
Which of the following pair of the molecule have identical shapes:
A.\[{\left[ {NiC{l_4}} \right]^{ - 2}}\],\[Xe{F_4}\]
B. \[{\left[ {Zn{{\left( {{H_2}O} \right)}_4}} \right]^{ + 2}}\], \[SiC{l_4}\]
C. \[\left[ {Fe{{\left( {CO} \right)}_5}} \right],XeO{F_4}\]
D. \[{\left[ {Ag{{\left( {N{H_3}} \right)}_2}} \right]^ + }{\rm{, }}S{F_2}\]
Answer
233.1k+ views
Hint: The shape of molecules is illustrated by the Valence Shell Electron Pair Repulsion (VSEPR) Theory based on the repulsion between the electron pairs in the valence shell of the atoms. The shape of coordination compounds is given by valence bond theory.
Complete Step by Step Answer:
VSEPR theory was given by Sidgwick and Powell in the year 1940 and was made better by Nyholm and Gillespie in the year 1957.
This theory states that:
The shape of the molecule depends on the no.of valence shell electron pairs around the main atom and whether or not they are bonded.
Pairs of electrons in the valence shell all are negatively charged and hence, repelled by one another.
These pairs of electrons try to reduce the repulsion and thus remain distant and separated from each other.
If we assume the valence shell as a sphere then the electron pairs are on the spherical exterior at the maximum space from one another.
Lone pair-lone pair repel each other the most. Then comes lone pair-bond pair repulsion and bond pair-bond pair repulsion.
Valence bond theory states that the metal-ligand bond arises by the donation of pairs of electrons by the ligand to the metal or ion.
These electrons are accommodated by the hybridised orbitals of equal energy of metal atoms.
Sometimes, the unpaired (n-1)d electrons pair up before hybridization thus producing some (n-1)d orbitals vacant. The central atom then makes available the no.of empty orbitals equal to its coordination number for the formation of metal-ligand bonds.
Here in this question, we have to find out which of the following molecules have identical shapes.
A. \[{\left[ {NiC{l_4}} \right]^{ - 2}}\],\[Xe{F_4}\]
\[{\left[ {NiC{l_4}} \right]^{ - 2}}\]
Ni has a +2 charge in this molecule.
So, the total no.of electrons on the central metal atom=8
These electrons lie in the 3d orbital of the central metal ion with the last two electrons unpaired.
Here \[C{l^ - }\] is a weak field ligand. So, it is, therefore, unable to pair up the unpaired electrons of the 3d orbital.
So, the one 4s and three 4p orbitals hybridise to form four\[s{p^3}\] hybrid orbitals.
These hybrid orbitals accommodate four pairs of electrons from chloride ions.
So, they have tetrahedral geometry.
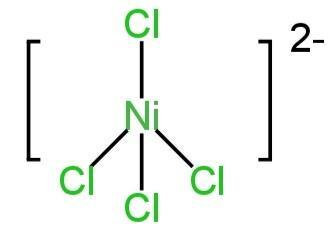
Image: Structure of \[{\left[ {NiC{l_4}} \right]^{ - 2}}\]
\[Xe{F_4}\]
Xe is a noble gas. It has filled atomic orbitals.
It has eight electrons in the outermost orbitals.
Four electrons are shared by four fluorine atoms. Two lone pairs of electrons remain on the Xe atom.
It has four Xe-F bonds and two lone pairs of electrons.
It has an \[s{p^3}{d^2}\] hybridization square planar structure.
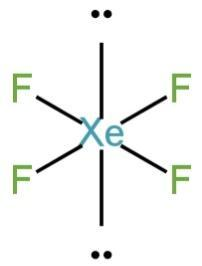
Image: Structure of \[Xe{F_4}\]
So, both the given anion and compound have different structures.
So, A is incorrect.
B. \[{\left[ {Zn{{\left( {{H_2}O} \right)}_4}} \right]^{ + 2}}\], \[SiC{l_4}\]
The atomic number of Zn is 30.
\[Z{n^{2 + }}\]ion has 10 electrons in 3d orbital with two electrons in each five orbital.
The four empty 4s and 4p orbitals hybridise to form four \[s{p^3}\] hybrid orbitals.
These hybrid orbitals accommodate four pairs of electrons from H2O.
So, they have tetrahedral geometry.

Image: Structure of \[{\left[ {Zn{{\left( {{H_2}O} \right)}_4}} \right]^{ + 2}}\]
\[SiC{l_4}\]
Silicon belongs to group 14 of the periodic table.
It contains 4 electrons in its outermost shell. Therefore, its valency is 4.
The valency of fluorine is 1.
So, four Si-Cl bonds are formed and there's no lone pair.
So, \[s{p^3}\]hybridization takes place.
It has a tetrahedral structure.
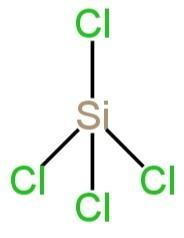
Image: Structure of \[SiC{l_4}\]
So, the structure of \[{\left[ {Zn{{\left( {{H_2}O} \right)}_4}} \right]^{ + 2}}\], \[SiC{l_4}\] is tetrahedral.
So, B is correct.
C. \[\left[ {Fe{{\left( {CO} \right)}_5}} \right],XeO{F_4}\]
\[\left[ {Fe{{\left( {CO} \right)}_5}} \right]\]
Here Fe is in a zero oxidation state. There are 6 electrons in the 3d orbital.
Out of six, four electrons are unpaired.
In the presence of ligand CO, being the strongest, the 3d electrons pair up leaving one d-orbital, one 4s, and three 4p orbitals undergoing \[ds{p^2}\] hybridization.
Each hybridised orbital accepts a lone pair of electrons from CO ligands.
So, it has a trigonal bipyramidal structure.
\[XeO{F_4}\]
Xe is a noble gas. It has filled atomic orbitals.
It has eight electrons in the outermost orbitals.
Four electrons are shared by four fluorine atoms. Oxygen and Xe form a double bond.
The molecule has one lone pair of electrons.
It has an \[s{p^3}{d^2}\]hybridization square pyramidal structure.
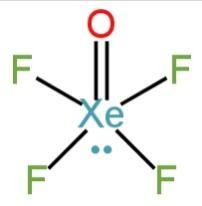
Image: Structure of \[XeO{F_4}\]
The structure of \[\left[ {Fe{{\left( {CO} \right)}_5}} \right],XeO{F_4}\] is trigonal bipyramidal and square pyramidal respectively.
So, C is incorrect.
D. \[{\left[ {Ag{{\left( {N{H_3}} \right)}_2}} \right]^ + }{\rm{, }}S{F_2}\]
Here Ag is the central metal atom.
The ground state electron configuration of
Ag is \[\left[ {Kr} \right]4{d^{10}}5{s^1}\].
To form the Ag+, an electron is withdrawn from the 5s sublevel.
So, the electron configuration of an\[A{g^ + }\] ion would be \[\left[ {Kr} \right]4{d^{10}}\].
Here are the 5s and 5p orbitals undergoing sp hybridization.
Electron pairs from the ligands are accommodated in the two sp hybrid orbitals.
So, the structure is linear.
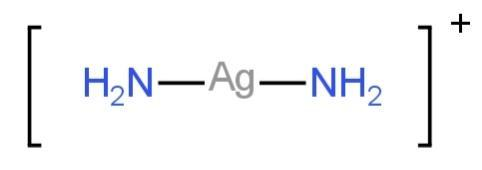
Image: Structure of \[{\left[ {Ag{{\left( {N{H_3}} \right)}_2}} \right]^ + }\]
\[S{F_2}\]
Here, the central atom is Sulphur, its atomic number is 16.
It has six electrons in the outermost shell.
There are two S-F bonds and two lone pairs of electrons in this molecule.
So, it has sp hybridization due to two bond pairs of electrons.
Due to the repulsion between lone pairs of electrons, it has a bent shape.

Image: Structure of \[S{F_2}\]
So, the \[{\left[ {Ag{{\left( {N{H_3}} \right)}_2}} \right]^ + }{\rm{, }}S{F_2}\] have linear and bent shapes respectively.
So, D is incorrect.
So, option B is correct.
Note: In coordination compounds, if after the formation of the complex, no unpaired electron is present, the complex is diamagnetic. If some unpaired electrons are present the complex is paramagnetic. Greater the no.of unpaired electrons greater the paramagnetic character.
Complete Step by Step Answer:
VSEPR theory was given by Sidgwick and Powell in the year 1940 and was made better by Nyholm and Gillespie in the year 1957.
This theory states that:
The shape of the molecule depends on the no.of valence shell electron pairs around the main atom and whether or not they are bonded.
Pairs of electrons in the valence shell all are negatively charged and hence, repelled by one another.
These pairs of electrons try to reduce the repulsion and thus remain distant and separated from each other.
If we assume the valence shell as a sphere then the electron pairs are on the spherical exterior at the maximum space from one another.
Lone pair-lone pair repel each other the most. Then comes lone pair-bond pair repulsion and bond pair-bond pair repulsion.
Valence bond theory states that the metal-ligand bond arises by the donation of pairs of electrons by the ligand to the metal or ion.
These electrons are accommodated by the hybridised orbitals of equal energy of metal atoms.
Sometimes, the unpaired (n-1)d electrons pair up before hybridization thus producing some (n-1)d orbitals vacant. The central atom then makes available the no.of empty orbitals equal to its coordination number for the formation of metal-ligand bonds.
Here in this question, we have to find out which of the following molecules have identical shapes.
A. \[{\left[ {NiC{l_4}} \right]^{ - 2}}\],\[Xe{F_4}\]
\[{\left[ {NiC{l_4}} \right]^{ - 2}}\]
Ni has a +2 charge in this molecule.
So, the total no.of electrons on the central metal atom=8
These electrons lie in the 3d orbital of the central metal ion with the last two electrons unpaired.
Here \[C{l^ - }\] is a weak field ligand. So, it is, therefore, unable to pair up the unpaired electrons of the 3d orbital.
So, the one 4s and three 4p orbitals hybridise to form four\[s{p^3}\] hybrid orbitals.
These hybrid orbitals accommodate four pairs of electrons from chloride ions.
So, they have tetrahedral geometry.

Image: Structure of \[{\left[ {NiC{l_4}} \right]^{ - 2}}\]
\[Xe{F_4}\]
Xe is a noble gas. It has filled atomic orbitals.
It has eight electrons in the outermost orbitals.
Four electrons are shared by four fluorine atoms. Two lone pairs of electrons remain on the Xe atom.
It has four Xe-F bonds and two lone pairs of electrons.
It has an \[s{p^3}{d^2}\] hybridization square planar structure.

Image: Structure of \[Xe{F_4}\]
So, both the given anion and compound have different structures.
So, A is incorrect.
B. \[{\left[ {Zn{{\left( {{H_2}O} \right)}_4}} \right]^{ + 2}}\], \[SiC{l_4}\]
The atomic number of Zn is 30.
\[Z{n^{2 + }}\]ion has 10 electrons in 3d orbital with two electrons in each five orbital.
The four empty 4s and 4p orbitals hybridise to form four \[s{p^3}\] hybrid orbitals.
These hybrid orbitals accommodate four pairs of electrons from H2O.
So, they have tetrahedral geometry.

Image: Structure of \[{\left[ {Zn{{\left( {{H_2}O} \right)}_4}} \right]^{ + 2}}\]
\[SiC{l_4}\]
Silicon belongs to group 14 of the periodic table.
It contains 4 electrons in its outermost shell. Therefore, its valency is 4.
The valency of fluorine is 1.
So, four Si-Cl bonds are formed and there's no lone pair.
So, \[s{p^3}\]hybridization takes place.
It has a tetrahedral structure.

Image: Structure of \[SiC{l_4}\]
So, the structure of \[{\left[ {Zn{{\left( {{H_2}O} \right)}_4}} \right]^{ + 2}}\], \[SiC{l_4}\] is tetrahedral.
So, B is correct.
C. \[\left[ {Fe{{\left( {CO} \right)}_5}} \right],XeO{F_4}\]
\[\left[ {Fe{{\left( {CO} \right)}_5}} \right]\]
Here Fe is in a zero oxidation state. There are 6 electrons in the 3d orbital.
Out of six, four electrons are unpaired.
In the presence of ligand CO, being the strongest, the 3d electrons pair up leaving one d-orbital, one 4s, and three 4p orbitals undergoing \[ds{p^2}\] hybridization.
Each hybridised orbital accepts a lone pair of electrons from CO ligands.
So, it has a trigonal bipyramidal structure.
\[XeO{F_4}\]
Xe is a noble gas. It has filled atomic orbitals.
It has eight electrons in the outermost orbitals.
Four electrons are shared by four fluorine atoms. Oxygen and Xe form a double bond.
The molecule has one lone pair of electrons.
It has an \[s{p^3}{d^2}\]hybridization square pyramidal structure.

Image: Structure of \[XeO{F_4}\]
The structure of \[\left[ {Fe{{\left( {CO} \right)}_5}} \right],XeO{F_4}\] is trigonal bipyramidal and square pyramidal respectively.
So, C is incorrect.
D. \[{\left[ {Ag{{\left( {N{H_3}} \right)}_2}} \right]^ + }{\rm{, }}S{F_2}\]
Here Ag is the central metal atom.
The ground state electron configuration of
Ag is \[\left[ {Kr} \right]4{d^{10}}5{s^1}\].
To form the Ag+, an electron is withdrawn from the 5s sublevel.
So, the electron configuration of an\[A{g^ + }\] ion would be \[\left[ {Kr} \right]4{d^{10}}\].
Here are the 5s and 5p orbitals undergoing sp hybridization.
Electron pairs from the ligands are accommodated in the two sp hybrid orbitals.
So, the structure is linear.

Image: Structure of \[{\left[ {Ag{{\left( {N{H_3}} \right)}_2}} \right]^ + }\]
\[S{F_2}\]
Here, the central atom is Sulphur, its atomic number is 16.
It has six electrons in the outermost shell.
There are two S-F bonds and two lone pairs of electrons in this molecule.
So, it has sp hybridization due to two bond pairs of electrons.
Due to the repulsion between lone pairs of electrons, it has a bent shape.

Image: Structure of \[S{F_2}\]
So, the \[{\left[ {Ag{{\left( {N{H_3}} \right)}_2}} \right]^ + }{\rm{, }}S{F_2}\] have linear and bent shapes respectively.
So, D is incorrect.
So, option B is correct.
Note: In coordination compounds, if after the formation of the complex, no unpaired electron is present, the complex is diamagnetic. If some unpaired electrons are present the complex is paramagnetic. Greater the no.of unpaired electrons greater the paramagnetic character.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




