




How to Identify and Apply Meso Compounds in Chemistry Problems
Meso compounds are symmetric or achiral compounds that have stereocenters. A meso compound, despite having two or more stereocenters, is optically inactive and possesses an inner symmetrical plane that allows it to be superimposed on its mirrored counterpart. Mesomers, therefore, are organic compounds with comparable chiral carbons on both sides, giving in zero net rotation.
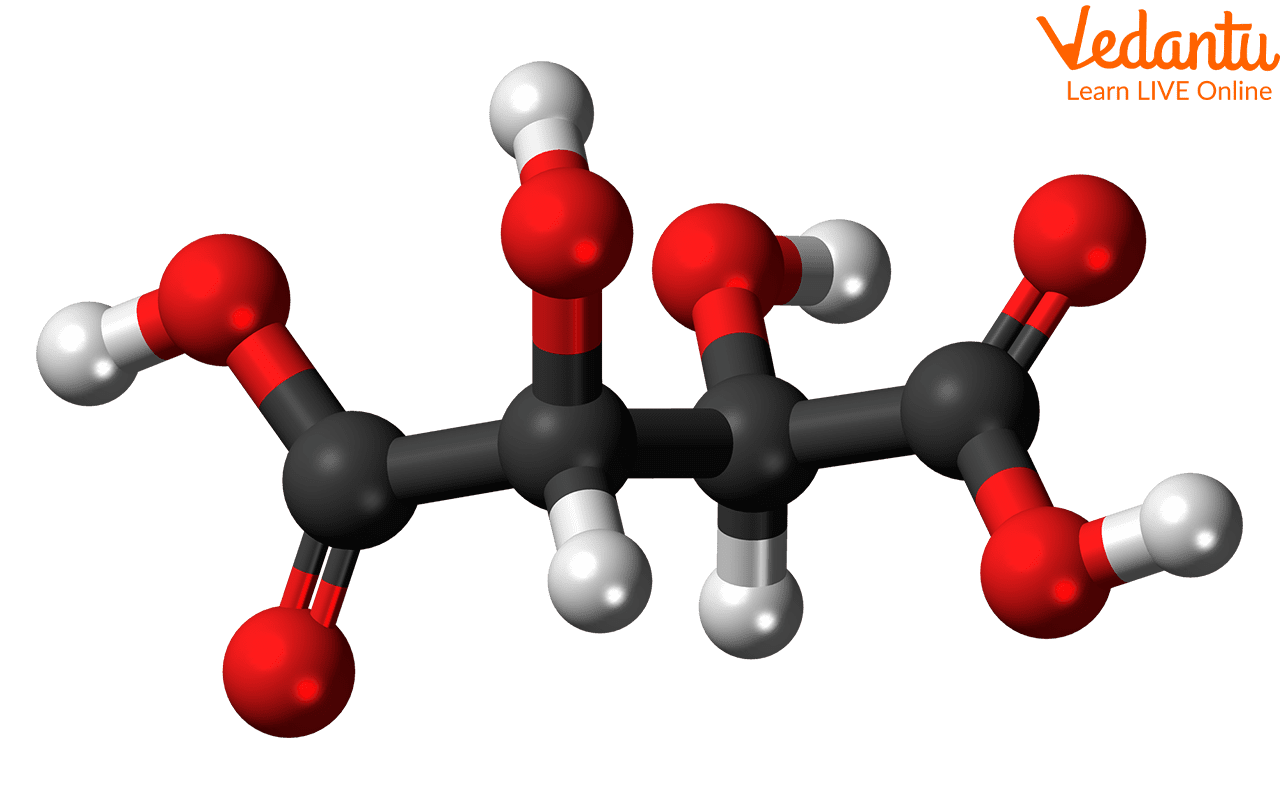
Meso Compounds
What are Meso Compounds?
Meso compounds are achiral compounds with multiple chiral centres. It is optically inactive despite possessing stereocenters since it is superimposed on its mirror counterpart. It also possesses an inner plane of symmetry.
A meso compound or meso isomer is a stereoisomer that has at least two optically active members but is otherwise inactive. Despite having two or more chiral carbon centres, this indicates that the compound is not chiral.
Identification of Meso Compounds
A symmetrical plane within the compound separates it in half. The inner mirror allows these two sides to reflect one another. In meso compounds, the stereochemistry around chiral centres must "cancel out." This indicates that when a compound is divided into two symmetrical halves by an internal plane, the stereochemistry of the left and right sides should be in opposition to one another and result in an optically inactive compound.
Let ‘A’ be a meso compound. We know that a meso compound must include R and S stereochemistry, two or more stereocenters, and an inner plane.
To find out if ‘A’ is meso, we must first search for an inner plane or inner mirror.
When evaluating if a chemical is a meso compound or not, stereochemistry is extremely important. Because a meso compound is optically inert, its stereochemistry tends to cancel out. If a meso compound has two stereocenters (R and S), then R must cancel out S.
Note:
Covalent bonds or \[s{{p}^{3}}\] orbitals have an interesting property that we can twist the substituted groups linked to a stereocenter to identify the inner plane. The stereochemistry of the compound does not vary as it is rotated.
The other scenario is when the entire structure is rotated by 180 degrees. Both of the components are still meso.
Meso Compounds Examples
As noted previously, a meso compound must have at least two chiral \[s{{p}^{3}}\] hybridized atoms and at least one inner plane that divides the molecule into two mirror images. Tetrahedral centres are present, which prevents the molecule from being planar. An achiral compound won't rotate planar polarized light even if it just contains one element of symmetry. Meso compounds lack optical activity because of this.
Exception: The nitrogen atom in tertiary amine is not \[s{{p}^{3}}\] hybridized, even though the compound is meso.
1. This compound is achiral because it possesses a plane of symmetry. But because it contains two chiral carbon atoms, it is a meso compound.
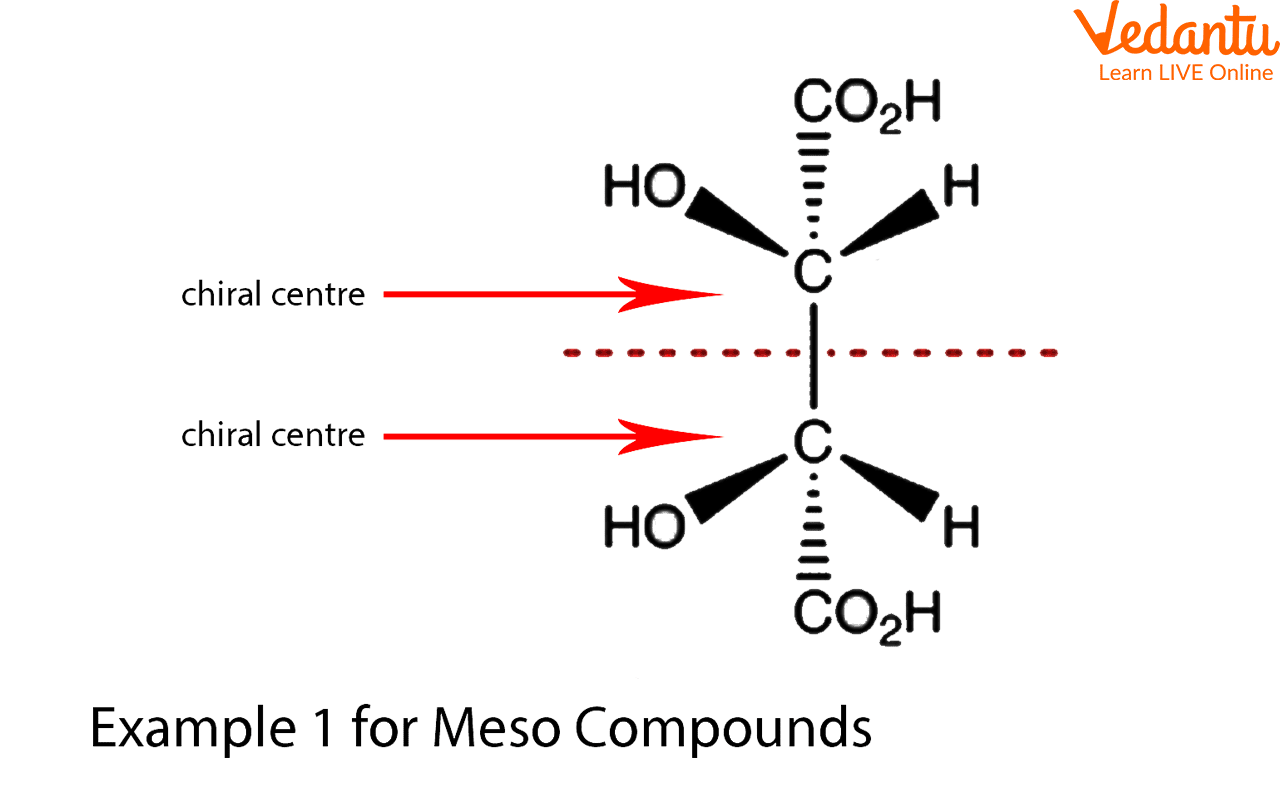
Example for Meso Compounds
2. This molecule has two chiral sites and is achiral since it possesses a symmetrical plane. It is a meso compound as a result.
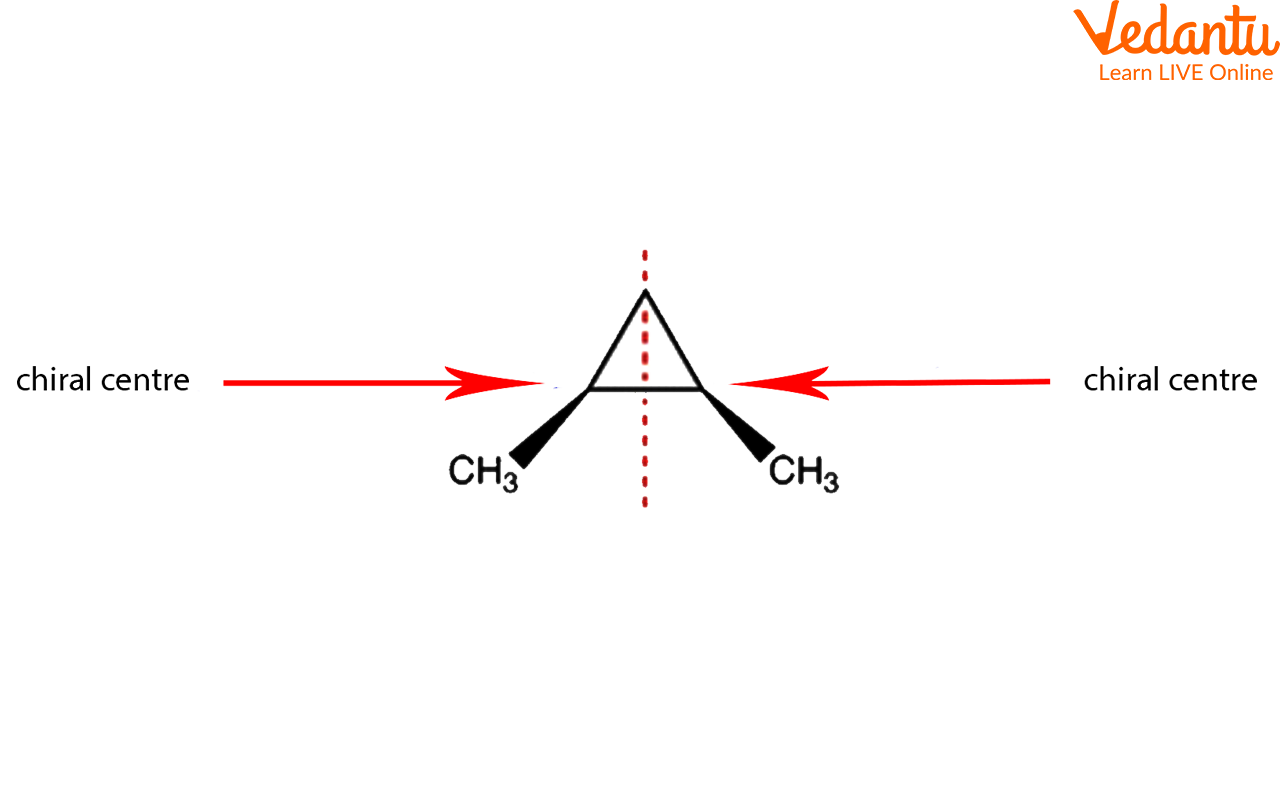
Example for Meso Compounds
Optical Activity of Meso Compounds
A meso compound or meso isomer is one of a group of stereoisomers that has at least two optically active members, although none of them is optically active. This indicates that despite having two or more chiral centres, the compound is not chiral. These substances are achiral compounds with several chiral centres. Despite having stereo centres, it is overlaid on its mirror counterpart and optically inert. It possesses a plane of inner symmetry that splits the molecule into halves. These two sides mirror one another through the inner mirror.
Stereochemistry in the stereocenter can balance out. This implies that the stereochemistry of the left and right sides should be opposed to one another if the molecule is divided into two symmetrical halves by an inner plane. Hence, the outcome is optically inactive. Technically, cyclic molecules are also meso. Since optical activity cancels out in the presence of a symmetrical plane, meso compounds lack optical activity.
Key Features
In this article, we have studied meso compounds, meso compound examples, and meso compound definitions.
Meso Compounds are non-optically active compounds, with at least two or more stereocenters
Meso Compounds examples include pentane, butane, etc.
Meso compounds can be identified by two or more stereocenters, an internal plane, and stereochemistry should be R or S.
Interesting Facts
Meso Compounds are optically inactive even after having two or chiral centres.
Many radioactive elements glow in the dark.
Helium balloons are lighter than air, that's why they float in the air.
Important Questions
1. What are enantiomers?
Enantiomers are defined as pairs of molecules that exist in two forms and both are non-superimposable mirror images of each other. Both enantiomers are chemically identical to each other.
2. Is chiral a meso compound?
A meso compound is an achiral compound with two or more chiral centres. A plane of symmetry is present in all meso compounds. The internal plane of symmetry makes a superimposable mirror image.
3. How do you know if a compound is meso?
There are certain characteristics and features of meso compounds. These are the presence of an internal plane of symmetry, two or more chiral centres or stereocenters, and R or S configuration.
FAQs on Meso Compounds Explained: Key Concepts & Tips
1. What is a meso compound? Please provide an example.
A meso compound is a molecule that contains two or more chiral centers (stereocenters) but is achiral overall, meaning it is superimposable on its mirror image. This is possible because it possesses an internal plane of symmetry or a center of inversion. Due to this internal symmetry, meso compounds are optically inactive. A classic example is meso-tartaric acid, where a plane of symmetry bisects the central carbon-carbon bond, making one half of the molecule a mirror image of the other.
2. How can a student identify if a molecule is a meso compound?
To identify a meso compound, you should check for the following three conditions:
- The molecule must have two or more chiral centers.
- There must be an internal plane of symmetry that divides the molecule into two identical, mirror-image halves.
- The stereochemical configurations (R/S) at the chiral centers must cancel each other out. For example, in a molecule with two chiral centers and a plane of symmetry between them, one center will have an 'R' configuration and the other will have an 'S' configuration.
3. Why are meso compounds optically inactive even though they possess chiral centers?
Meso compounds are optically inactive due to a phenomenon called internal compensation. While each chiral center in the molecule does rotate plane-polarised light, the internal plane of symmetry ensures that for every chiral center causing a rotation in one direction (e.g., clockwise), there is another equivalent chiral center causing an equal rotation in the opposite direction (e.g., counter-clockwise). The net result is a zero optical rotation, rendering the entire molecule optically inactive.
4. What is the main difference between a meso compound and a chiral compound?
The main difference lies in their overall symmetry and interaction with light. A chiral compound lacks an internal plane of symmetry, is non-superimposable on its mirror image, and is optically active. In contrast, a meso compound possesses an internal plane of symmetry, is superimposable on its mirror image (making it achiral), and is optically inactive, despite containing individual chiral centers.
5. How do meso compounds relate to enantiomers and diastereomers?
The relationship is key to understanding stereoisomerism:
- Enantiomers are pairs of chiral molecules that are non-superimposable mirror images of each other. A meso compound cannot have an enantiomer because its mirror image is identical to itself.
- A meso compound is a diastereomer of the chiral isomers of the same molecule. For example, (2R, 3S)-tartaric acid (the meso form) is a diastereomer of both (2R, 3R)-tartaric acid and (2S, 3S)-tartaric acid because they are stereoisomers but not mirror images of each other.
6. Can a molecule with only one chiral center ever be a meso compound?
No, a molecule with only one chiral center can never be a meso compound. The definition of a meso compound requires the presence of at least two chiral centers. A single chiral center will always make a molecule chiral, as there is no second center to create an internal plane of symmetry and cause the internal compensation of optical rotation.
7. Can cyclic compounds also be meso compounds?
Yes, cyclic structures can be meso compounds. The same rules apply: the molecule must contain at least two chiral centers and have an internal plane of symmetry. A common example is cis-1,2-dimethylcyclohexane. It has two chiral carbons (C-1 and C-2), but a plane of symmetry runs through the molecule, making it achiral and therefore a meso compound. Its trans-isomer, however, exists as a pair of enantiomers.
8. What is the practical importance of identifying meso compounds in chemistry?
Identifying meso compounds is crucial for several reasons:
- Predicting Reaction Products: In stereospecific reactions, knowing whether a meso compound can be formed helps predict the exact number and type of stereoisomers in the product mixture.
- Purification and Separation: A meso compound has different physical properties (like melting point and boiling point) from its diastereomers, allowing it to be separated using techniques like distillation or crystallization.
- Pharmaceuticals and Biology: Since different stereoisomers can have different biological effects, correctly identifying a compound as an achiral meso form is vital for understanding its interaction with biological systems.
























