
Which of the following on hydrolysis forms acetic acid
A.\[{\rm{C}}{{\rm{H}}_{\rm{3}}}{\rm{CN}}\]
B.\[{\rm{C}}{{\rm{H}}_{\rm{3}}}{\rm{OH}}\]
C.\[{{\rm{C}}_{\rm{2}}}{{\rm{H}}_{\rm{5}}}{\rm{OH}}\]
D.\[{{\rm{C}}_{\rm{2}}}{{\rm{H}}_{\rm{5}}}{\rm{N}}{{\rm{H}}_2}\]
Answer
233.1k+ views
Hint: Hydrolysis refers to the breaking down of chemical bonds by a molecule of water. Water or hydroxyl ion acts as a nucleophile in this type of reaction.
Complete Step by Step Solution:
Acetic acid, also recognized as ethanoic acid, is an acidic, uncoloured liquid.
It is the simplest carboxylic acid containing two carbon atoms and thus has the name 'ethanoic acid'.
A. \[{\rm{C}}{{\rm{H}}_{\rm{3}}}{\rm{CN}}\]
This is ethanenitrile or acetonitrile also known as methyl cyanide.
This is an uncoloured liquid and is the simplest organic nitrile.
The acidic hydrolysis of acetonitrile will produce acetic acid.
This hydrolysis happens in the presence of hydrochloric acid.
The initial step involves the formation of ethanamide.
The next step involves the formation of ethanoic acid and ammonium chloride.
The reaction happens as follows: \[{\rm{C}}{{\rm{H}}_{\rm{3}}}{\rm{CN + 2}}{{\rm{H}}_{\rm{2}}}{\rm{O + HCl}} \to {\rm{C}}{{\rm{H}}_{\rm{3}}}{\rm{COOH + N}}{{\rm{H}}_{\rm{4}}}{\rm{Cl}}\]
So, A is correct.
B. \[{\rm{C}}{{\rm{H}}_{\rm{3}}}{\rm{OH}}\]
This is methanol or methyl alcohol.
This is the simplest organic alcohol compound.
Methanol on hydrolysis does not give acetic acid.
So, B is incorrect.
C. \[{{\rm{C}}_{\rm{2}}}{{\rm{H}}_{\rm{5}}}{\rm{OH}}\]
This is ethanol or ethyl alcohol.
This is the next organic alcohol compound after methanol.
Ethanol on hydrolysis produces ethanal or acetaldehyde.
The reaction occurs as follows:
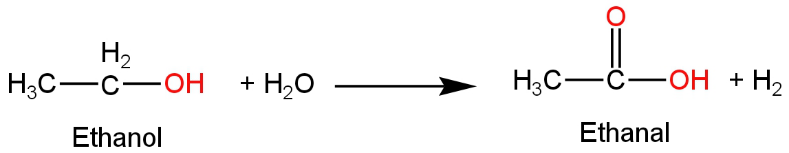
Image: Hydrolysis of ethanol
This does not produce acetic acid as the product.
So, C is incorrect.
D. \[{{\rm{C}}_{\rm{2}}}{{\rm{H}}_{\rm{5}}}{\rm{N}}{{\rm{H}}_2}\]
This is ethanamine or ethylamine.
It is a simple amine compound.
Ethylamine on diazotization by nitrous acid preceded by hydrolysis will give ethanol.
When this compound is treated with sodium nitrite and hydrochloric acid, the amine group is transformed into ethyl diazonium salt which then on hydrolysis will give ethanol.
The reaction happens as follows:
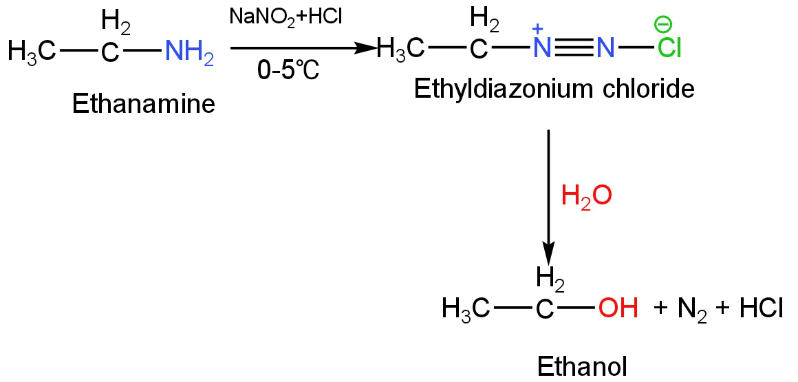
Image: Reaction of ethyl amine with nitrous acid.
So, this compound on hydrolysis will not give acetic acid.
So, D is incorrect.
So, option A is correct.
Note: Acetylene in the reaction with HgOH and dilute sulfuric acid leads to the formation of vinyl alcohol. Vinyl alcohol tautomerizes to form acetaldehyde. Acetaldehyde on oxidation forms acetic acid.
Complete Step by Step Solution:
Acetic acid, also recognized as ethanoic acid, is an acidic, uncoloured liquid.
It is the simplest carboxylic acid containing two carbon atoms and thus has the name 'ethanoic acid'.
A. \[{\rm{C}}{{\rm{H}}_{\rm{3}}}{\rm{CN}}\]
This is ethanenitrile or acetonitrile also known as methyl cyanide.
This is an uncoloured liquid and is the simplest organic nitrile.
The acidic hydrolysis of acetonitrile will produce acetic acid.
This hydrolysis happens in the presence of hydrochloric acid.
The initial step involves the formation of ethanamide.
The next step involves the formation of ethanoic acid and ammonium chloride.
The reaction happens as follows: \[{\rm{C}}{{\rm{H}}_{\rm{3}}}{\rm{CN + 2}}{{\rm{H}}_{\rm{2}}}{\rm{O + HCl}} \to {\rm{C}}{{\rm{H}}_{\rm{3}}}{\rm{COOH + N}}{{\rm{H}}_{\rm{4}}}{\rm{Cl}}\]
So, A is correct.
B. \[{\rm{C}}{{\rm{H}}_{\rm{3}}}{\rm{OH}}\]
This is methanol or methyl alcohol.
This is the simplest organic alcohol compound.
Methanol on hydrolysis does not give acetic acid.
So, B is incorrect.
C. \[{{\rm{C}}_{\rm{2}}}{{\rm{H}}_{\rm{5}}}{\rm{OH}}\]
This is ethanol or ethyl alcohol.
This is the next organic alcohol compound after methanol.
Ethanol on hydrolysis produces ethanal or acetaldehyde.
The reaction occurs as follows:

Image: Hydrolysis of ethanol
This does not produce acetic acid as the product.
So, C is incorrect.
D. \[{{\rm{C}}_{\rm{2}}}{{\rm{H}}_{\rm{5}}}{\rm{N}}{{\rm{H}}_2}\]
This is ethanamine or ethylamine.
It is a simple amine compound.
Ethylamine on diazotization by nitrous acid preceded by hydrolysis will give ethanol.
When this compound is treated with sodium nitrite and hydrochloric acid, the amine group is transformed into ethyl diazonium salt which then on hydrolysis will give ethanol.
The reaction happens as follows:

Image: Reaction of ethyl amine with nitrous acid.
So, this compound on hydrolysis will not give acetic acid.
So, D is incorrect.
So, option A is correct.
Note: Acetylene in the reaction with HgOH and dilute sulfuric acid leads to the formation of vinyl alcohol. Vinyl alcohol tautomerizes to form acetaldehyde. Acetaldehyde on oxidation forms acetic acid.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




