
Of the following the formula which represents a saturated cyclic compound is
A. \[{C_3}{H_6}\]
B. \[{C_3}{H_8}\]
C. \[{C_8}{H_{10}}\]
D. \[{C_8}{H_{12}}\]
Answer
232.8k+ views
Hint: A cyclic hydrocarbon is a hydrocarbon in which the carbon chain is in the form of a ring. A saturated cyclic hydrocarbon is a compound in which all the carbon atoms are joined together by single bonds.
Complete Step by Step Answer:
Here in this question, we are given the chemical formula of four different compounds, we have to figure out which one is a saturated cyclic compound.
A. \[{C_3}{H_6}\]
It is the chemical formula of cyclopropane.
It is a saturated cyclic organic compound with the molecular formula \[{\left( {C{H_2}} \right)_3}\], containing three methylene groups \[\left( {C{H_2}} \right)\]attached to form a ring.
So, A is correct.
The structure of cyclopropane is as follows:
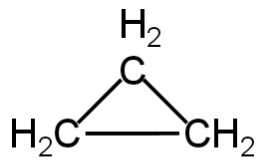
Image: Cyclopropane
B. \[{C_3}{H_8}\]
This is propane which is a saturated aliphatic hydrocarbon with the chemical formula \[C{H_3}C{H_2}C{H_3}\].
So, B is incorrect.
C. \[{C_8}{H_{10}}\]
This is the formula of ethylbenzene.
It has an ethyl group \[\left( { - {C_2}{H_5}} \right)\] attached to the benzene \[\left( { - {C_6}{H_5}} \right)\] group.
So, it is an unsaturated compound.
So, B is incorrect.
D. \[{C_8}{H_{12}}\]
It is a cyclooctadiene compound.
A cyclooctadiene is a cyclic diene with the formula \[{\left( {C{H_2}} \right)_4}{\left( {{C_2}{H_{\bf{2}}}} \right)_2}\].
It has two double bonds.
So, although it is a cyclic hydrocarbon it has unsaturation.
So, D is incorrect.
So, option A is correct.
Note: Cyclopropane was initially produced through a Wurtz coupling reaction. 1,3-dibromopropane when treated with sodium produced cyclopropane. The yield of this reaction can be enhanced by the usage of zinc as the dehalogenation agent and sodium iodide as a catalyst.
\[BrC{H_2}C{H_2}C{H_2}Br + 2Na \to {\left( {C{H_2}} \right)_3} + 2NaBr\]
This reaction is named cyclopropanation.
Complete Step by Step Answer:
Here in this question, we are given the chemical formula of four different compounds, we have to figure out which one is a saturated cyclic compound.
A. \[{C_3}{H_6}\]
It is the chemical formula of cyclopropane.
It is a saturated cyclic organic compound with the molecular formula \[{\left( {C{H_2}} \right)_3}\], containing three methylene groups \[\left( {C{H_2}} \right)\]attached to form a ring.
So, A is correct.
The structure of cyclopropane is as follows:

Image: Cyclopropane
B. \[{C_3}{H_8}\]
This is propane which is a saturated aliphatic hydrocarbon with the chemical formula \[C{H_3}C{H_2}C{H_3}\].
So, B is incorrect.
C. \[{C_8}{H_{10}}\]
This is the formula of ethylbenzene.
It has an ethyl group \[\left( { - {C_2}{H_5}} \right)\] attached to the benzene \[\left( { - {C_6}{H_5}} \right)\] group.
So, it is an unsaturated compound.
So, B is incorrect.
D. \[{C_8}{H_{12}}\]
It is a cyclooctadiene compound.
A cyclooctadiene is a cyclic diene with the formula \[{\left( {C{H_2}} \right)_4}{\left( {{C_2}{H_{\bf{2}}}} \right)_2}\].
It has two double bonds.
So, although it is a cyclic hydrocarbon it has unsaturation.
So, D is incorrect.
So, option A is correct.
Note: Cyclopropane was initially produced through a Wurtz coupling reaction. 1,3-dibromopropane when treated with sodium produced cyclopropane. The yield of this reaction can be enhanced by the usage of zinc as the dehalogenation agent and sodium iodide as a catalyst.
\[BrC{H_2}C{H_2}C{H_2}Br + 2Na \to {\left( {C{H_2}} \right)_3} + 2NaBr\]
This reaction is named cyclopropanation.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




