
Mineral Nutrition in Plants Revision Notes Free PDF for NEET Botany Preparation
Class 11 Botany has an excellent chapter based on mineral nutrition that plants need for proper growth and to maintain their physiological functions. The inorganic substances plants need and absorb from various sources will be explained in this chapter. It will also describe how to study mineral nutrition using specific methods. To understand the importance of this chapter, refer to the Mineral Nutrition Class 11 notes prepared by the subject experts of Vedantu.
These notes have been designed to offer a simpler version of this chapter. Students will find a concise description of the fundamental concepts related to minerals required by plants for various purposes. These notes will help NEET aspirants to quickly revise this chapter and reduce the preparation time considerably.
Access NEET Revision Notes Biology Mineral and Nutrition
Introduction
To complete their life cycle, organisms require a wide range of organic and inorganic elements. Their nutrition consists of all such items obtained from outside sources.
Organisms are classified as heterotrophs or autotrophs based on their nutritional requirements.
Most of the non-green plants and animals, including humans, are heterotrophs.
Mineral nutrition is a type of nutrition in which autotrophic green plants get their nourishment from inorganic compounds found in soil in the form of minerals, often known as mineral elements or mineral nutrients.
- Using this strategy, essential components were identified and their deficiency symptoms were revealed.
Hydroponics:
Hydroponics has been used to commercially produce vegetables such as tomato, seedless cucumber, and lettuce with great success.
It is critical to emphasise that the nutrient solutions be appropriately aerated in order to achieve optimal growth.
The practice of soilless cultivation is often known as tank farming because the plants are grown in enormous tanks.
Knop was the first to create a hydroponic culture solution. Knop's solution, Hoagland's solution, Arnon's solution, and Sach's solution are some of the most well-known nutritional solutions.
The importance of mineral nutrients can be determined via hydroponic or soilless culture.
The symptoms of deficiency arose as a result of the lack of availability of specific nutrients.
When an element is present in excess in a plant, it becomes hazardous.
The potential for interaction between several components found in plants
The function of key components in plant metabolism
Hydroponics is beneficial in locations where the soil is thin, unproductive, and arid. They save water, regulate the appropriate pH for a certain crop, control pests, and disease, prevent problems through weeding, and lower labor costs.
Types of Elements:
Essential elements are substances that plants require for optimal growth and development and without which they cannot complete their life cycle.
Essential element deficiency causes trouble because essential elements are incorporated by plants in the production of structural or functional molecules.
Plants have about 50-60 elements in their bodies, but only 16-17 of them are deemed essential. C, H, O, N, K, S, Ca, Fe, Mg, P, Cu, Mn, B, Cl, Zn, Mo, Ni, Ca, Fe.
Non-essential elements are those that are present in the plant body but are not required by plants. For example, Na, Si, Al, Se, Sr, and V.
On the basis of plant macronutrient and micronutrient requirements, Arnon separated these required mineral elements into two groups.
Macronutrients/major elements: It must have a concentration of $1-10 \mu \mathrm{g} \mathrm{L}^{-1} / 10$ m mole $\mathrm{kg}^{-1}$ dry matter.
$\mathrm{C}, \mathrm{H}, \mathrm{O}, \mathrm{N}, \mathrm{K}, \mathrm{S}, \mathrm{Ca}, \mathrm{Fe}, \mathrm{Mg}, \mathrm{P}$, and so on.
In comparison, they are required in vast quantities.
Protoplasm is made up primarily of the elements $C, H, O, N$, and $P$. (organic materials).
As a result, they are referred to as protoplasmic elements. In mineral nutrition, $\mathrm{C}$ and $\mathrm{O}$ are received from the environment, whereas $\mathrm{H_2} \mathrm{O}$ is obtained from the soil. Nucleic acid, proteins, enzymes, carbohydrates, and lipids all contain the atoms C, H, and O. (Framework elements).
Micronutrients/minor elements:Its concentration is less than $1.0-0.1 \mu \mathrm{g} \mathrm{L} / 10 \mathrm{~m}$ mole $\mathrm{kg}^{-1}$ of dry matter (minor element/micronutrients).
$\mathrm{Cu}, \mathrm{Zn}, \mathrm{Mn}, \mathrm{B}, \mathrm{Cl}, \mathrm{Mo}$, and $\mathrm{Ni}$, for example.
- In comparison, they are required in smaller quantities.
Essential Elements are Classified Into Four Groups Based on Their Function:
Biomolecule components (e.g., C, $\mathrm{H}, \mathrm{O}, \mathrm{N}$ ), biomolecule components (e.g., $\mathrm{C}, \mathrm{H}, \mathrm{O}$, $N$ ), and biomolecule components (e.g., $C, H, O, N$ ), and biomolecule components (e.g., C, Mg in chlorophyll and phosphorus in ATP are examples of energy-related chemical molecules.|
$\mathrm{Mg}^{2+}$ promotes ribulose bisphosphate carboxylase, oxygenase, and phosphoenolpyruvate carboxylase, and inhibits phosphoenolpyruvate carboxylase.
During nitrogen metabolism, $\mathrm{Zn}^{2+}$ activates alcohol dehydrogenase and Mo of nitrogenase.
Potassium, for example, is critical in the opening and closing of stomata because it alters the cell's osmotic potential.
Beneficial nutrients are mineral elements other than essential elements that meet certain supplementary nutrient requirements of specific plants.
An example of a halophyte is Atriplex which is also a C4 plant.
Silicon (Si) provides mechanical strength in Grasses.
- Cobalt (Co) in Leguminous plants and Selenium (Se) in Astragalus helps root nodule formation.
Minerals' Common Functions:
Protoplasmic elements include carbon, hydrogen, oxygen, nitrogen, phosphorus, and sulfur.
Maintain the cell's osmotic pressure.
Ca and K play an antagonistic (balancing) role by neutralizing the toxicity of hazardous chemicals.
- Ca+ and K+ regulate cell membrane permeability.
The Impact of Macro and Micronutrients
It is beneficial to understand the structure and functions of macro and micronutrients in order to obtain a better understanding of them and recognize their roles.
- Nitrogen, phosphorus, and potassium are the most significant crop nutrients since they directly affect plant growth and the formation of various plant parts.
Nitrogen
Plants require it for a variety of reasons.
Metabolism is a vital substance that includes amino acids, proteins, and enzymes.
Germination and vegetative development are influenced.
It serves a key function in photosynthesis as a component of chlorophyll.
The quick growth of foliage is due to this.
- It gives the plants a green hue.
Iron
Iron deficiency in plants causes reduced crop growth, chlorosis (leaf yellowing), and weakening during fruiting and flowering, all of which result in lower yields.
- Too much nitrogen is bad for plants because it creates dark green leaf colouration, lush growth, crop lodging, and reduced fruit quality.
Phosphorus
Plants require it for a variety of reasons.
The formation of roots
Maintaining high flowering, fruiting, and seed production quality.
Energy storage and transportation
Disease resistance.
- Plant development is stunted, roots are weakened, shoots are thin, and leaves are dark green/purple/red due to phosphorus deficiency. Excess phosphorus can lead to decreased reception and shortage in other elements such as Zn, Fe, Cu, Mn, and B.
Potassium
Potassium is a mineral found in many foods (K)
Plants require it for the following reasons:
Having an impact on water intake.
Drought tolerance improvement.
Cold hardiness has improved.
Providing fungal disease and insect pest resistance.
Protein, sugar, and fat synthesis.
- Potassium deficiency in plants causes stunted growth, burning or yellowing of the leaf margins, and dead patches on older leaves. Excess levels are also harmful because they interfere with the absorption of other nutrients like magnesium, calcium, and nitrogen.
Plant Micronutrients and Their Functions
The most important micronutrients in a plant organism are boron, iron, manganese, and zinc. Let's have a look:
Boron
It is necessary for:
Transport of sugar.
Production of amino acids.
Formation of the cell wall
Reproduction of the crop.
Fruiting.
Flowering.
Crop quality improvement.
Stunted growth of immature crops, deformation of leaves, death of growing points, dark brown lesions on leaves, poor flowering, and chlorosis or yellowing of foliage are all indications of boron shortage in plants. It is critical that boron is applied prior to the blossoming stage of crops; adding it later is not advantageous.
Iron
Iron is necessary for:
Production of chlorophyll.
Photosynthesis.
Composition of enzymes.
Energy transfer, nitrogen reduction, and fixation are all affected.
Formation of lignin.
Plants with an iron deficiency suffer from yellowing between the veins, which is harmful to younger leaves.
Manganese
Manganese is a mineral that is found in abundance in (Mn)
Plants Require It for the Following Reasons:
Influences the formation of chloroplasts.
Participating actively in the photosynthetic process.
Enzyme activation and influence on germination and crop maturity.
Mn deficiency can also cause chlorosis, which is the yellowing of veins in younger leaves.
Zinc
Plants Require it for the Following Reasons:
Early phases of development.
Root, seed, and fruit development
During the photosynthetic process.
Hormone balance in plants.
Symptoms of Deficiency
When an element's concentration falls below the critical level, it is said to be deficient.
The critical concentration of essential components is the level below which plant growth is slowed.
Deficiency Symptoms are highly evident pathological disorders that occur as a result of a lack or deficiency of a vital nutritive component.
Deficiency symptoms of highly mobile elements such as N,P,K, and Mg in plants first occur in older plant sections. These minerals are present as structural constituents of adult plant parts' proteins, which are broken down as the plant parts age, making these elements available to younger plant parts.
Immobile element deficiency symptoms, such as Ca and S, emerge initially in young plant parts and are not transmitted from older plant parts.
Any mineral ion concentration in plant tissue that reduces the dry weight of tissue by around 10% is considered harmful, and this impact is referred to as toxicity.
Because the amount of micronutrients required by plants is so small, the majority of them become harmful. Other necessary elements are inhibited by the excess concentration.
Excess Mn (Manganese) can lead to iron, magnesium, and calcium shortage, as well as the formation of brown blotches with chlorotic veins. Mn competes for absorption with iron (Fe) and magnesium (Mg), as well as binding to enzymes with Mg. Mn also prevents calcium from reaching the shoot apex, causing crinkle leaf disease.
As a result, the symptoms of Mn poisoning may be confused with those of Fe, Mg, and Ca insufficiency.
Mechanism of Element Absorption
Mineral salts are primarily found in soil. The (sub-terminal) meristematic area of the roots absorbs the majority of these mineral salts.
Mineral salts are absorbed in two ways: passively and actively.
Minerals Passive Absorption (Without Expenditure of Atp)
Simple Diffusion: Mineral ions from the soil solution may diffuse into root cells via this mechanism.
Mineral ion absorption happens with the flow of water under the influence of transpiration, according to this approach.
Ion Exchange: This involves mineral ions exchanging with ions of the same charge.
When mineral ions come into contact with H+ and OH– ions, this is known as contact exchange.
Carbonic acid exchange occurs when mineral ions interact with carbonic acid ions.
Donnan Equilibrium:
With ATP, the passive buildup of ions against a concentration gradient or electrochemical potential (ECP) is explained by Donnan equilibrium.
- Some anions are fixed or non-diffusible at the inner side of the cell membrane that separates from the outside (external medium), and the membrane is impermeable to these anions, whereas cations are diffusible.
Absorption of Active Ion (By Expenditure of Atp)
The following are examples of evidence in favour of active mineral absorption:
When a plant is placed in a mineral solution, its rate of respiration increases (salt respiration).
Factors that slow the rate of respiration, such as a lack of oxygen, CO, or CN, also slow the absorption of mineral ions in plants.
- In Nitella algae, K+ ion absorption is found against a concentration gradient.
Cytochrome Pump Theory By Lundegardh Burstorm, 1933
Only anions are absorbed by active mechanisms through cytochrome pumping, according to this view, while cation absorption is a passive process.
Concept of the Carrier: (By Vanden Honert)
According to this notion, the cell membrane of the root cell contains some unique carrier molecules made up of proteins that absorb both ions and create an ion-carrier complex. With the expenditure of energy, this complex fractures inside the cell membrane.
Mineral Uptake Factors
The following elements, such as temperature and light, have an impact on the mineral absorption process.
Temperature: Temperature has a direct relationship with the rate of absorption of salts and minerals.
When the temperature reaches its maximum limit, mineral ion absorption is impeded, possibly due to enzyme denaturation.
Light: The more light there is, the more photosynthesis takes place. As a result, there is more dietary energy available, and salt intake rises.
Oxygen: A lack of ${O_2}$ results in a decrease in the rate of mineral absorption. It's most likely due to ATP shortages. Greater oxygen tension aids in increased salt uptake.
pH: pH regulates the availability of ions in the body, which impacts the rate of mineral absorption. Monovalent ions are absorbed more quickly at normal physiological pH, whereas bivalent and trivalent ions are absorbed more quickly at alkaline pH.
The absorption of one type of ion is altered by the absorption of another type. $\mathrm{Ca}^{++}, \mathrm{Mg}^{++}$, and other polyvalent ions have an effect on $\mathrm{K}^{+}$ absorption. It's most likely owing to a lack of binding sites on the carrier. In the presence of $\mathrm{Ca}^{++}$ions, however, $\mathrm{K}^{+}$and $\mathrm{Br}^{-}$absorption is conceivable. In the absorption of $\mathrm{K}, \mathrm{Rb}$, and $\mathrm{Cs}$ ions, there is a mutual competition.
- The surface area, number of cells, and number of mineral ion binding sites all increase as a result of healthy growth. Mineral absorption is improved as a result.
Translocation of Solutes (Mineral Salts)
It has been proven that inorganic compounds travel up the plant through xylem using radioisotopes. The transpiration pull pulls these chemicals along with the water.
The rate of inorganic solute translocation through the xylem is equal to the rate of water translocation. Ions can reach the xylem via two paths after mineral absorption by roots: apoplast and symplast pathways.
Soil is as Essential Element in Reservoir
Plants develop in soil because it provides them with anchoring, air, water, and minerals.
The majority of the nutrients required for plant growth and development are made available to the roots as a result of rock weathering and breakdown.
Mineral nutrition refers to their significance in plant nutrition because they are derived from rock minerals.
Soil is made up of a vast range of components. Soil not only contains minerals but also bacteria that fix nitrogen and other microorganisms.
Because essential mineral deficiency has an impact on crop productivity, fertilisers are frequently used to supplement them.
Fertilisers contain both macro-nutrients (N, P, K, S, etc.) and micro-nutrients (Cu, Zn, Fe, Mn, etc.) that are administered as needed.
Nitrogen Metabolism
Nitrogen is found in the environment as oxides, organic amines, and other compounds. The amount of nitrogen in the environment is 78.8% by volume.
Plants are unable to absorb nitrogen in its molecular state. Plants absorb it in two forms: nitrate $\left(\mathrm{NO}^{-}\right)$and ammonium $\left(\mathrm{NH}^{+}\right)$.
Cycle of Nitrogen
The most important element is nitrogen. Nitrogen is the most abundant element in living creatures, followed by carbon, hydrogen, and oxygen.
Nitrogen can be present in proteins, nucleic acids, growth regulators, and a variety of vitamins.
Plants and microorganisms struggle for the limited nitrogen available in soil. As a result, nitrogen is a nutrient that is limited in both natural and farmed environments.
Nitrogen is made up of two nitrogen atoms connected by a strong triple covalent bond (N ≡ N).
The process of nitrogen fixation converts atmospheric N2 gas into ammonia.
Lightning and ultraviolet radiation give enough energy for nitrogen to be converted to nitrogen oxides in nature (NO, NO2, N2O).
Industrial combustion, forest fires, automotive exhaust, and power plants are all sources of nitrogen oxides in the atmosphere.
The nitrogen cycle ensures that the plants receive a consistent supply of nitrogen. The nitrogen cycle refers to the constant movement of nitrogen among living organisms.
Nitrogen fixation, ammonification, nitrification, and denitrification are the four processes that make up the nitrogen cycle.
Nitrogen Fixation
Nitrogen fixation is the process of converting inert atmospheric nitrogen (N2) into usable nitrogen compounds such as nitrate, ammonia, and amino acids. The biological and abiological ways of nitrogen fixation are the two types of nitrogen fixation.
In the atmosphere, physical or biological nitrogen-fixing takes place in four stages.
Lightning causes nitrogen to be converted to nitric oxide.
Nitrogen dioxide is produced through the oxidation of nitrogen oxide (Nitrogen dioxide)
When nitrogen dioxide is dissolved in rainwater, it forms nitrous and nitric acid in the soil.
These react with soil alkali to generate nitrates which are absorbable form. nitrates
On a large scale, abiological fixation is accomplished using the Habers–Bosch nitrates process, which is carried out at high pressure and temperature.
Biological nitrogen fixation is the conversion of gaseous nitrogen into nitrogenous compounds by living organisms such as bacteria and cyanobacteria.
Ammonification
In the soil, nitrogenous organic molecules in plants and animals' dead bodies are transformed to ammonia or ammonium ions. Ammonifying bacteria are responsible for this.
Bacillus mycoides, Bacillus yugaris, Bacillus ramosus, and other ammonifying bacteria cause ammonification.
Ammonification is a type of mineralisation.
Protein is a type of food (from dead cells) Amino acids are the building blocks of proteins.
Microorganisms use the organic acid generated in this process for their own metabolism.
Some of the ammonia is volatilized and re-emitted into the atmosphere, but the majority is transformed to nitrate.
If plants do not take ammonia directly, it is transformed to nitrate through the nitrification process.
Nitrification
Nitrification is the process of converting $\mathrm{NH} 3$ in soil to nitrates $\left(\mathrm{NO}_{3}{ }^{-}\right)$and nitrites $\left(\mathrm{NO}_{2}{ }^{-}\right.$).
Nitrosomonas, Nitrosococcus (converts $\mathrm{NH}_{3}$ to nitrites), and Nitrobacter are examples of nitrifying bacteria (converts nitrites into nitrates).
Nitrifying bacteria are chemoautotrophs, meaning they benefit from the energy created during oxidation and utilised in chemosynthesis.
The activity of ammonifying and nitrifying bacteria is reported to be highest at soil temperatures of $30^{\circ} \mathrm{C}-35^{\circ} \mathrm{C}$ in alkaline soils with sufficient moisture and aeration.
Plants absorb the resulting nitrate and transmit it to their leaves. It is reduced in leaves to generate ammonia, which then forms the amine group of amino acids.
Denitrification:
The conversion of nitrates and nitrites in soil into atmospheric ${N_2}$ is called denitrification, which is done by denitrifying bacteria, e.g., Micrococcus denitrificans and Bacillus denitrificans, Pseudomonas & Thiobacillus.
Denitrification occurs in four steps –
Nitrifying bacteria are chemoautotrophs, which means they get energy from oxidation and use it for chemosynthesis. The activity of ammonifying and nitrifying bacteria is reported to be maximum in alkaline soils with appropriate moisture and aeration at soil temperatures of $30^{\circ} \mathrm{C}-35^{\circ} \mathrm{C}$.
The resultant nitrate is absorbed by the plants and transmitted to their leaves. It is reduced in leaves to make ammonia, which subsequently forms the amino acid's amine group.
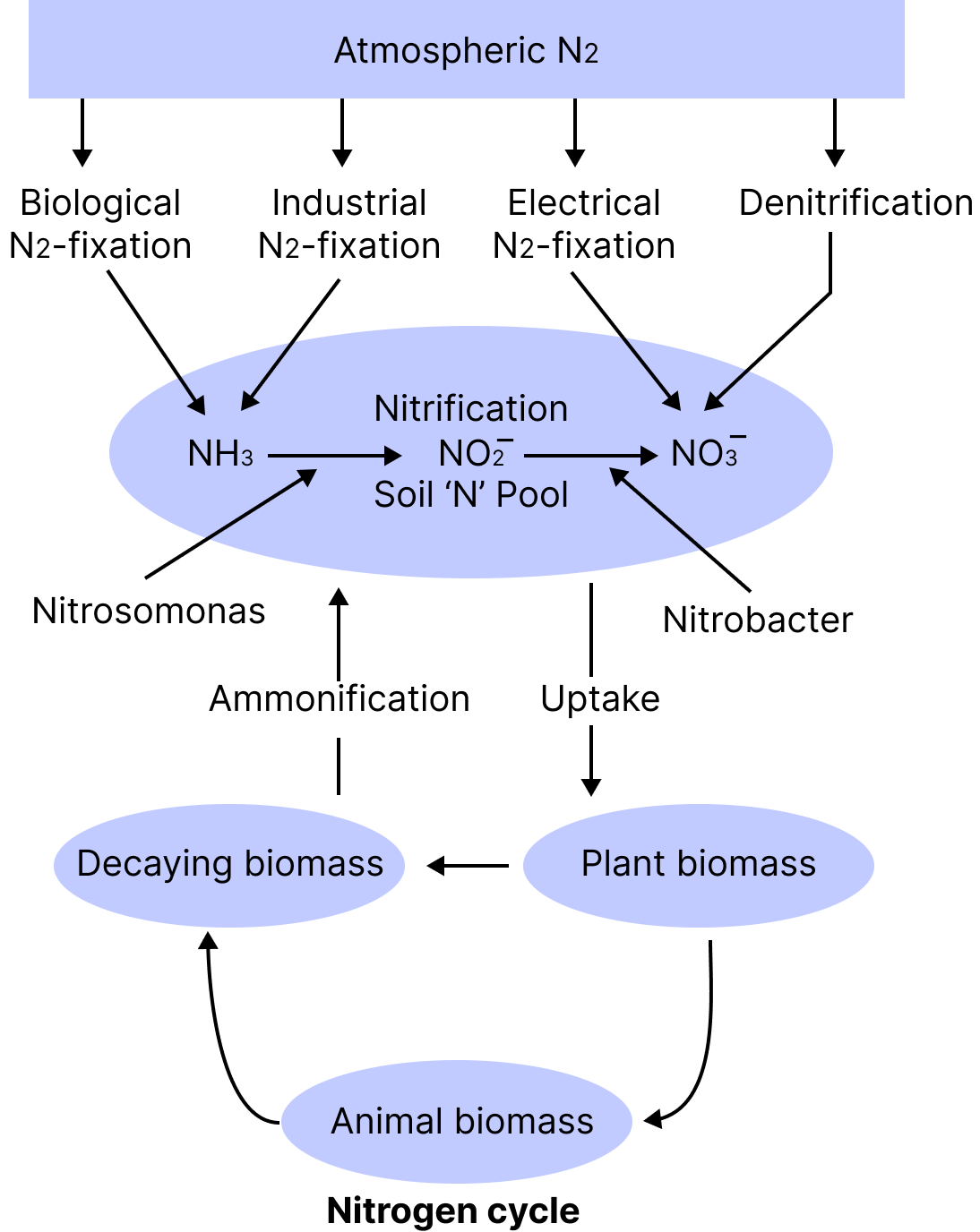
Nitrogen Cycle
Nitrogen Fixation in Biological Systems
Biological nitrogen fixation is the process of living organisms converting atmospheric nitrogen into usable (inorganic nitrogenous compound) form.
Nitrogenase, an enzyme capable of nitrogen reduction, is found in prokaryotes. These bacteria are known as ${N_2}$-fixers.
Free-living (asymbiotic) or symbiotic microorganisms are two examples.
Free-living bacteria that fix nitrogen. Bacillus, Klebsiella, Clostridium (both aerobic) and Azotobacter, Beijernickia (both aerobic) (anaerobic).
Cyanobacteria that fix nitrogen in the wild. Anabaena, Nostoc, Calothrix, Lyngbya, Aulosira, Cylindrospermum, and Trichodesmium are examples of free-living blue-green algae (BGA) or cyanobacteria that fix nitrogen.
Cyanobacteria are primarily responsible for preserving rice fields' fertility and productivity. In sugarcane and maize fields, for example, Nostoc, Anabaena, and Cylindrospermum are active.
Cyanobacteria that fix nitrogen in a symbiotic relationship. Symbionts in lichens include Anabaena and Nostoc species.
Bacteria that fix nitrogen in a symbiotic relationship.
The link between legumes and bacteria is the most well-known. Alfalfa, sweet clover, sweet pea, lentils, garden pea, broad bean, clover beans, and other legumes have such a connection with rod-shaped Rhizobium species.
Nodules are the most prevalent root association. The nodules on the roots are tiny outgrowths.
On the roots of non-leguminous plants, the bacterium Frankia creates nitrogen-fixing nodules (e.g., Alnus).
${N_2}$ fixation takes place in nodules. It contains the pink pigment leghaemoglobin as well as the enzyme nitrogenase (Mo-Fe protein).
Rhizobium and Frankia are both free-living in soil, but they can fix atmospheric nitrogen as symbionts.
Formation of Nodules
The production of nodules is the result of a series of interactions between Rhizobium and the roots of the host plant.
The following are the major phases in the creation of a nodule:
Rhizobia colonise the environment around roots and connect to epidermal and root hair cells.
When the root hair of leguminous plants comes into touch with Rhizobium, the bacteria's chemical compound deforms the hair's curves, resulting in the formation of nodules.
Bacteria residing in such nodules consume glucose from the host cell and convert absorbed nitrogen from the air into ammonia.
A combination of cytokinin produced by infected bacteria and auxin produced by plant cells is thought to accelerate cell proliferation and extension, resulting in the creation of nodules.
Plant nod genes and the bacterial nod, nif, and fix gene cluster control the formation of root nodules and nitrogen fixation.
The following ingredients are required for nitrogen fixation:
A strong reducing agent (FAD).
The enzyme system ATP
The atmospheric ${N_2}$ (dinitrogen) is decreased by adding hydrogen atoms to ammonia during this process.
Amino Acids Synthesis
The first products of nitrogen absorption are amino acids. Both nitrate and ammonium ions ($N{H^{4+}}$) can be assimilated by most plants.
In plants, $N{H^{4+}}$ is used to synthesise amino acids.
The following are the two processes for amino acid synthesis:
Amination through reductive oxidation -Glutaric acid is formed when ammonia interacts with ketoglutaric acid.
Transamination is the process of the transaminase enzyme transferring the amino group of one amino acid to the keto group of another amino acid.
Glutamic acid is the primary amino acid from which the NH2 amino group is transferred and additional (17) amino acids are produced via transamination.
Amides
Amides are the structural component of most proteins and contain more nitrogen than amino acids.
Amides, such as asparagine and glutamine, are doubly aminated keto acids. They are created by adding another amino group to two amino acids, notably aspartic acid and glutamic acid, respectively.
Another NH2– radical replaces the hydroxyl portion of the acid. Because amides contain more nitrogen than amino acids, they are transported by xylem vessels to other areas of the plant.
In addition, the nodules of some plants (e.g., soyabean) export the fixed nitrogen as ureides together with the transpiration stream. In addition, the nitrogen to carbon ratio of these molecules is unusually high.
Key Points to Remember:
Inorganic nutrients are obtained by plants from the air, water, and soil.
Plants are capable of absorbing a wide range of mineral elements.
Plants do not require all of the mineral elements that they absorb. Less than 21 of the more than 105 elements observed so far are required and valuable for growth and development of a normal plant.
The elements necessary in large quantities are referred to as macronutrients, while those required in small amounts or in trace amounts are referred to as micronutrients.
These elements are either necessary components of proteins, carbohydrates, fats, nucleic acids, and so on, or they participate in various metabolic processes.
Deficiencies in any of these crucial components can cause symptoms known as deficiency symptoms. Some common deficiency symptoms include chlorosis, necrosis, impaired cell division, stunted growth, and so on.
Plants absorb minerals either passively or actively through their roots. They are transported to all parts of the plant via xylem, along with water.
Nitrogen is critical for the survival of life. Plants cannot directly utilise atmospheric nitrogen. However, some plants, particularly legume roots, can fix atmospheric nitrogen into physiologically usable forms in collaboration with N2-fixing bacteria.
Nitrogen fixation necessitates the use of a strong reducing agent as well as energy in the form of ATP.
Nitrogen Fixing microbes, primarily Rhizobium, are used to achieve N2-fixation. Nitrogenase, an enzyme involved in biological N2-fixation, is extremely sensitive to oxygen. The majority of the processes occur in an anaerobic environment.
The required energy, ATP, is supplied by the host cells' respiration. The amino group is formed when ammonia is formed as a result of N2-fixation.
Importance of NEET Class 11 Botany Mineral Nutrition
Mineral nutrition is a chapter in Class 11 Botany that covers the various aspects of sources of minerals plants need, their modes of absorption, proper distribution processes, and metabolism. It will also explain how these elements are needed for the development, physiology, structure, and reproduction of the plants.
The chapter explains how we can study the absorption and use of specific minerals required by plants using various proven methods. One such method is hydroponics, where plants are grown in a water-based platform with proper mineral solutions.
You will discover that plants need as many as 65 different minerals or elements for all the physiological functions. It will also explain the essentiality of elements considering a few factors. These elements will then be divided into macro and micronutrients based on the amount required. It will also classify into different classes based on its functions.
This chapter elaborately covers all the aspects of minerals used by the plants for various purposes. Its importance in botany is immense as it offers the crucial factors that plant physiology needs to survive. To understand these concepts, refer to the Mineral Nutrition Class 11 NEET notes.
Benefits of Vedantu’s Mineral Nutrition Class 11 NEET Notes
These notes are designed by following the Class 11 NEET syllabus and CBSE Class 11 level. The chapter has been converted into a concise format to offer a simpler version to the NEET aspirants. Using these notes will ensure you can revise this chapter within a shorter period.
There is no need to struggle with making notes as the experts have already done it for you. You can refer to these notes to revise and complete the chapter at your convenience.
Resolve queries using Mineral Nutrition in Plants notes PDF faster and utilise your time to solve sample and mock test papers.
The notes have an easy format to understand and recall what you have studied. There is no need to remember the vast format of the textbook for this chapter. In fact, you can remember the right answer to all the fundamental questions by using these notes to revise and score more in the NEET Exam.
Download Free Mineral Nutrition Class 11 Notes for NEET PDF
Get the free PDF version for these notes and complete your study material for this chapter. Prepare mineral nutrition, a significant chapter of Class 11 Botany, and understand its importance in the NEET syllabus. Perform Mineral Nutrition Class 11 notes PDF download and use these notes whenever you want. You can make your study sessions more productive with such notes.
Other Important Links
Other Important Links for NEET Mineral Nutrition |
NEET Biology Revision Notes -Chapter Pages
NEET Biology Chapter-wise Revision Notes | |
Mineral Nutrition Notes | |
FAQs on NEET Notes for Biology Mineral Nutrition
1. What are mineral nutrients?
The elements that a plant needs for its various physiological functions are called mineral nutrients. They are absorbed in the form of ions from their salts present in soil and water.
2. What are deficiency symptoms in plants?
The visible abnormal developments in the plants due to a lack of minerals are called deficiency symptoms. For example, Chlorosis occurs due to a lack of sodium, potassium, magnesium, manganese, iron, etc.
3. What are micronutrients?
The minerals or elements that are required in trace amounts are called micronutrients. Example: iron, copper, boron, etc.
4. What are macronutrients?
The minerals or elements that are required in larger elements and are present in the tissues of plants are called macronutrients. Example: carbon, hydrogen, nitrogen, etc.
























