
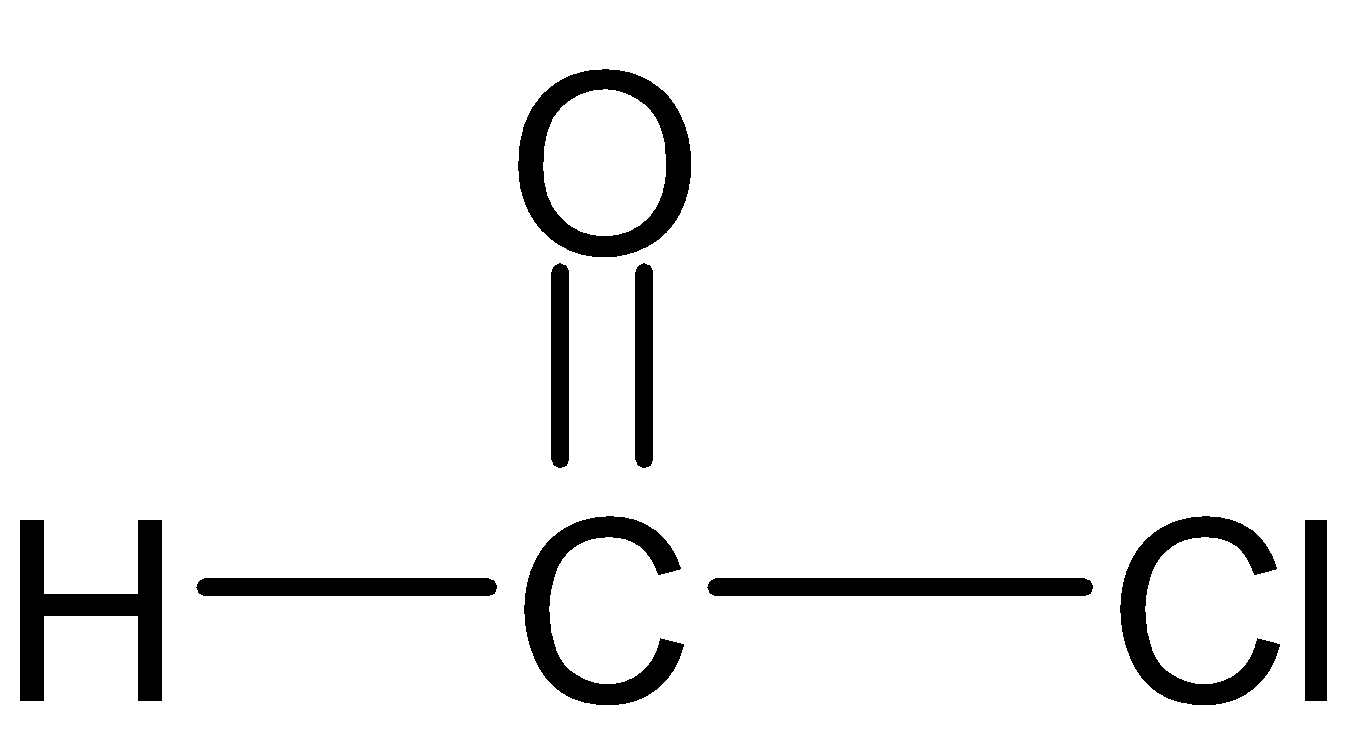
 Is called
Is called
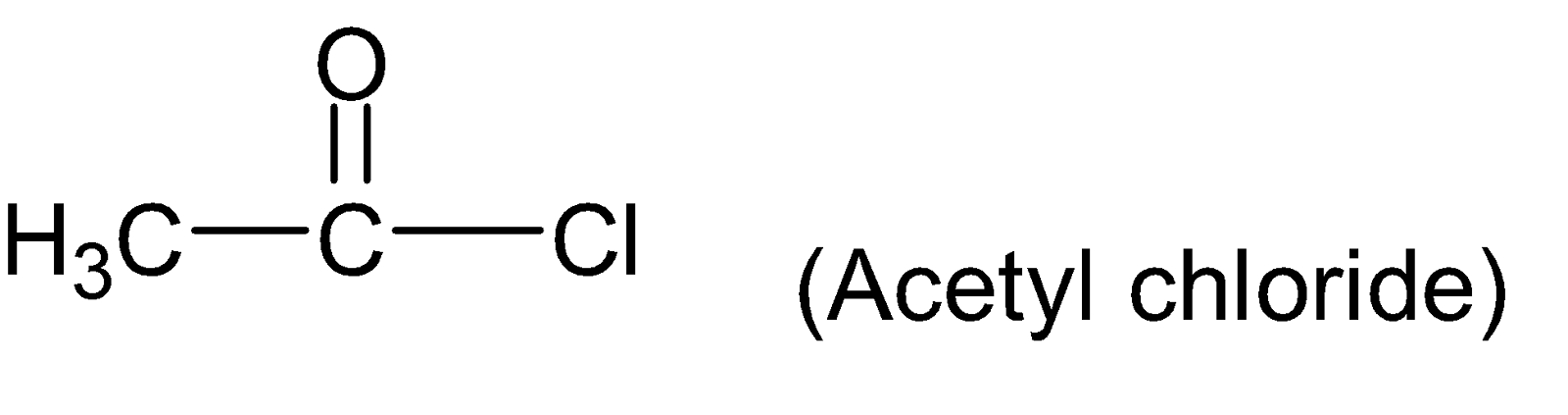
A.Acetyl chloride
B.Formyl chloride
C.Chloretone
D.Oxochloromethane
Answer
233.1k+ views
Hint: In this question we have an organic compound in which a carbonyl group $(C=O)$is attached to a hydrogen atom $(-H)$ and a chlorine atom $(-C\,l\,)$. Generally in organic chemistry, the functional group $R-\underset{|}{\mathop{C}}\,=O$ is called the acyl group which $R-$represents any alkyl or aryl group.
Complete answer:As the given compound consists of an acyl group and a halide group that is chlorine hence it can be named acyl halide or acyl chloride. Or this can be explained in another way that the hydroxyl group of a carboxyl group is replaced by halogen, which is named by placing the name of the corresponding halide after that of the acyl group.
Therefore, the general structure of acyl halide can be represented as:
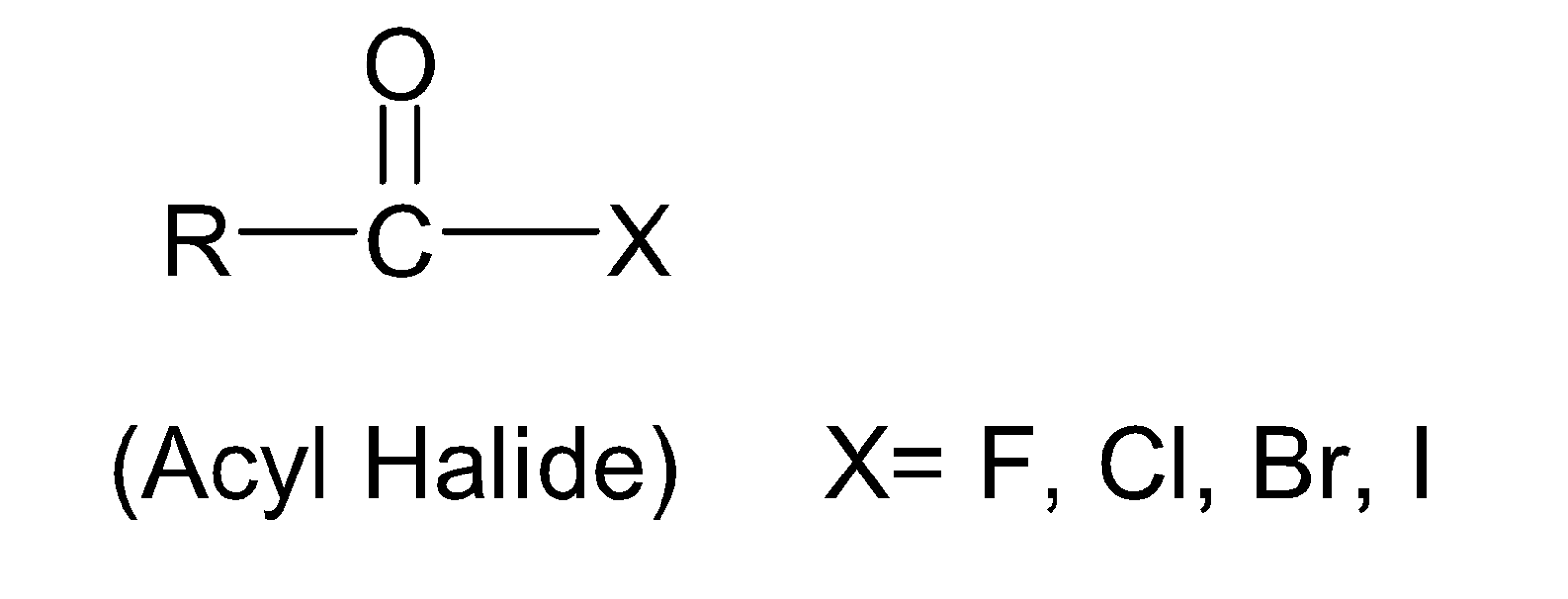
The nomenclature of acyl halide can be done by following the rules given in below:
(i)Alkyl or aryl group $+$the suffix of $-oyl$group followed by the halogen.
(ii)At first select the longest continuous carbon chain, containing the acyl group.
(iii)Then number the carbon chain, beginning at the end nearest to the acyl group.
(iv)Finally number the substitution and write the name, listing substituting alphabetically.
For example:
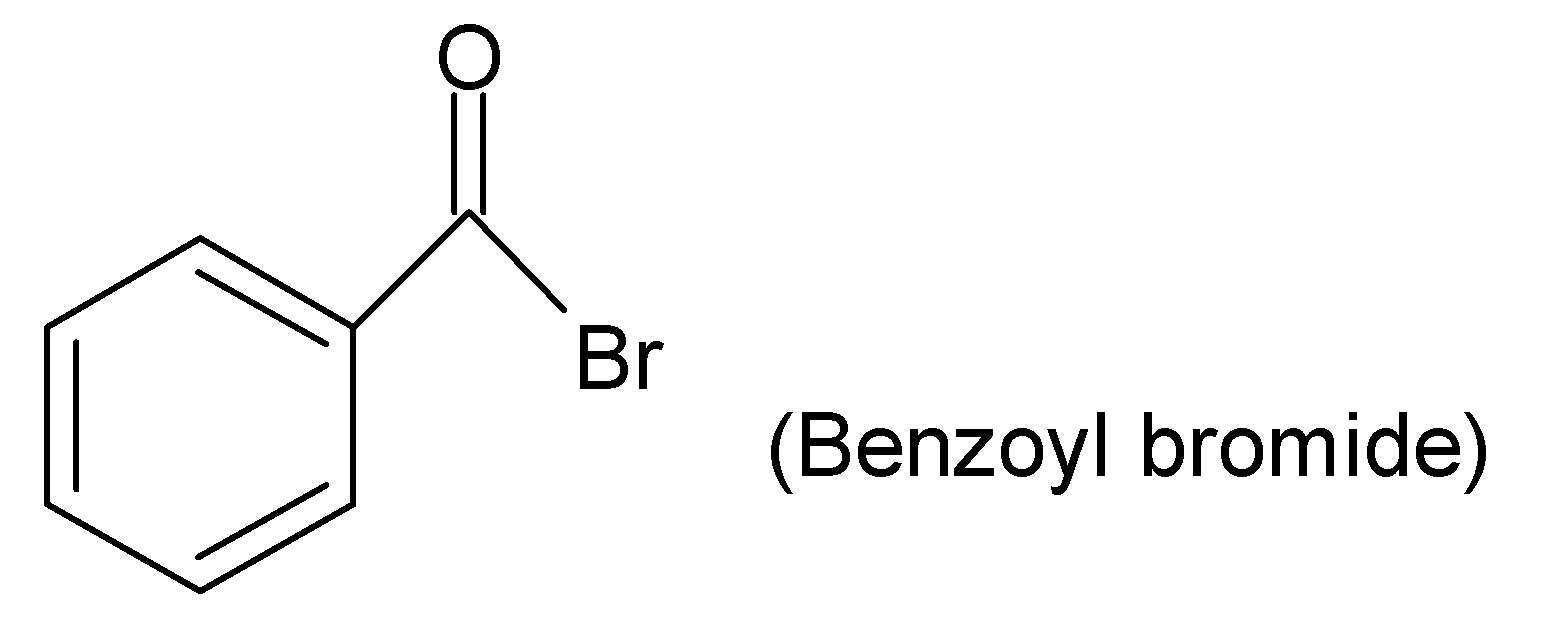
As in the given compound, there is no alkyl or aryl chain attached to the acyl group but hydrogen is there, therefore, this acyl chloride is named Formyl chloride (Since $H-\underset{|}{\mathop{C}}\,=O$group is called formyl group).
Thus, option (B) is correct.
Note: Acyl halide is an organic compound that can be prepared by the reaction of carboxylic acids with reagents such as $PC\,{{l}_{5}}$ thionyl chloride for acyl chloride but for the bromide, the reagent phosphorus tribromide is used. Also, aromatic acyl chlorides are prepared by Friedel crafts acylation using $HCHO$as the reagent.
Complete answer:As the given compound consists of an acyl group and a halide group that is chlorine hence it can be named acyl halide or acyl chloride. Or this can be explained in another way that the hydroxyl group of a carboxyl group is replaced by halogen, which is named by placing the name of the corresponding halide after that of the acyl group.
Therefore, the general structure of acyl halide can be represented as:
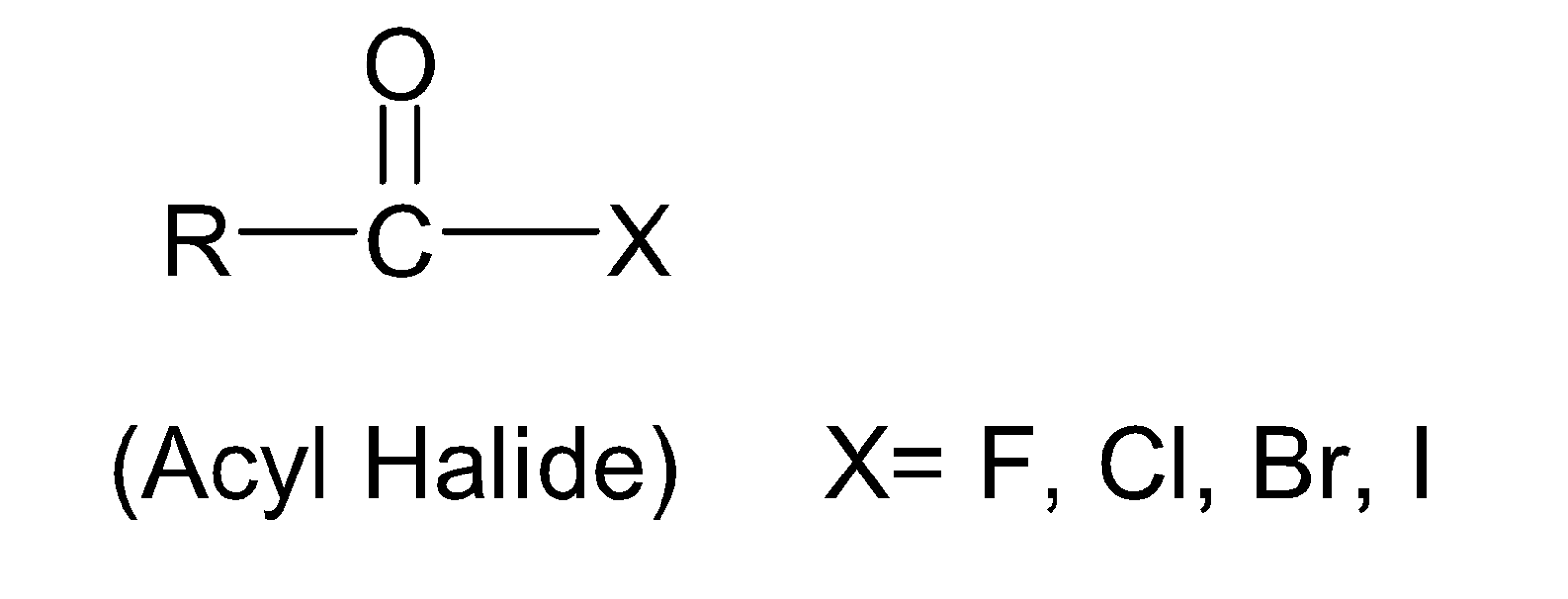
The nomenclature of acyl halide can be done by following the rules given in below:
(i)Alkyl or aryl group $+$the suffix of $-oyl$group followed by the halogen.
(ii)At first select the longest continuous carbon chain, containing the acyl group.
(iii)Then number the carbon chain, beginning at the end nearest to the acyl group.
(iv)Finally number the substitution and write the name, listing substituting alphabetically.
For example:

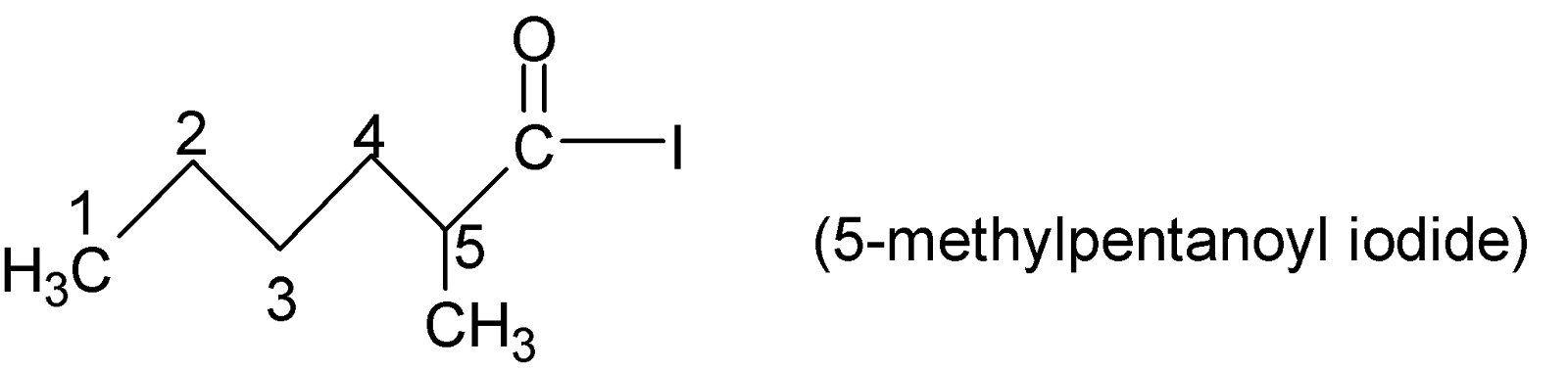

As in the given compound, there is no alkyl or aryl chain attached to the acyl group but hydrogen is there, therefore, this acyl chloride is named Formyl chloride (Since $H-\underset{|}{\mathop{C}}\,=O$group is called formyl group).
Thus, option (B) is correct.
Note: Acyl halide is an organic compound that can be prepared by the reaction of carboxylic acids with reagents such as $PC\,{{l}_{5}}$ thionyl chloride for acyl chloride but for the bromide, the reagent phosphorus tribromide is used. Also, aromatic acyl chlorides are prepared by Friedel crafts acylation using $HCHO$as the reagent.
Recently Updated Pages
Types of Solutions in Chemistry: Explained Simply

JEE General Topics in Chemistry Important Concepts and Tips

JEE Extractive Metallurgy Important Concepts and Tips for Exam Preparation

JEE Amino Acids and Peptides Important Concepts and Tips for Exam Preparation

JEE Atomic Structure and Chemical Bonding important Concepts and Tips

Electricity and Magnetism Explained: Key Concepts & Applications

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




