Where Can You Find Metals And Non Metals Class 10 Questions And Answers
NCERT Solutions For Class 10 Science Chapter 3 Metals And Non Metals - 2025-26
FAQs on NCERT Solutions For Class 10 Science Chapter 3 Metals And Non Metals - 2025-26
1. What are the main topics covered in the NCERT Solutions for Class 10 Science Chapter 3 Metals and Non-metals?
The NCERT Solutions for Class 10 Science Chapter 3 cover:
- Physical and chemical properties of metals and non-metals
- The reactivity series of metals
- Formation of oxides and displacement reactions
- Occurrence and extraction of metals
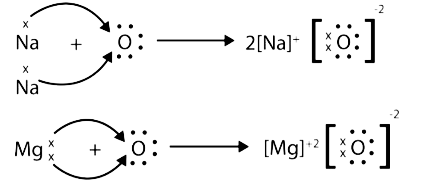
- Properties of ionic compounds
- Corrosion and its prevention
2. How do the NCERT Solutions help students solve exercise questions in Chapter 3 Science Class 10?
The NCERT Solutions provide clear, stepwise answers for all exercise questions of Chapter 3, following the latest CBSE marking scheme. They guide students on how to approach, solve, and present chemical equations and conceptual questions, improving understanding and answer-writing skills for exams.
3. According to NCERT Solutions, what are the key differences between metals and non-metals discussed in Class 10 Science Chapter 3?
Metals:
- Shiny, malleable, ductile, and good conductors of heat and electricity
- Form basic or amphoteric oxides
- Displace hydrogen from acids (more reactive metals)
- Dull, brittle, poor conductors (except graphite), not malleable or ductile
- Form acidic or neutral oxides
- Do not displace hydrogen from acids
4. What is the reactivity series and why is it important in this chapter's solutions?
The reactivity series is a list of metals arranged in decreasing order of their chemical reactivity. It is important for:
- Predicting which metals can displace others in single displacement reactions
- Explaining trends like which metals form oxides more readily or react vigorously with acids and water
- Selecting suitable methods for extraction from ores
5. What are amphoteric oxides as covered in NCERT Solutions for Class 10 Science Chapter 3?
Amphoteric oxides are oxides that react with both acids and bases to form salts and water.
- Examples include zinc oxide (ZnO) and aluminium oxide (Al2O3).
6. What is the process of electrolysis in metal extraction as explained in the solutions?
Electrolysis is a method used to extract metals high in the reactivity series (e.g., sodium, aluminium) from their molten salts.
- The process involves passing an electric current to separate the metal from the solution.
- This method is explained with examples and relevant chemical equations in the solutions as per CBSE guidelines.
7. Why are metals like sodium and potassium stored under oil? (CBSE FUQ)
Sodium and potassium are highly reactive metals. They are stored under oil to prevent them from reacting with oxygen and moisture in the air, which could cause fire or explosions. This is a frequently asked conceptual question in Class 10 Science Chapter 3 NCERT Solutions.
8. How does the NCERT Solution explain the prevention of corrosion and rusting of metals?
The solutions describe rust prevention methods such as:
- Painting or greasing: Forms a barrier to air and moisture.
- Galvanization: Coating iron with zinc to protect it.
- Alloying: Mixing metals to improve corrosion resistance.
9. What are the stepwise guidelines for writing chemical equations in Class 10 Science Chapter 3 solutions?
To write chemical equations as per NCERT Solutions:
- Balance both sides of the equation for atoms
- Mention physical states (s, l, g, aq) where required
- Write observations (colour change, gas release) if asked
10. What are some misconceptions about metals and non-metals clarified by the NCERT Solutions in this chapter? (HOTS/FUQ)
Common misconceptions addressed include:
- Not all metals react with acids or water; e.g., copper and gold do not.
- Some metals are less reactive than hydrogen and won’t displace it from acids.
- Non-metals can have various physical states (e.g., bromine is a liquid).
11. How do the NCERT Solutions approach application-based questions in the extraction and refining of metals? (FUQ)
The solutions use real-life contexts (e.g., why aluminium is used for utensils despite being reactive), step-by-step chemical reactions, and the logic behind selecting specific extraction or refining methods depending on metal reactivity, making complex topics accessible and exam-ready.
12. How can using the NCERT Solutions for Science Chapter 3 help improve exam performance?
Practising NCERT Solutions for Chapter 3:
- Improves concept clarity and problem-solving skills
- Develops proper answer structures as expected in CBSE exams
- Boosts confidence to tackle both direct and higher-order application questions




















 Watch Video
Watch Video