




Key Properties and Applications of Sodium Azide
Sodium azide is used as a chemical preservative in hospitals and laboratories. An organic azide is an organic compound containing the azide (N3) functional group. Because of the hazards associated with their use, few azides are used commercially although they exhibit interesting reactivity for researchers. Some inorganic azides and alkyl azides are used as initiating explosives in detonators and percussion caps.
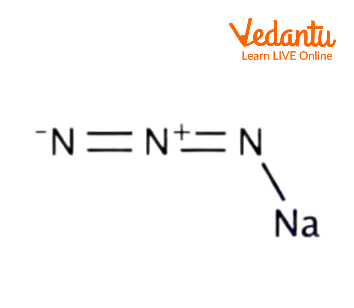
Ionic Structure for Sodium Azide
What is Azide Group?
An organic azide group is an organic compound containing the chemical compound (N3) as a functional group. Owing to the hazards related to their use, few azides are used commercially though they exhibit attention-grabbing reactivity for researchers.
For example Compounds, R-N3 is named Phenyl azide when R is a phenyl group, in radicofunctional terminology, by putting the word "azide" after the name of the radical R . In ( Azidobenzene ), in substitutive nomenclature, by adding the prefix "azido-" to the name of the compound RH.

Azido Benzene
Synthesis of Sodium Azide
Sodium azide is an ionic compound. The NaN3 compounds are very soluble in water. These solutions contain some quantity of hydrogen azide as shown within the following chemical equation:
\[N_{3}^{-}+{{H}_{2}}O\rightleftharpoons H{{N}_{3}}K={{10}^{-4.6}}\]
The sodium azide is present in the form of a white solid.

Sodium Azide
Preparation
The common synthesis technique used is the "Wislicenus method," which takes place in 2 steps from ammonia. in the beginning, ammonia is converted to metal \[NaN{{H}_{2}}\].
$2Na+2N{{H}_{3}}\to 2NaN{{H}_{2}}+{{H}_{2}} \\$
$2NaN{{H}_{2}}+{{N}_{2}}O\to Na{{N}_{3}}+NaOH+N{{H}_{3}} \\$
The NaNH2 reacts with nitrous oxide.
Alternatively, the salt is obtained by the reaction of nitrate with metal NaNH2.
Reactions of Sodium Azide
Treating sodium azide with sturdy acids provides hydrazoic acid as a product:
\[{{H}_{2}}S{{O}_{4}}+Na{{N}_{3}}\to H{{N}_{3}}+NaHS{{O}_{4}}\]
Sodium azide doesn't soften however decomposes smartly to Na metal and N gas at approximately 300 °C. An electrical charge triggered by automobile impact heats the salt to explosively unleash N gas within the airbag:
\[2Na{{N}_{3}}\to 2Na+3{{N}_{2}}\]
In the above reaction, the formed shape may be a hazard itself. In automobile airbags, it's converted by reaction with alternative ingredients, like KNO3and SiO2, into an inert alkaline salt 'glass'.
Sodium azide is destroyed by treatment with acidic sodium nitrite resolution.
\[2Na{{N}_{3}}+2HN{{O}_{2}}\to 3{{N}_{2}}+2NO+NaOH\]
Sodium azide is employed in organic synthesis to introduce the azide functional group by displacement of a salt.
Biochemical Uses of Sodium Azide
Sodium azide may be a helpful probe chemical agent and preservative. In hospitals and laboratories, it's a biocide; it's particularly vital in bulk reagents and stock solutions which can otherwise support microorganism growth where the sodium azide acts as a bacteriostatic by inhibiting hemoprotein enzyme in gram-negative bacteria; gram-positive (streptococci, pneumococci, lactobacilli) are resistant. it's additionally used in agriculture (farming) for pest management.
Azide inhibits hemoprotein enzyme by binding irreversibly to the haem cofactor in a very similar method almost like the action of CO(Carbon monoxide). sodium azide significantly affects organs that endure high rates of respiration, like the heart and also the brain.
Toxic Effects
Sodium azide is usually comparable to cyanide, as they provide similar symptoms. Exposure to sodium azide has some or all of the subsequent symptoms within minutes: fast respiration, restlessness, dizziness, weakness, headache, nausea, vomit, fast pulse, red eyes (gas or mud exposure), clear drainage from the nose, cough, skin burns, and blisters (explosion or direct skin contact. Exposure to a high amount of Na azide could cause these alternative health effects as well: convulsions, low blood pressure, low pulse, loss of consciousness, and respiratory organ injury, respiratory failure resulting in death.
Interesting Facts
Sodium azide could be a rapidly acting, deadly chemical that exists as an odorless white solid.
When it's mixed with water or acid, Sodium azide changes quickly to a poisonous gas with a pungent (sharp) odor.
The odor of the gas might not be sharp enough, however, to provide individuals sufficient warning of the danger.
Important Questions
1. Why NaN3 compound is used in airbags?
Ans. Crashes trip sensors in cars that send an electrical signal to an ignitor. the heat generated causes sodium azide to decompose into Na metal and N gas, which inflates the car's airbags. under normal circumstances, this molecule is kind of stable.
2. Azides are acid sensitive or not?
Ans. heavy metal azide salts tend to be extremely heat and shock-sensitive explosives. Avoid water and robust acids which might cause the formation of hydrogen azide, which is very poisonous, volatile, and explosive.
Summary
Azide is the ion with the formula N-3. Sodium azide is the compound with the formula NaN3. This colorless salt is the gas-forming element in legacy automobile airbag systems. NaN3 is present as a colorless crystalline solid. Its density is 1.85 g / cm3. It causes burns within the air and may explode if high quantities are involved. It's harmful if consumed. Sodium azide is often used as a bacteriostatic preservative for biological samples.
FAQs on Sodium Azide: Complete Chemistry Guide
1. What exactly is Sodium Azide and what is its chemical formula?
Sodium Azide is an inorganic chemical compound that is the sodium salt of hydrazoic acid. Its chemical formula is NaN₃. It is an ionic substance that appears as a colourless, crystalline solid and is known for being highly soluble in water and slightly soluble in ammonia.
2. What are the most common real-world applications of Sodium Azide?
Sodium Azide is used in several specific applications due to its unique properties. The most common examples include:
- As the primary chemical in automobile airbags for rapid inflation.
- A chemical preservative in hospitals and laboratories to inhibit bacterial growth in solutions.
- As a propellant in explosives and detonators.
- In organic chemistry, it is used as a reagent to introduce the azide functional group into molecules.
3. Why is Sodium Azide the chosen chemical for car airbags?
Sodium Azide is ideal for airbags because it decomposes extremely quickly upon receiving an electrical signal, such as from a car's impact sensor. This rapid thermal decomposition produces a large volume of harmless nitrogen gas (N₂) almost instantly. This gas inflates the airbag in milliseconds, providing a crucial safety cushion during a collision.
4. What makes Sodium Azide and other related azides explosive?
The explosive nature of azides is due to the inherent instability of the azide anion (N₃⁻). The bonds between the three nitrogen atoms are under significant strain. When subjected to heat or shock, these bonds break very easily, releasing a large amount of energy and stable dinitrogen gas (N₂). This sudden and massive release of gas and energy is what causes the explosion.
5. Is Sodium Azide harmful to humans, and what safety precautions are important?
Yes, Sodium Azide is extremely toxic if ingested, inhaled, or absorbed through the skin. It acts as a potent poison by inhibiting a key enzyme in cellular respiration. Therefore, it must be handled with strict safety measures, including protective gear and proper ventilation. It should also be stored away from acids and heavy metals (like lead or copper) because it can form even more sensitive and explosive compounds with them.
6. How is the structure of Sodium Azide different from Sodium Nitride?
Although their names sound similar, Sodium Azide and Sodium Nitride are structurally very different. Sodium Azide (NaN₃) is an ionic compound formed from a sodium ion (Na⁺) and a linear azide anion (N₃⁻). In contrast, Sodium Nitride (Na₃N) is formed from three sodium ions (3Na⁺) and a single nitride anion (N³⁻). Their chemical properties and reactions are completely distinct due to these different anionic structures.
7. How does Sodium Azide behave when dissolved in water?
When Sodium Azide is dissolved in water, it dissociates into its constituent ions: the sodium cation (Na⁺) and the azide anion (N₃⁻). The resulting solution is slightly basic because the azide ion can react with water in a hydrolysis reaction to produce a small amount of hydrazoic acid (HN₃) and hydroxide ions (OH⁻).
























