




What Affects Ionisation Enthalpy in Transition Metals?
Elements with partially filled d orbitals are referred to as transition elements according to IUPAC, a transition element is an element with a partially filled d subshell of electrons or an element with a partially filled d orbital that can form stable cations. Any element that falls under the periodic table's d-block, which includes groups 3–12, is typically regarded as a transition element. Even the lanthanides and actinides, which are members of the f-block, can be categorised as transition elements. The ionisation enthalpy of an element can be defined as the amount of energy required to remove an electron from an isolated gaseous atom in its gaseous state.
Electronic Configuration of Transition Elements
It should be noted that the electron configuration in some of these elements corresponds to (n-1)$${{d}^{5}}$$ n$${{s}^{1}}$$ or (n-1)$${{d}^{10}}$$ n$${{s}^{1}}$$. This is due to the stability that the partially or entirely filled electron orbitals offer. In the below image, you can see the electronic configuration of some transition elements. Ionisation enthalpies of transition metals are intermediate between those of s-block and p- block elements as they are placed between s-block and p-block in the periodic table.
Many transitional elements, such as chromium, do not obey the Aufbau principle. The comparatively small energy difference between the 3d and 4s orbitals and the 4d and 5s orbitals is thought to be the cause of this.
Properties of Transition Elements
Since their electrical structures differ from other transition metals, the elements zinc, cadmium, and mercury are not regarded as transition elements, as was previously said. The properties of the remaining d-block elements, however, are quite comparable, and this similarity may be seen along each particular row of the periodic table. Below is a list of these characteristics of the transitional elements.
These substances and ions are created by these elements. The electron d-d transition provides an explanation for its colour.
The energy difference between these elements' potential oxidation states is quite small. As a result, the transition elements have a variety of oxidation states.
These elements produce a large number of paramagnetic compounds due to the unpaired electrons in the d orbital.
These elements can be bound to a wide range of ligands. As a result, transition elements can create a wide range of stable complexes.
These substances have a high charge to radius ratio.
When compared to other elements, transition metals have relatively high densities and a tendency to be hard.
Due to the delocalized d electrons' involvement in metallic bonding, these elements have high melting and boiling temperatures.
The delocalized d electrons metallic bonding also makes the transition elements excellent electrical conductors.
What is Ionisation Enthalpy?
The amount of energy required to liberate the most loosely bound electron from a single gaseous atom in order to produce a gaseous ion is known as the ionisation enthalpy or ionisation energy.
It is given in kJ/mol, a calorie-like energy unit.
Any particular atom's outermost valence electrons will ionise with a lower energy than its inner-shell electrons.
Ionisation Enthalpy of Transition Elements
The quantity of energy required to be supplied to an element in order to remove a valence electron is referred to as the ionisation enthalpy. The ionisation potential of an element increases with the effective nuclear charge acting on the electrons. This explains why transition elements typically have higher ionisation enthalpies than s-block elements. An element's atomic radius and ionisation energy are somewhat inversely connected. Smaller atoms often have higher ionisation enthalpies than atoms with comparatively larger radii. While advancing along the row, the transition metals' ionisation energies rise.
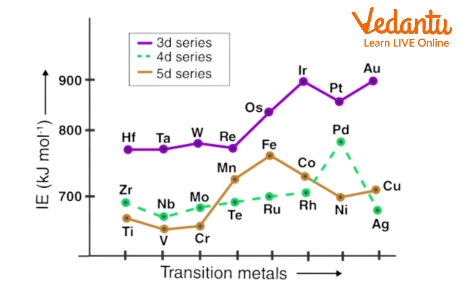
Ionisation Enthalpy of Transition Elements in Graph
Important Questions
1. What is lanthanoid contraction?
Ans: The general term for the decrease in atomic and ionic radii with increasing atomic number is called lanthanoid contraction. With the addition of electrons, the effective nuclear charge increases along the lanthanide series, and the more electrons in the f-subshell lead to insufficient shielding that is unable to counteract the effect of the rising nuclear charge. The effect is a reduction in size.
2. Describe the preparation of potassium dichromate from chromite ore. What is the effect of change of pH on dichromate ions?
Ans: Preparation of Potassium dichromate $({K}_{2}{Cr}_{2}{O}_{7})$:
Potassium dichromate is prepared from chromite ore $$({Fe}{Cr}_{2}{O}_{4})$$ in the following steps.
Step (1):
Preparation of sodium chromate
$${4}{Fe}{Cr}_{2}{O}_{4} {+} {16}{Na}{OH} {+} {7}{O}_{2} \to {8}{Na}{Cr}{O}_{4} {+} {2}{Fe}_{2}{O}_{3} {+} {8}{H}_{2}{O}$$
Step (2):
Conversion of sodium chromate into sodium dichromate
$${2}{Na}_{2}{Cr}{O}_{4} {+} {conc.}{H}_{2}{SO}_{4} \to {Na}_{2}{Cr}_{2}{O}_{7} {+} {Na}_{2}{SO}_{4} {+} {H}_{2}{O}$$
Step(3):
Conversion of sodium dichromate to potassium dichromate
$${Na}_{2}{Cr}_{2}{O}_{7} {+} {2}{K}{Cl} \to {K}_{2}{Cr}_{2}{O}_{7} {+} {2}{Na}{Cl}$$
Potassium dichromate being less soluble than sodium chloride is obtained in the form of orange coloured crystals and can be removed by filtration.
Multiple Choice Questions
1. The first transition element is
a. Copper
b. Nickel
c. Scandium
d. Vanadium
Answer: (c)
2. Transition elements exhibit variable valency because they release electrons from
a. ns orbitals
b. np orbitals
c. (n-1)d orbitals
d. (n-1)d & ns orbitals
Answer: C
Conclusion
Transition elements are substances with partially filled d orbitals, also referred to as transition metals. Transition elements are those that, despite having an incomplete d orbital, can nevertheless form stable cations, according to the International Union of Pure and Applied Chemistry (IUPAC). Ionisation enthalpies are higher for smaller atoms than for larger ones. Along the row, the ionisation energies of the transition metals increase (due to the increase in atomic number).
FAQs on Ionisation Enthalpy of Transition Elements-Key Concepts & Tips
1. What is meant by the ionisation enthalpy of transition elements?
The ionisation enthalpy of a transition element is the minimum energy required to remove the most loosely bound electron from an isolated gaseous atom in its ground state. It is typically measured in kJ/mol. For transition elements, this usually involves removing an electron from the outermost ns orbital first. The values are generally intermediate between those of s-block and p-block elements.
2. What is the general trend of first ionisation enthalpy across a transition series?
The general trend for the first ionisation enthalpy (IE₁) across a transition series (like the 3d series from Sc to Zn) is a gradual increase from left to right. This is due to an increase in the effective nuclear charge as the atomic number increases, which pulls the electrons more tightly. However, this trend is not regular and has some anomalies.
3. Why is the trend in first ionisation enthalpies of the 3d series irregular?
The irregular trend in the first ionisation enthalpies of the 3d series is due to the interplay between increasing nuclear charge and the shielding effect of (n-1)d electrons. Key irregularities include:
- A higher than expected value for Chromium (Cr) and Copper (Cu) because of the stability associated with their half-filled (3d⁵) and fully-filled (3d¹⁰) electronic configurations, respectively.
- A very high value for Zinc (Zn), as the electron is removed from a fully-filled 4s orbital and its 3d¹⁰ configuration is extremely stable.
- A dip in the value for Manganese (Mn) compared to the general trend, which is often attributed to its stable d⁵ configuration making it slightly harder to remove an electron.
4. Why do transition elements have higher ionisation enthalpies than s-block elements but lower than p-block elements in the same period?
Transition elements have ionisation enthalpies that fall between s-block and p-block elements for these reasons:
- Higher than s-block elements: Across a period, transition elements have a greater nuclear charge and smaller atomic radii compared to the s-block elements, making it more difficult to remove an electron.
- Lower than p-block elements: Within the same period, the outermost electron in a p-block element is in a p-orbital, which penetrates less and is more effectively shielded than an s-orbital. The increasing nuclear charge in p-block elements is also more pronounced, making electron removal significantly harder than from the ns or (n-1)d orbitals of transition metals.
5. How does ionisation enthalpy influence the variable oxidation states of transition elements?
Ionisation enthalpy directly explains the ability of transition elements to show variable oxidation states. The energy difference between successive ionisation enthalpies (IE₁, IE₂, IE₃, etc.) is relatively small. This is because electrons can be removed from both the outermost ns orbital and the inner (n-1)d orbital, which have very similar energy levels. As long as the cumulative energy required for removing electrons is not prohibitively high, the element can exhibit multiple oxidation states. For instance, manganese can show states from +2 to +7 by losing electrons from both 4s and 3d orbitals.
6. What is lanthanoid contraction, and how does it affect the ionisation enthalpies of the 5d transition series?
Lanthanoid contraction is the steady decrease in the size of lanthanoid atoms and ions with increasing atomic number. This is caused by the poor shielding effect of the 4f electrons. This contraction has a significant impact on the properties of the subsequent transition elements in the 5d series (like Hf, Ta, W). Because of lanthanoid contraction, the atomic radii of 5d elements are almost identical to their 4d counterparts. Consequently, the 5d elements have a very high effective nuclear charge, leading to significantly higher ionisation enthalpies than would otherwise be expected.
7. How can successive ionisation enthalpies explain why the Mn²⁺ ion is more stable than Mn³⁺?
The stability of Mn²⁺ over Mn³⁺ is clearly explained by analysing successive ionisation enthalpies. The first two ionisation enthalpies (IE₁ and IE₂) for Manganese (Mn) are relatively low, allowing the removal of two 4s electrons to form Mn²⁺, which has a stable, half-filled d-orbital configuration (3d⁵). However, the third ionisation enthalpy (IE₃) is exceptionally high. This is because removing a third electron requires breaking this highly stable half-filled d⁵ configuration. This large energy barrier makes the formation of Mn³⁺ much less favourable than Mn²⁺.
























