
Find the isoelectric point pI of Lysine:
(A) 5.56
(B) 9.74
(C) 6.25
(D) 0
Answer
233.1k+ views
Hint: (1) By isoelectric point we mean the characteristic pH at which net electric charge of a molecule such as amino acid is zero.
(2) At the isoelectric point, the zwitterions form of the amino acid is dominant.
Complete step-by-step answer: If an amino acid has only one amino acid and only one carboxyl group, then the isoelectric point pI is calculated from the mean of the pKas of the amino acid molecule.
\[pI = \dfrac{{p{K_{a1}} + p{K_{a2}}}}{2}\]
At a pH lower than their pI, the molecule will carry a net positive charge and at a pH higher than their pI, the molecule will carry a net negative charge. At pH equal to pI charge will be zero.
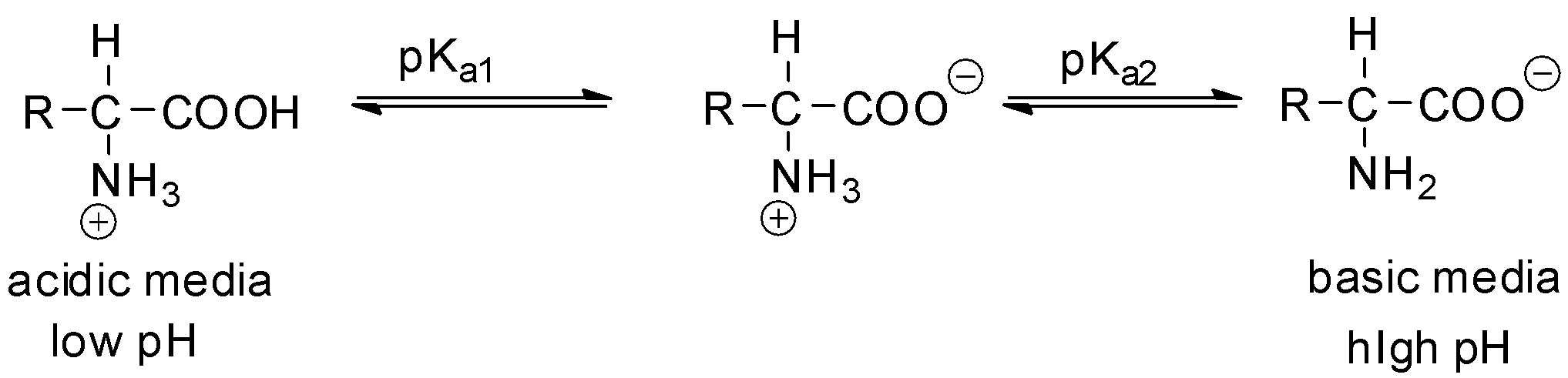
pKa1 and pKa2 are the pKas of the carboxylic acid and the amine respectively.
For amino acids having acidic and basic side chains, a third acid dissociation constant pKa3 is used to describe the ionisable groups in the side chain R.
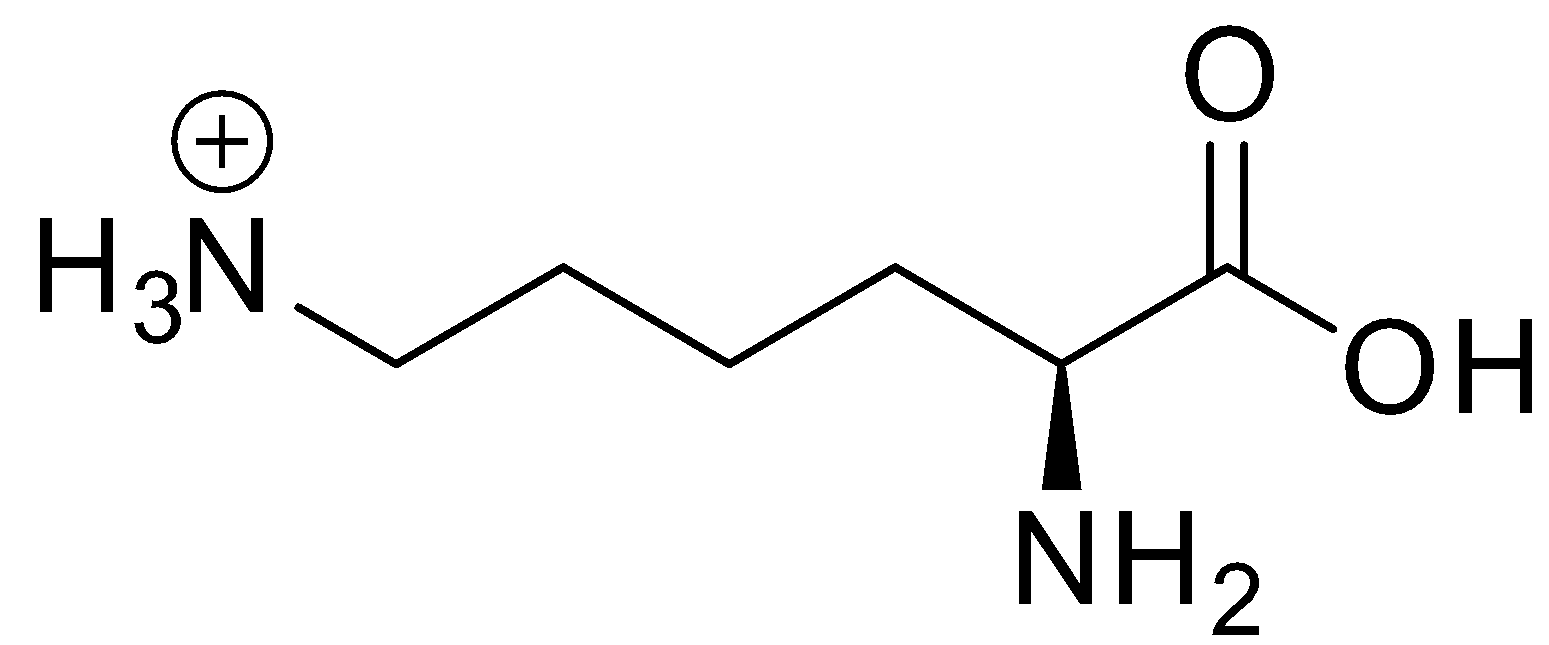
Lysine is a basic amino acid. It is represented by the symbol Lys or K. Its structure is shown below:
Therefore, for lysine, we can write:
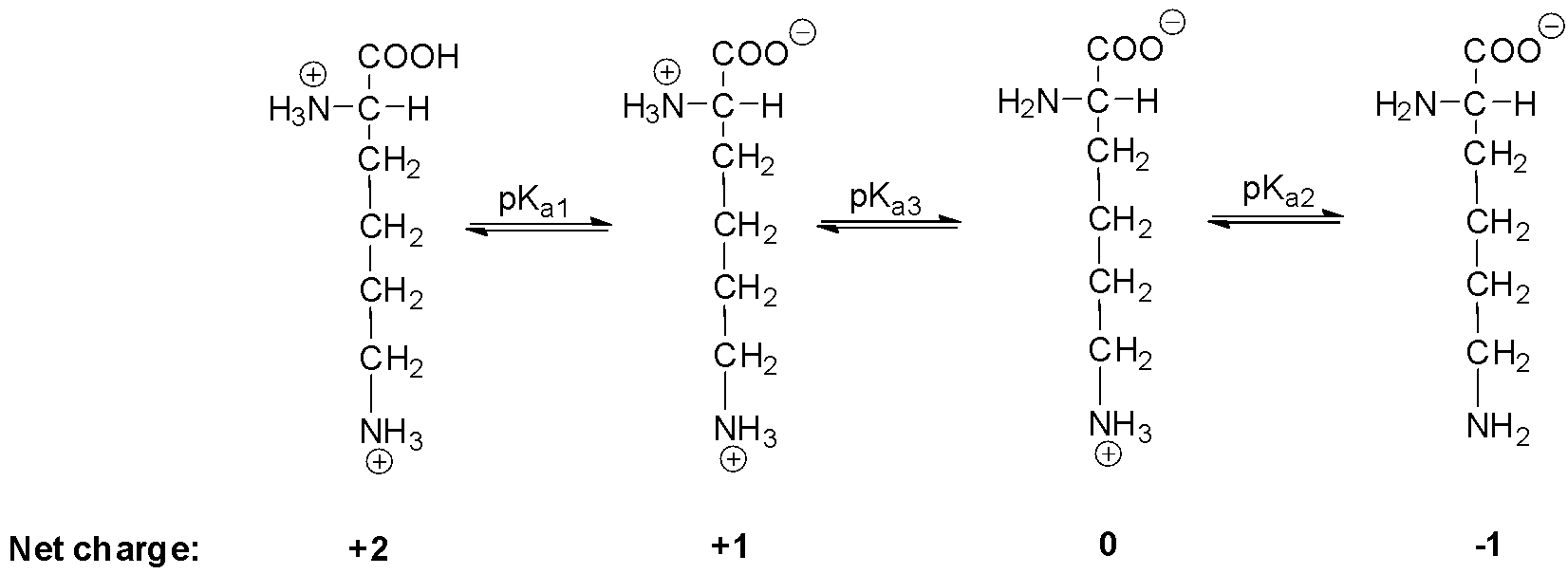
Since the isoelectric point is given by the average of the pKa values that involve the zwitterion, so we can write the formula for lysine as:
\[pI = \dfrac{{p{K_{a3}} + p{K_{a2}}}}{2}\]
Now, for lysine, the pKa1 is equal to 2.18, pKa2 is equal to 8.95 and pKa3 is equal to 10.53.
Therefore, by replacing the equation by these values we will get, the isoelectric point for lysine is:
\[\] \[pI = \dfrac{{10.53 + 8.95}}{2} = 9.74\]
So, the correct option is (B).
Note: The isoelectric point is given by the average of the pKa values that involve the zwitterions, not just by the pKa values that describe the carboxylic acid group and the amine group. For neutral amino acids, the side chains are neutral and the isoelectric point is given simply by the average of the pKa values of carboxylic acid and amine. For acidic amino acids, the isoelectric point will be at lower pH as the acidic side chain will introduce an extra negative charge and for basic amino acids, the isoelectric point will be at higher pH as the basic side chain will introduce an extra positive charge.
(2) At the isoelectric point, the zwitterions form of the amino acid is dominant.
Complete step-by-step answer: If an amino acid has only one amino acid and only one carboxyl group, then the isoelectric point pI is calculated from the mean of the pKas of the amino acid molecule.
\[pI = \dfrac{{p{K_{a1}} + p{K_{a2}}}}{2}\]
At a pH lower than their pI, the molecule will carry a net positive charge and at a pH higher than their pI, the molecule will carry a net negative charge. At pH equal to pI charge will be zero.

pKa1 and pKa2 are the pKas of the carboxylic acid and the amine respectively.
For amino acids having acidic and basic side chains, a third acid dissociation constant pKa3 is used to describe the ionisable groups in the side chain R.
Lysine is a basic amino acid. It is represented by the symbol Lys or K. Its structure is shown below:

Therefore, for lysine, we can write:

Since the isoelectric point is given by the average of the pKa values that involve the zwitterion, so we can write the formula for lysine as:
\[pI = \dfrac{{p{K_{a3}} + p{K_{a2}}}}{2}\]
Now, for lysine, the pKa1 is equal to 2.18, pKa2 is equal to 8.95 and pKa3 is equal to 10.53.
Therefore, by replacing the equation by these values we will get, the isoelectric point for lysine is:
\[\] \[pI = \dfrac{{10.53 + 8.95}}{2} = 9.74\]
So, the correct option is (B).
Note: The isoelectric point is given by the average of the pKa values that involve the zwitterions, not just by the pKa values that describe the carboxylic acid group and the amine group. For neutral amino acids, the side chains are neutral and the isoelectric point is given simply by the average of the pKa values of carboxylic acid and amine. For acidic amino acids, the isoelectric point will be at lower pH as the acidic side chain will introduce an extra negative charge and for basic amino acids, the isoelectric point will be at higher pH as the basic side chain will introduce an extra positive charge.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




