




How Does Back Bonding Affect the Properties of BF3?
Covalent bonds are formed by sharing of electrons. Electron clouds in an atom are arranged in orbitals. The orbitals overlap to form bonds. Sometimes electron pairs from an electron rich element drift back into an empty orbital of comparable energy of the other atom, this donation of electrons back to an atom results in a secondary bonding interaction. This bonding is known as back bonding.
BF3 is a planar covalent compound which displays a pi-back bonding. Lone pair of fluorine is donated to the empty orbital of boron, to form a pi-bonding interaction.
Physical Properties
BF3 is an inorganic covalent compound, it is found in gaseous form. It is colourless, pungent, and toxic and forms white fumes when it comes in presence of moisture.
Table: Properties of BF3
Types of Covalent Bonding
Sigma Bonding: It happens for single bonds where the atomic orbitals overlap head-to-head. It’s the first covalent bond that forms between any atoms.
Pi Bonding: It happens for double and triple bonds. The orbitals participate in the sideways overlap. The second and third covalent bonds are formed in this manner.
Sigma bonds can be formed by hybridised orbital and they are much stronger than pi bonds. The pi bonds are easier to break.
BF3 Molecule
BF3 is known as boron trifluoride. It is a trigonal planar molecule with three fluorine atoms attached to a boron. The fluorine atom occupies the three vertices of the triangle with boron in the middle. The fluorine atoms are singly bonded to boron.
Trigonal Planar Boron Trifluoride
Let’s examine the electronic configuration of boron and fluorine.
Boron, atomic number 5: 1s2 2s2 2p1
Fluorine, atomic number 9: 1s2 2s2 2p5
We can see fluorine is one electron short of its octet, while boron is one electron excess of its octet. Both the atoms first undergo sp2 hybridisation, where the 2s orbital and two 2p orbital hybridise to form three sp2 hybrid orbital. In boron, each three sp2 orbitals contain one electron each bond is directed in three directions in a plane forming a planar triangular shape. The fluorine atom also undergo sp2 hybridisation, the three hybrid orbital contain five electrons, two of the three hybrid orbital contain a pair of electrons each, the third sp2 orbital has one unpaired electron which is extended towards the boron. The sp2 orbital of fluorine and boron containing one electron each overlap head on to form a sigma bond. Three fluorine atom bonds in this manner with the three sp2 orbitals of boron to form the BF3 molecule.
Back Bonding in BF3
In both boron and fluorine, the two 2p orbital and one 2s orbital are engaged in sp2 hybridization, leaving one p orbital free. Boron hosts one unhybridized free p orbital which is empty, while the free p orbital on fluorine contains a pair of electrons. This filles p-orbital over fluorine partially interacts with the empty p-orbital of boron, in a sideway overlap resulting in a pi-bonding interaction. This is the back bonding observed in BF3.
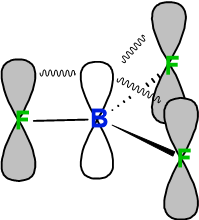
Back Bonding in BF3
Comparing the electronegativity of fluorine and boron, fluorine being highly electronegative pulls the bonding electron towards itself resulting in a partial positive charge over boron and partial negative charge over fluorine, the excess charge is stabilised by the backward pi electron drift from fluorine to boron by the back bonding.
BF3 Chemistry
The BF3 molecule is a perfect trigonal planar molecule with F—B—F angle 120°. The BF bond is shorter than the expected single bond length, this is attributed to the strong pi back bonding. Each B-F bond is polar, but the dipole moment of each B—F bond cancels each other, and as a result, the BF3 molecule is overall non polar. It is categorised as an electron deficient molecule and hence can act as lewis acid.
Some reaction of BF3 demonstrates its ability to form adduct with lewis bases (such as fluorides and ethers)
CsF + BF3 → CsBF4
C2H5—O—C2H5 + BF3 → BF3.O(C2H5)
BF3 reacts with water to form an aquo adduct, which subsequently loses HF to give fluoroboric acid. Hydrolysis of BF3 produces the well-known boric acid [(B(OH)3].
4 BF3 + 3H2O → 3HBF4 + B(OH)3
Uses of BF3
BF3 serves as reagent in organic chemistry, where it acts as lewis acid.
BF3 serves as the building block of all boron compounds.
Used in fumigation.
Used in soldering magnesium.
It is used as a neutron detector.
Key Features
BF3 is an inorganic covalent compound.
BF3 has a trigonal planar structure.
Boron has an empty p-orbital and fluorine has a filled p-orbital.
The p-electron from fluorine drifts into empty p-orbital boron resulting in a pi-bonding interaction, which is known as back bonding.
Interesting Facts
Back bonding is a type of intermolecular lewis acid-base interaction.
BF3 was discovered in 1808 by Joseph Louis Gay-Lussac and Louis Jacques Thenard.
FAQs on Back Bonding in BF3 Explained
1. What exactly is back bonding in the BF3 molecule?
Back bonding in Boron Trifluoride (BF3) is a special type of chemical bonding where a lone pair of electrons from a fluorine atom is donated to the vacant p-orbital of the central boron atom. This creates a partial pπ-pπ bond, which coexists with the main sigma bond. It helps to stabilize the electron-deficient boron atom.
2. What are the main consequences of back bonding on the properties of BF3?
Back bonding significantly impacts the properties of BF3 in two key ways:
- Bond Length: It gives the B-F bond a partial double-bond character. This makes the bond shorter and stronger than what would be expected for a pure single bond.
- Lewis Acidity: The electron donation from fluorine to boron reduces boron's electron deficiency. This makes BF3 a weaker Lewis acid (electron pair acceptor) than other boron halides like BCl3 or BBr3.
3. What general conditions are necessary for back bonding to occur in a molecule?
For back bonding to take place, two primary conditions must be met:
- One of the bonded atoms must have a vacant orbital of suitable energy, like the empty 2p orbital of Boron in BF3.
- An adjacent atom bonded to it must possess a lone pair of electrons available for donation, like the lone pairs in the 2p orbital of Fluorine.
For the bond to be strong, the overlapping orbitals should be of similar size and energy.
4. Why is back bonding stronger in BF3 compared to BCl3 and BBr3?
The strength of back bonding follows the order BF3 > BCl3 > BBr3. This is because of the effectiveness of orbital overlap. In BF3, the overlap occurs between Boron's 2p orbital and Fluorine's 2p orbital. Since these orbitals are of similar size and energy, the 2p-2p overlap is very strong. In contrast, the overlap in BCl3 (2p-3p) and BBr3 (2p-4p) is much less effective due to differences in orbital size and energy, resulting in weaker back bonding.
5. How does back bonding explain the surprising trend in Lewis acidity among boron halides?
Although fluorine is the most electronegative halogen, BF3 is the weakest Lewis acid among the boron halides. This is explained by back bonding. The strong 2p-2p back bonding in BF3 effectively reduces the electron deficiency on the boron atom. In BCl3 and BBr3, back bonding is much weaker, leaving their boron atoms more electron-deficient and thus more eager to accept an electron pair, making them stronger Lewis acids.
6. What type of orbital overlap is involved in BF3 back bonding?
The back bonding in BF3 is a classic example of pπ-pπ overlap. The 'pπ' indicates that a pi bond is formed by the sideways overlap of two p-orbitals. Specifically, it's the filled 2p orbital of a fluorine atom overlapping with the empty 2p orbital of the boron atom.
7. Why is the BF3 molecule planar and non-polar despite having polar bonds?
The central boron atom in BF3 is sp2 hybridized, which results in a trigonal planar geometry with bond angles of 120°. While each individual B-F bond is polar due to the difference in electronegativity, the molecule as a whole is non-polar. This is because the three polar bonds are arranged symmetrically, and their individual dipole moments cancel each other out, leading to a net dipole moment of zero.
























