gold plating; and $\mathrm{K}_{3}$ $\left[\mathrm{Cu}(\mathrm{CN})_{4}\right]$ is used as an electrolyte in copper plating.
Coordination complexes are important biological compounds. Chlorophyll, for example, is a $\mathrm{Mg}^{2+}$ complex. This green pigment is essential for photosynthesis in plants. Similarly, haemoglobin, the red pigment found in blood, is a $\mathrm{Fe}^{2+}$ coordination complex, and vitamin B12, an essential nutrient, is a $\mathrm{Co}^{3+}$ complex.
6. Medicinal uses: In the treatment of metal poisoning, complexing or chelating agents are used, in which a coordination complex is formed between the toxic metal in excess metal and the complexing agent. EDTA, for example, is used to treat lead poisoning. When EDTA is injected intravenously into the bloodstream, it traps lead, forming a compound that is excreted in the urine. Mercury, arsenic, aluminum, chromium, cobalt, manganese, nickel, selenium, zinc, tin, and thallium are other heavy metal poisonings that can be treated similarly with chelation therapy. Similarly, D-penicillamine and desferrioxamine B, chelating ligands, are used to remove excess copper and iron, respectively.
13. Coordination Compounds and Complex Ions
Coordination compounds are those in which the central metal atom is linked to a number of ligands (ions or neutral molecules) via coordinate bonds, i.e. by these ligands donating lone pairs of electrons to the central metal atom ion.
If such a compound has a positive or negative charge, it is referred to as a complex ion, for example, ${\left[ {{\text{Fe}}{{\left( {{\text{CN}}} \right)}_{\text{6}}}} \right]^{{\text{4--}}}}{\text{, }}{\left[ {{\text{Cu}}{{\left( {{\text{N}}{{\text{H}}_{\text{3}}}} \right)}_{\text{4}}}} \right]^{{\text{2 + }}}}{\text{.}}$ Hence Co-ordination compounds are also those that contain complex ions, such as $K_4 [Fe(CN)_6]$, $[Cu(NH_3)_4]SO_4$, and so on. In general, a complex ion is denoted by ${\left[ {{\text{M}}{{\text{L}}_{\text{n}}}} \right]^{ \pm x}}$ where M is the metal ion, L represents ligands, n is the coordination number of metal ion and x is the net charge on the complex.
Four types of complexes are shown below:
Cation as complex ion, (carrying a net positive charge) e.g., $\left[\mathrm{Cr}\left(\mathrm{NH}_{3}\right)_{6}\right]^{3+}$ in $\left[\mathrm{Cr}\left(\mathrm{NH}_{3}\right)_{6}\right.$ ] $\mathrm{Cl}_{3} .$
Anion as complex ion, (carrying a net negative charge) e.g., $\left[\mathrm{Fe}(\mathrm{CN})_{6}\right]^{3-}$ in $\mathrm{K}_{3}\left[\mathrm{Fe}(\mathrm{CN})_{6}\right]$.
Cation and anion both as complex ions. Carrying both positive and negative change.
For e.g., $\left[\mathrm{Pt}(\mathrm{Py})_{4}\right]\left[\mathrm{PtCl}_{4}\right]$
Neutral complex (A complex carrying no net charge) e.g., $\left[\mathrm{Ni}(\mathrm{CO})_{4}\right]$ etc.
14. Terminology of Coordination Compounds
14.1 Centre of Coordination (Central atom/ion or Acceptor atom/ion):
The centre of coordination is the cation or neutral atom to which one or more ligands (neutral molecules or anions) are attached or coordinated. As an acceptor, the central atom/ion must have empty orbitals in order to accommodate electron pairs donated by the ligand's donor atom. This explains why transition metals with empty d-orbitals readily form coordination compounds.
For example in the complexes $\left[\mathrm{Ni}\left(\mathrm{NH}_{3}\right) 6\right]^{2+}$ and $\left[(\mathrm{CN})_{6}\right]^{3-}, \mathrm{Ni}^{2+}$ and $\mathrm{Fe}^{3+}$ respectively are the central ions.
14.2 Ligands
A ligand or coordinating group is an atom, ion, or molecule that can donate at least two electrons to the central atom to form a coordinate bond (or dative linkage). The atom in a ligand that actually donates the electron pair is referred to as the donor atom. The ligands function as Lewis bases by donating one or more electron pairs to the central metal atoms or ions, which function as Lewis acids by accepting electrons.
14.2.1 Types of Ligands:
Ligands are classified based on how many lone pair electrons they donate to the central metal atom or ion.
Monodentate or Unidentate Ligands: These ligands have a single donor atom that donates only one electron pair to the central metal atom.
Bidentate Ligands: Ligands with two donor atoms and the ability to link with the central metal in two positions are referred to as bidentate ligands.
Tridentate Ligand: The ligands that possess three donor atoms are called tridentate ligands
Tetradentate Ligand: These ligands have four donor atoms.
Pentadentate Ligands: They have five donor atoms
Hexadentate Ligands: They have six donor atoms.
14.2.2 Chelating Ligands:
A bidentate or polydentate ligand is referred to as a chelating ligand if it forms a cyclic ring structure upon coordination. Chelates are the complexes that result from this process. Chelates with 5 or 6 membered rings are more stable. Due to steric hindrance, larger group ligands form more unstable rings than smaller group ligands.
14.2.3 Ambidentate Ligands:
The ligands that have two donor atoms but only one donor atom are attached to the metal atom at a time when forming complexes. These ligands are known as ambidentate ligands. As an example:
$\mathrm{M} \leftarrow \mathrm{NO}_{2}$ Nitrito-N
$\mathrm{M} \leftarrow \mathrm{CN}$ Cyano-C
$\mathrm{M} \leftarrow \mathrm{SCN}$ Thiocyanato-S
$\mathrm{M} \leftarrow \mathrm{ONO}$ Nitrito-O
$\mathrm{M} \leftarrow \mathrm{NC}$ Isocyano
$\mathrm{M} \leftarrow \mathrm{NCS}$ Thiocyanato-N
15. Coordination Number (C.N)
The number of atoms in the ligands that are directly bound to the central metal atom or ion by coordinate bonds is known as the metal atoms or ion's coordination number. It is also the same as secondary valency.
What are Coordination Compounds?
Coordination compounds contain ions or molecules linked or coordinated to a transition metal. A few examples of the coordination compounds include [Ni(H2O)6]Cl2, [Cr(NH3)5(NO2)]2+. Ag(CN)2-, CuCl42- are called the coordination complexes or the complex ions. Ligands are the ions or molecules that combine with the transition metal ions to produce these complexes further.
The coordination number of any of the Coordination compounds is given by the total number of ligands that are associated with the transition metal ion. Coordination compounds include substances such as chlorophyll, haemoglobin, vitamin B12, catalysts, and dyes, used in the preparation of the organic substances. The Coordination compounds are also used as catalysts for several biological and industrial processes having much importance in the qualitative and quantitative chemical analysis within the field of analytical chemistry.
Important Applications of the Coordination Compounds
Let us look at the essential applications of the Coordination Compounds.
Because of the formation of cyanide complexes (dicyanoargentate and dicyanoaurate), noble metals, such as silver and gold are extracted from their ore
The haemoglobin is one of the coordination compounds of iron
In the ethene polymerization, the Ziegler Natta catalyst (a combination of titanium tetrachloride and the triethyl aluminum) is used
A catalyst of a complex metal is used in the hydrogenation of alkenes
When the aqueous ammonia is mixed with the copper sulphate solution, a deep blue complex is formed, which is soluble in water. This reaction is helpful to detect the cupric ions present in the salt
Class 12 Chemistry Chapters 5 Formulas and Concepts
1. Werner's Coordination Theory: Werner proposed the theory of coordination compounds, stating that metal ions exhibit two types of valencies - primary and secondary. Primary valency determines the oxidation state of the metal ion, while secondary valency determines the coordination number and the number of ligands attached to the metal ion.
2. Stability Constant (Kₛ): The stability constant (also known as formation constant) is a measure of the stability of a complex ion in solution. It is defined as the equilibrium constant for the formation of the complex ion from its constituent ions.
3. Isomerism: Coordination compounds exhibit various types of isomerism including structural isomerism (geometric isomerism, linkage isomerism), and stereoisomerism (optical isomerism, geometrical isomerism).
4. Crystal Field Theory (CFT): CFT explains the electronic structure and properties of transition metal complexes by considering the interaction between the d orbitals of the metal ion and the ligand's electron pairs.
Class 12 Chemistry Chapter 5 Coordination Compounds Notes Important Topics and Subtopics
S.No. | Topics | Subtopics |
1 | Introduction to Coordination Compounds | Definition, Examples, Importance |
2 | Ligands | Types of ligands (monodentate, bidentate, polydentate), Chelating ligands |
3 | Coordination Number | Definition, Common coordination numbers (2, 4, 6) |
4 | Nomenclature of Coordination Compounds | IUPAC rules for naming coordination compounds |
5 | Isomerism in Coordination Compounds | Structural isomerism, Stereoisomerism (Geometrical and Optical) |
6 | Bonding in Coordination Compounds | Valence Bond Theory (VBT), Crystal Field Theory (CFT) |
7 | Stability of Coordination Compounds | Factors affecting stability, Stability constants |
8 | Applications of Coordination Compounds | Biological roles (hemoglobin, chlorophyll), Industrial applications (catalysis) |
Importance of Revision Notes for Class 12 Chemistry Chapter 5
Summarises Key Points: Condenses important concepts for quick review.
Saves Time: Provides a fast way to revise before exams.
Highlights Essentials: Focuses on crucial topics and definitions.
Improves Memory: Helps in better retention of information.
Enhances Exam Prep: Targets weak areas for more effective study.
Clarifies Concepts: Simplifies complex ideas for easier understanding.
Includes Visuals: Uses diagrams and charts for better grasp.
Boosts Confidence: Prepares students thoroughly for exams.
Tips for Learning the Class 12 Chapter 5
Focus on core processes with illustrations and examples.
Draw and label diagrams for clarity.
Create summaries of each process.
Connect concepts to everyday examples.
Solve past exam questions to test understanding.
Explain concepts to others to reinforce learning.
Revisit material frequently to retain information.
Utilise platforms like Vedantu for additional support.
Conclusion
The Class 12 Chemistry Chapter 5: Coordination Compounds is a vital chapter that explains the nature and structure of coordination compounds, their importance in various fields, and their complex bonding mechanisms. Vedantu’s notes simplify the challenging concepts and make them easy to understand. Download the FREE PDF from Vedantu to help with exam preparation and for better retention of the important concepts covered in this chapter.
Related Study Materials for Class 12 Chemistry Chapter 5
S.No. | Important Study Material Links for Class 12 Chemistry Chapter 5 |
1. | Class 12 Chemistry Coordination Compounds Important Questions |
2. | |
3. | Class 12 Chemistry Coordination Compounds Exemplar Solutions |
Revision Notes Links for Class 12 Chemistry Revision Notes
S.No. | Chapter-wise Links for Class 12 Chemistry Revision Notes |
1 | |
2 | |
3 | |
4 | |
5 | |
6 | |
7 | |
8 | |
9 |
Other Important Study Material Links for Class 12 Chemistry
S.No. | Related Study Materials for Class 12 Chemistry |
1. | |
2. | |
3. | |
4. | |
5. | |
6. |
FAQs on CBSE Notes Class 12 Chemistry Chapter 5 - Coordination Compounds - 2025-26
1. What are the key concepts covered in the Coordination Compounds Class 12 revision notes?
The revision notes for Coordination Compounds in Class 12 Chemistry cover core concepts such as definition and importance of coordination compounds, types of ligands, coordination number, isomerism, nomenclature, bonding theories (VBT and CFT), stability of complexes, their applications, and summary tables of formulas as per CBSE 2025–26 syllabus.
2. How do revision notes help in preparing for the Class 12 Chemistry Chapter on Coordination Compounds?
Revision notes condense the chapter into essential points, making them ideal for quick last-minute preparation. They simplify complex ideas, highlight exam-relevant keywords and definitions, and provide structured summaries—helping students review the chapter’s structure, formulas, and interconnections efficiently.
3. What is the recommended order to revise topics in Coordination Compounds using short notes?
A suggested revision order is:
- Introduction and definitions (coordination compound, ligand, coordination number)
- Types of ligands and nomenclature rules
- Isomerism (structural and stereoisomerism)
- Bonding theories (Valence Bond Theory and Crystal Field Theory)
- Stability and applications (biological and industrial)
4. What are the most important key terms and definitions to remember for quick revision in Coordination Compounds?
Key terms include:
- Coordination Sphere: The central atom/ion plus attached ligands, shown in square brackets.
- Ligand: Ion/molecule donating electron pairs to central metal.
- Coordination Number: Number of ligand atoms attached to central atom.
- Isomerism: Compounds with same formula but different structures (geometric, optical, linkage).
- Valence Bond Theory (VBT) and Crystal Field Theory (CFT): Models explaining bonding in complexes.
5. How can I use concept maps or summary sheets to enhance my revision of Class 12 Coordination Compounds?
Concept maps and summary sheets visually link main topics—like types of ligands, isomerism, and bonding theories—making it easier to recall relationships and differences. Creating diagrams for geometrical arrangements, coordination spheres, and isomer structures helps reinforce memory retention and clarity during revision.
6. What common misconceptions should I avoid when revising Coordination Compounds for quick exams?
Common misconceptions include mixing up primary and secondary valency, confusing coordination number with oxidation state, misunderstanding the difference between double salts and coordination compounds, and errors in isomerism classification. Focus on definitions and their specific examples as given in the revision notes.
7. What types of questions should I focus on while revising Coordination Compounds for board exams?
Prioritize questions on:
- Defining and explaining key terms
- Nomenclature exercises
- Distinguishing types of isomerism
- Drawing and identifying structures of complexes
- Application-based short answer questions on bonding and stability
8. How are the concepts of isomerism and bonding connected in Coordination Compounds as per revision notes?
Isomerism in coordination compounds arises due to the arrangement of ligands around the central metal and how these ligands bond (as explained by VBT and CFT). Understanding bonding theories helps predict possible geometries and thus which types of geometrical or optical isomers can exist for a given complex.
9. What are quick memory aids or tips provided for effective revision of Coordination Compounds?
Some quick tips are:
- Use mnemonics for remembering ligand types and nomenclature rules
- Create diagrams showing coordination spheres and 3D structures
- Relate biological examples (like haemoglobin, chlorophyll) for practical understanding
- Practise sample questions and visualize isomerism
10. Why is the revision of Coordination Compounds essential before the Chemistry board exam?
This chapter is rich in definitions, formulas, and conceptual variety. Quick revision ensures that all key points—like types of ligands, bonding, and applications—are fresh in memory, helping you answer both direct and application-based questions efficiently in the exam.


























