
Which of the following is optically active
A. Ethylene glycol
B. Oxalic acid
C. Glycerol
D. Tartaric acid
Answer
233.1k+ views
Hint: The compound which rotates the plane polarised light in a clockwise or in an anticlockwise direction is called an optically active compound. For a compound to be optically active it must contain at least one chiral carbon.
Complete answer:The optical activity of any compound can be determined by passing the plane-polarised light through the compound, if the compound rotates the plane-polarised light in a clockwise direction it is called dextrorotatory and if the compound rotates the plane-polarised light in an anti-clockwise direction it is called levorotatory. For a compound to be optically active compound must contain a chiral carbon or a chiral center, a chiral carbon is a carbon that is attached to four different substituents and a chiral center is a stereogenic center containing four different substituents.
The structures of the given compounds are –
1. Ethylene glycol
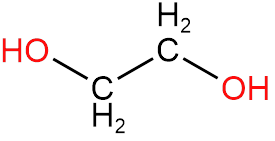
In ethylene glycol, we can see that there is no chiral carbon because with both the carbons two hydrogens are attached.
2. Oxalic Acid
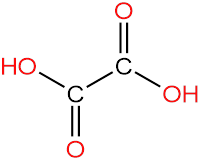
In oxalic acid, we can see that there is no chiral carbon because oxygen is attached with a double bond with both carbons.
3. Glycerol
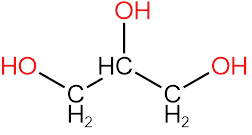
In glycerol as well there is no chiral carbon because CH2OH is a common substituent attached to the center carbon.
4. Tartaric Acid
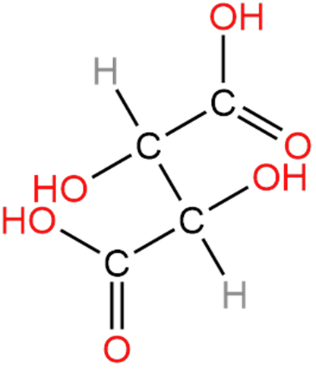
In tartaric acid, we can see that there are two chiral carbons so tartaric acid is optically active.
Thus, Option (D) is correct
Note: Tartaric acid have three optical isomerism. D- tartaric acid, l- tartaric acid and meso- tartaric acid. Among these three isomers D- tartaric acid and l- tartaric acid are optically active and meso- tartaric acid is optically inactive.
Complete answer:The optical activity of any compound can be determined by passing the plane-polarised light through the compound, if the compound rotates the plane-polarised light in a clockwise direction it is called dextrorotatory and if the compound rotates the plane-polarised light in an anti-clockwise direction it is called levorotatory. For a compound to be optically active compound must contain a chiral carbon or a chiral center, a chiral carbon is a carbon that is attached to four different substituents and a chiral center is a stereogenic center containing four different substituents.
The structures of the given compounds are –
1. Ethylene glycol

In ethylene glycol, we can see that there is no chiral carbon because with both the carbons two hydrogens are attached.
2. Oxalic Acid

In oxalic acid, we can see that there is no chiral carbon because oxygen is attached with a double bond with both carbons.
3. Glycerol

In glycerol as well there is no chiral carbon because CH2OH is a common substituent attached to the center carbon.
4. Tartaric Acid

In tartaric acid, we can see that there are two chiral carbons so tartaric acid is optically active.
Thus, Option (D) is correct
Note: Tartaric acid have three optical isomerism. D- tartaric acid, l- tartaric acid and meso- tartaric acid. Among these three isomers D- tartaric acid and l- tartaric acid are optically active and meso- tartaric acid is optically inactive.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




