
Which of the following is not used as an explosive?
(A) Trinitrotoluene
(B) Trinitrobenzene
(C) Picric acid
(D) Nitrobenzene
Answer
233.1k+ views
Hint: An explosive substance is a solid, liquid, or mixture that, by chemical reaction, is capable of releasing gas at a temperature, pressure, and speed high enough to harm the environment. An organic compound is said to be explosive if its explosion leads to highly stable compounds with the release of energy.
Complete Step by Step Solution:
Trinitrotoluene (TNT) is a solid organic compound that is mostly used as an explosive. It is a pale yellow colour. It is not very sensitive to shock and requires a detonator to explode. It is the most popular chemical explosive and is widely employed in weapons and demolitions for these reasons.
Trinitrobenzene is a strong explosive when it is dry. Harmful nitrogen oxides are produced when trinitrobenzene is exposed to heat or fire. Though more explosive than TNT, trinitrobenzene is too costly. Military and commercial mining operations utilise it mainly as a highly explosive compound.
Picric acid is also mainly used as an explosive. Dried-out picric acid should be handled with caution because it could explode if exposed to heat, flame, friction, or shock. When combined with bases, metals, ammonia, concrete, and other oxidising substances, picric acid can react violently and produce salts that are unstable.
However, nitrobenzene is not explosive. It is a stable compound up to about${{450}^{o}}C$.
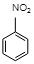
Correct option: (D) Nitrobenzene.
Note: Di or tri nitro compounds are explosive, but mono nitro compounds are not. Nitrobenzene is used in lubricants used in machinery and motors. Nitrobenzene is a very minor component of the pigments, medicines, insecticides, and rubber product manufacturing processes.
Complete Step by Step Solution:
Trinitrotoluene (TNT) is a solid organic compound that is mostly used as an explosive. It is a pale yellow colour. It is not very sensitive to shock and requires a detonator to explode. It is the most popular chemical explosive and is widely employed in weapons and demolitions for these reasons.
Trinitrobenzene is a strong explosive when it is dry. Harmful nitrogen oxides are produced when trinitrobenzene is exposed to heat or fire. Though more explosive than TNT, trinitrobenzene is too costly. Military and commercial mining operations utilise it mainly as a highly explosive compound.
Picric acid is also mainly used as an explosive. Dried-out picric acid should be handled with caution because it could explode if exposed to heat, flame, friction, or shock. When combined with bases, metals, ammonia, concrete, and other oxidising substances, picric acid can react violently and produce salts that are unstable.
However, nitrobenzene is not explosive. It is a stable compound up to about${{450}^{o}}C$.

Correct option: (D) Nitrobenzene.
Note: Di or tri nitro compounds are explosive, but mono nitro compounds are not. Nitrobenzene is used in lubricants used in machinery and motors. Nitrobenzene is a very minor component of the pigments, medicines, insecticides, and rubber product manufacturing processes.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




