Amines Class 12 important questions with answers PDF download


FAQs on Important Questions For Class 12 Chemistry Chapter 9 Amines - 2025-26
1. What are some of the most important named reactions from the Amines chapter for the CBSE Class 12 board exam 2025-26?
For the Class 12 Chemistry board exam, the following named reactions from the Amines chapter are frequently asked in 2- or 3-mark questions:
- Hofmann Bromamide Reaction: A method to prepare primary amines with one carbon atom less than the parent amide.
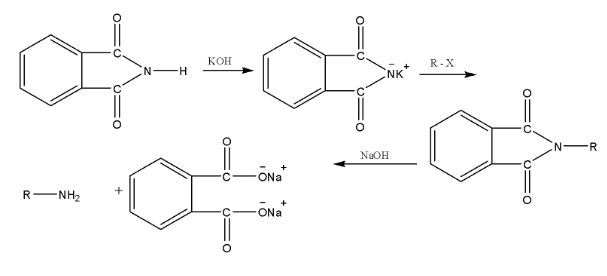
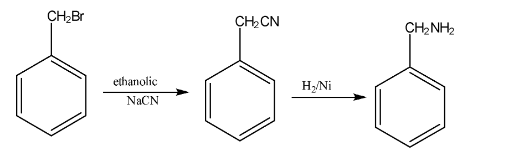
- Gabriel Phthalimide Synthesis: A preferred method for preparing pure primary aliphatic amines from primary alkyl halides.
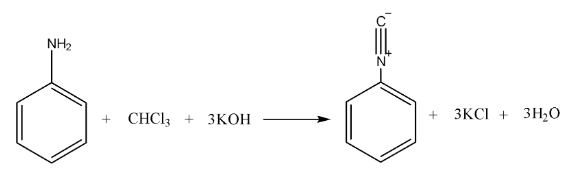
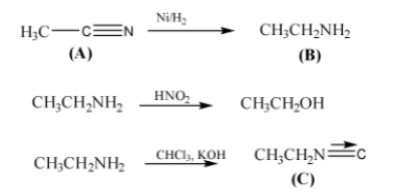
- Carbylamine Reaction (Isocyanide Test): A definitive test for primary amines, which produce a foul-smelling isocyanide.
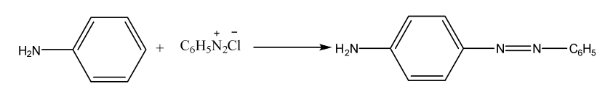
- Coupling Reaction: The reaction of diazonium salts with phenols or aromatic amines to form brightly coloured azo dyes.
2. How can you distinguish between primary, secondary, and tertiary amines using a single chemical test as per the CBSE curriculum?
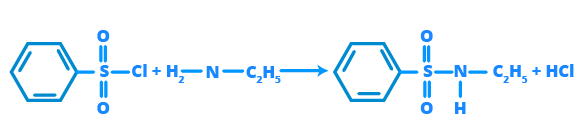
The Hinsberg's Test is the standard method used to distinguish between primary, secondary, and tertiary amines. It uses benzenesulphonyl chloride (Hinsberg's reagent).
- Primary Amines react to form a sulphonamide that is soluble in alkali (like NaOH or KOH).
- Secondary Amines react to form a sulphonamide that is insoluble in alkali.
- Tertiary Amines do not react with Hinsberg's reagent.
This difference in reactivity and solubility is a crucial concept for practical chemistry and board exam questions.
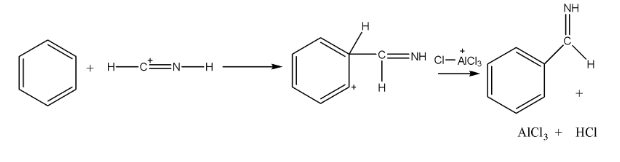
3. Explain why aniline does not undergo the Friedel-Crafts reaction, a typical electrophilic substitution reaction.
Aniline does not undergo the Friedel-Crafts reaction (both alkylation and acylation) due to a specific interaction with the catalyst. The catalyst used is a Lewis acid, typically anhydrous AlCl₃. Aniline, being a Lewis base due to the lone pair of electrons on the nitrogen atom, reacts with the Lewis acid catalyst to form a salt. This deactivates the benzene ring by pulling electrons away from it, making it highly resistant to further electrophilic substitution.
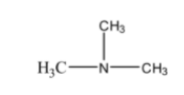
4. Arrange the following compounds in the increasing order of their basic strength in an aqueous solution and justify your answer: C₂H₅NH₂, (C₂H₅)₂NH, (C₂H₅)₃N, NH₃.
The correct increasing order of basic strength in an aqueous solution is: NH₃ < (C₂H₅)₃N < C₂H₅NH₂ < (C₂H₅)₂NH. The basicity of amines in water is determined by a combination of three factors:
- Inductive Effect (+I): The electron-donating ethyl groups increase the electron density on the nitrogen atom, enhancing basicity.
- Solvation Effect: The ammonium cation formed after accepting a proton is stabilised by hydrogen bonding with water. More H-atoms on nitrogen lead to better stabilisation.
- Steric Hindrance: Bulky alkyl groups can block the protonation of the amine.
The secondary amine, (C₂H₅)₂NH, is the strongest base because it has the most favourable combination of the +I effect and solvation, with moderate steric hindrance.
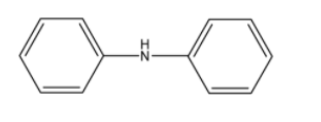
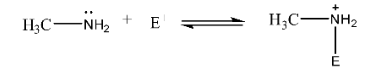
5. Why are aliphatic amines stronger bases than aromatic amines like aniline?
Aliphatic amines are stronger bases than aromatic amines due to the availability of the lone pair of electrons on the nitrogen atom.
- In aliphatic amines (e.g., ethylamine), the electron-donating alkyl group (+I effect) increases the electron density on the nitrogen, making the lone pair more available for donation.
- In aromatic amines (e.g., aniline), the lone pair of electrons on the nitrogen atom is delocalised into the benzene ring through resonance. This makes the lone pair less available for protonation, thus reducing its basic strength.
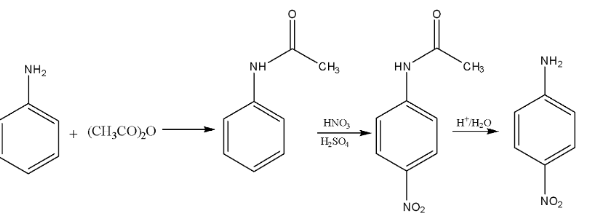
6. Although the amino group is ortho- and para-directing, the nitration of aniline gives a substantial amount of m-nitroaniline. Why is this an important conceptual question?
This is a classic higher-order thinking (HOTS) question. Nitration is carried out in a strongly acidic medium (conc. HNO₃ and conc. H₂SO₄). In this medium, the basic aniline molecule gets protonated to form the anilinium ion (-NH₃⁺). The anilinium group is a powerful electron-withdrawing and deactivating group, which directs the incoming electrophile (NO₂⁺) to the meta-position. This is why a significant amount of m-nitroaniline (around 47%) is formed, along with ortho and para isomers.
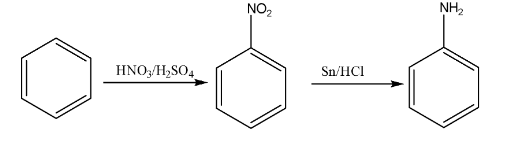
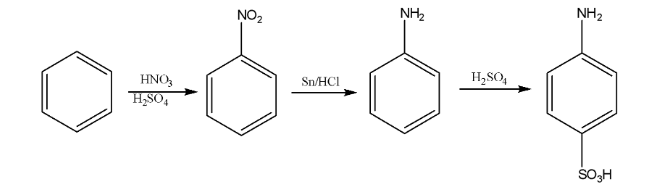
7. How would you carry out the conversion of Benzene to Aniline? This is a frequently tested 2-mark conversion.
The conversion of Benzene to Aniline is a two-step process:
- Step 1: Nitration of Benzene. Benzene is treated with a nitrating mixture (concentrated Nitric acid and concentrated Sulphuric acid) at 323-333 K to form Nitrobenzene.
- Step 2: Reduction of Nitrobenzene. The nitro group is reduced to an amino group using a reducing agent like Tin and concentrated HCl (Sn/HCl) or Hydrogen gas with a Palladium catalyst (H₂/Pd). This step yields Aniline.
8. Explain why the boiling points of primary amines are higher than tertiary amines of comparable molecular mass.
Primary amines (R-NH₂) have higher boiling points than tertiary amines (R₃N) because primary amines can form strong intermolecular hydrogen bonds with each other due to the presence of two hydrogen atoms bonded to the nitrogen. Tertiary amines lack hydrogen atoms bonded to nitrogen and therefore cannot form hydrogen bonds. More energy is required to overcome these strong intermolecular forces in primary amines, resulting in a higher boiling point.
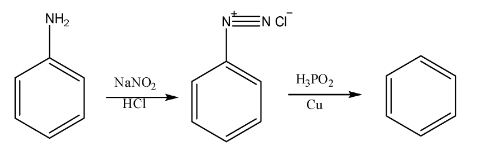
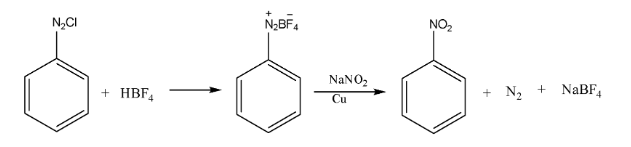
9. What is the significance of diazonium salts in organic chemistry, making them a crucial topic for board exams?
Benzene diazonium chloride is a highly valuable intermediate in organic synthesis because the diazonium group (-N₂⁺Cl⁻) is an excellent leaving group and can be easily replaced by various nucleophiles. This allows for the synthesis of a wide range of substituted benzene compounds that are difficult to prepare directly. Important reactions include:
- Sandmeyer Reaction: Replacement by -Cl, -Br, or -CN.
- Gattermann Reaction: Replacement by -Cl or -Br using copper powder.
- Replacement by -I, -F, -OH, and -H.
- Coupling Reactions: To form azo dyes.
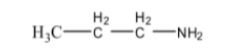
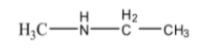
10. An organic compound 'A' with molecular formula C₃H₉N reacts with benzenesulphonyl chloride to form a product soluble in NaOH. Identify compound 'A'.
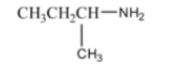
This question tests the application of the Hinsberg's Test. The fact that the product is soluble in NaOH indicates that the amine is a primary amine. The primary amine with the molecular formula C₃H₉N is Propan-1-amine (CH₃CH₂CH₂NH₂) or Propan-2-amine ((CH₃)₂CHNH₂). Both are valid answers as they are primary amines and isomers.
11. Why can't aromatic primary amines like aniline be prepared by the Gabriel Phthalimide synthesis?
The Gabriel Phthalimide synthesis is not suitable for preparing aromatic primary amines like aniline for a key reason related to reaction mechanisms. The method relies on the nucleophilic substitution of an alkyl halide by the phthalimide anion. However, the C-X bond in aryl halides (like chlorobenzene) has a partial double-bond character due to resonance, making it very difficult to break. Therefore, aryl halides do not undergo this nucleophilic substitution reaction, preventing the formation of aniline.
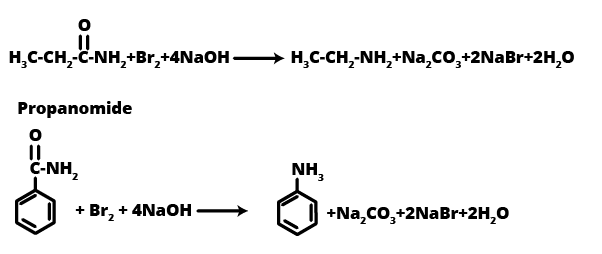
12. Identify A, B, and C in the following reaction sequence, a common 3-mark problem: CH₃CH₂I --(NaCN)--> A --(OH⁻/Partial Hydrolysis)--> B --(Br₂/NaOH)--> C
This is a typical multi-step synthesis problem often asked in exams. The sequence of products is as follows:
- Compound A is Propanenitrile (CH₃CH₂CN), formed by nucleophilic substitution of iodide by cyanide.
- Compound B is Propanamide (CH₃CH₂CONH₂), the result of partial hydrolysis of the nitrile.
- Compound C is Ethanamine (CH₃CH₂NH₂), formed via the Hofmann Bromamide degradation reaction, where the amide is converted to a primary amine with one less carbon atom.