
The compound formed when an alcoholic solution of ethylene dibromide is heated with granulated zinc is:
(A) Ethene
(B) Ethyne
(C) Ethane
(D) Bromoethane
Answer
233.1k+ views
Hint:An elimination reaction is a type of organic reaction in which two atoms or substituents that are on adjacent carbon atoms are removed from a molecule in either one step or two-step mechanism
Complete step by step solution:
> In the question it is given that ethylene dibromide is heated with granulated zinc.
- The structure of the ethylene dibromide is as follows.
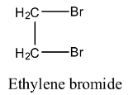
- The reaction of an alcoholic solution of ethylene dibromide is heated with granulated zinc is as follows
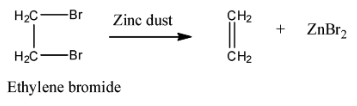
- The above reaction is called a dehalogenation reaction or elimination reaction.
- Zinc removes bromine atoms as zinc bromide (\[ZnB{{r}_{2}}\]).
Coming to given options, option B, Ethyne, means acetylene. But the product formed is not ethylene. So, it is wrong.
Coming to option C, ethane, but the product formed is acetylene. So, it is wrong.
Coming to option D, bromo ethane, it is also wrong,
Coming to option A, Ethene, means ethylene. This option is correct because the product formed in the above reaction is ethylene.
So, the correct option is A.
Note: Don’t be confused between ethane, Ethene, Ethyne. All are not the same.
Ethane-it is called alkane and a saturated compound.
Ethene-it is called alkene and ethylene. It is an unsaturated compound.
Ethyne- it is called as alkyne and called as acetylene. It is an unsaturated compound.
Complete step by step solution:
> In the question it is given that ethylene dibromide is heated with granulated zinc.
- The structure of the ethylene dibromide is as follows.

- The reaction of an alcoholic solution of ethylene dibromide is heated with granulated zinc is as follows

- The above reaction is called a dehalogenation reaction or elimination reaction.
- Zinc removes bromine atoms as zinc bromide (\[ZnB{{r}_{2}}\]).
Coming to given options, option B, Ethyne, means acetylene. But the product formed is not ethylene. So, it is wrong.
Coming to option C, ethane, but the product formed is acetylene. So, it is wrong.
Coming to option D, bromo ethane, it is also wrong,
Coming to option A, Ethene, means ethylene. This option is correct because the product formed in the above reaction is ethylene.
So, the correct option is A.
Note: Don’t be confused between ethane, Ethene, Ethyne. All are not the same.
Ethane-it is called alkane and a saturated compound.
Ethene-it is called alkene and ethylene. It is an unsaturated compound.
Ethyne- it is called as alkyne and called as acetylene. It is an unsaturated compound.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




