




How Is Nitrogen Sesquioxide Prepared? Methods & Reactions
Nitrogen Sesquioxide is one of the oxides of nitrogen, its chemical name is dinitrogen trioxide. The chemical formula of nitrogen sesquioxide is N2O3. It appears as a blue liquid and has a distinctly unpleasant odour. It is obtained at a very low temperature at around -30oC by condensing equal parts of NO (nitric oxide) and NO2 (nitrogen dioxide). At a higher temperature above -30oC, it partially dissociates into NO and NO2. At a very low temperature (~ -100 oC), N2O3 can be isolated in the solid phase, it condenses as a pale blue solid.
The liquid N2O3 undergoes self-ionization to form nitrosonium ion (NO+):
N2O3 ⇌ NO+ + NO2-
It is a strong oxidizing agent and is highly corrosive in nature. It comes under the category of hazardous chemicals and can be extremely harmful, and potentially fatal if inhaled. Contact with soft surfaces like skin, eyes, or mucous membranes can cause strong irritation.
Physical Properties
The compound, N2O3, is highly unstable as it dissociates at higher temperatures. This makes it difficult to predict the physical properties accurately.
Table: Properties of N2O3
N2O3 Structure
The N2O3 molecule has two nitrogen atoms bound with a single bond; they form the centre of the molecule. One of the nitrogen is connected to two oxygen atoms through a single bond and a double bond; the second nitrogen has one doubly bonded oxygen atom.
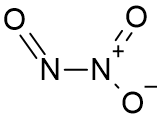
N2O3 Structure
Spectral studies have revealed the geometry. of nitrogen sesquioxide as planar in both solid as well as other states.
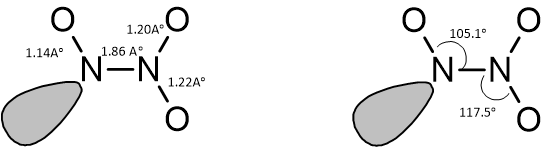
Bond Lengths and Bond Angles of Dinitrogen Trioxide
The N—N bond in dinitrogen trioxide is rather long (1.86Ao) when compared with the bond length of hydrazine NH2—NH2 (1.45Ao). The compound is diamagnetic in nature indicating the absence of odd electrons.
Another proposed structure of N2O3 in the complete anhydrous condition is assumed as shown below, it is also thought to take such a shape upon irradiation by light of appropriate wavelength. Although it was not right.
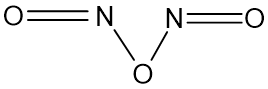
Proposed Structure of Anhydrous N2O3
Preparation
The most common method of preparation of N2O3 is by condensing equimolar NO and NO2 at very low temperatures. NO2 can be produced in situ by adding an appropriate amount of O2 to NO.
2NO + N2O4 → 2N2O3 (in cold condition)
4NO + O2 → 2N2O3
N2O3 can also be prepared by reducing nitric acid (1:1) by As2O3 (at 70oC).
2HNO3 + 2 H2O + As2O3 → N2O3 + 2H3AsO4
However, the drawback of the above method is that product yields are not completely anhydrous, due to the presence of water in the reaction. Dehydration of the product is difficult in this condition.
Chemical Reactions
N2O3 is an acidic oxide, it is the anhydride form of nitrous acid (HNO2). In the presence of water, it forms nitrous acid.
N2O3 + H2O ⇌ 2HNO2
In alkaline solution, it is converted to nitrites. For example, dinitrogen trioxide reacts with sodium hydroxide to give sodium nitrite (NaNO2)
N2O3 + 2NaOH ⇌ 2NaNO2 + H2O
Uses of N2O3
It is one of the sources of nitrosonium ion NO+ that has extensive chemistry.
In the organic synthesis of proton and water-sensitive products that involve nitrosation and nitration of the substrate, nitric acid is replaced by solvent-stabilized dinitrogen trioxide (readily prepared in high concentrations in dry organic solvent at the normal working condition). This allows acid and water-free reaction conditions.
N2O3 is used as special-purpose rocket fuel due to its combustible nature. However, exposure to heat for a long time may rupture the container and the rocket violently. Due to its strong oxidizing ability, it is used as an oxidizing agent.
Key Features
N2O3 compound name is nitrogen sesquioxide
N2O3 chemical name is Dinitrogen trioxide
N2O3 is one of the simplest oxides of nitrogen
N2O3 is a strongly oxidizing agent. It is highly corrosive and hazardous.
N2O3 is used as special fuel in rocket engines.
N2O3 forms the part of simple nitrogen oxides (NOx) formed in the internal combustion of engines due to the combustion of fossil fuel. It contributes to air pollution from vehicular emissions.
Like other oxides of nitrogen, dinitrogen trioxide is found in nature as a part of the biogeochemical cycle of nitrogen on earth.
FAQs on Nitrogen Sesquioxide: Detailed Guide for Students
1. What exactly is nitrogen sesquioxide?
Nitrogen sesquioxide, also known as dinitrogen trioxide, is an oxide of nitrogen with the chemical formula N₂O₃. The term 'sesquioxide' indicates a 2:3 ratio of an element to oxygen atoms. It is an unstable, deep blue compound that is considered the anhydride of nitrous acid (HNO₂), meaning it reacts with water to form this acid.
2. What are the key properties of nitrogen sesquioxide (N₂O₃)?
The key properties of nitrogen sesquioxide are primarily defined by its instability and physical state at different temperatures. Here are its main characteristics:
- Appearance: It is a deep blue liquid or solid at very low temperatures.
- Stability: N₂O₃ is highly unstable and only exists in pure form at low temperatures (below 3.5 °C). At higher temperatures, it dissociates into nitric oxide (NO) and nitrogen dioxide (NO₂).
- Acidic Nature: It is an acidic oxide. When dissolved in water, it forms nitrous acid (HNO₂).
- Oxidation State: The nitrogen atoms in N₂O₃ have an oxidation state of +3.
- Bonding: The molecule contains covalent bonds between the nitrogen and oxygen atoms.
3. How is nitrogen sesquioxide prepared?
Nitrogen sesquioxide (N₂O₃) is typically prepared by mixing equimolar amounts of nitric oxide (NO) and nitrogen dioxide (NO₂) or dinitrogen tetroxide (N₂O₄). The reaction is performed at low temperatures, generally around -20 °C, to condense the resulting deep blue liquid. The chemical equation for this reversible reaction is: NO + NO₂ ⇌ N₂O₃.
4. What is the oxidation state of nitrogen in N₂O₃ and how is it calculated?
The oxidation state of nitrogen in nitrogen sesquioxide (N₂O₃) is +3. This can be calculated by assigning the standard oxidation state of -2 to each oxygen atom. Since the overall charge of the molecule is zero, the calculation is as follows:
Let 'x' be the oxidation state of nitrogen.
2(x) + 3(-2) = 0
2x - 6 = 0
2x = +6
x = +3
5. What are some important uses of dinitrogen trioxide?
Despite its instability, dinitrogen trioxide has specialised applications, including:
- Chemical Synthesis: It is used to prepare pure nitrous acid (HNO₂), which is otherwise unstable.
- Specialised Oxidizer: It can be used in special rocket propellants and fuels that ignite spontaneously (hypergolic fuels).
- Organic Chemistry: It serves as a reagent in reactions that require the introduction of a nitrosonium ion (NO⁺).
6. Why is nitrogen sesquioxide (N₂O₃) only stable at very low temperatures?
Nitrogen sesquioxide's instability at room temperature is due to a reversible equilibrium where it dissociates into nitric oxide (NO) and nitrogen dioxide (NO₂). The formation of N₂O₃ is an exothermic process. According to Le Chatelier's principle, lowering the temperature favours the exothermic forward reaction, thus stabilising the N₂O₃ molecule. Above its boiling point of 3.5 °C, the equilibrium shifts significantly towards dissociation, making it unstable.
7. How does nitrogen sesquioxide (N₂O₃) differ from other nitrogen oxides like N₂O₄?
While both are oxides of nitrogen, N₂O₃ and N₂O₄ have distinct differences:
- Oxidation State: Nitrogen is in the +3 oxidation state in N₂O₃, whereas it is in the +4 oxidation state in N₂O₄ (dinitrogen tetroxide).
- Colour: N₂O₃ is a characteristic deep blue solid or liquid. In contrast, N₂O₄ is a colourless solid or liquid that exists in equilibrium with the brown gas NO₂.
- Chemical Product: N₂O₃ is the anhydride of nitrous acid (HNO₂), while N₂O₄ is the anhydride of both nitrous acid and nitric acid (HNO₃).
8. Why is the N-N bond in dinitrogen trioxide considered unusually long and weak?
The N-N bond in dinitrogen trioxide is approximately 186 picometres (pm), which is significantly longer than a typical N-N single bond (~145 pm). This long bond length makes it very weak, which is a primary reason for the molecule's tendency to easily dissociate into NO and NO₂. The weakness is attributed to a combination of steric hindrance and electronic repulsion between the non-bonding electron pairs and the oxygen atoms attached to each nitrogen, which stretch and weaken the central N-N bond.
























