
Which of the following is not correct regarding terylene?
A. Condensation Polymer
B. Synthetic fibre
C. Step growth polymer
D. Thermosetting plastic
Answer
233.1k+ views
Hint: The polymer is large molecules which are also called macromolecules. They are made from the addition or condensation of small monomers which may be monofunctional or bifunctional. Monofunctional monomers give us additional polymers while bifunctional monomers give us condensation polymers.
Complete Step by Step Solution:
Terylene is a form of condensation polymer arised from the condensation of ethylene glycol and terephthalic acid. The condensation has an outcome in the form of loss of water molecules. The condensation is written as:
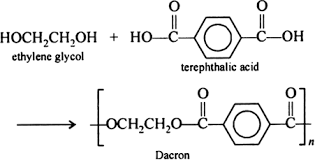
Terylene is synthesised by the condensation of an alcohol i.e. ethylene glycol and an acid i.e. terephthalic acid. The reaction between alcohol and acid results in the development of an ester bond. As the monomers of ethylene glycol and terephthalic acid are associated by ester linkages and the ester is used for preparing clothes. So we call terylene a polyester fibre.
Terylene is a fibre , and not a thermosetting plastic because when we heat them they melt and do not show plastic property.
Terylene is made by the condensation of repeating units of bifunctional monomers to form long-chain polymers. In this whole process, the monomers merged to form dimmers then trimers then oligomers, and finally polymers. So terylene is a step-growth polymer.
Thus, Option (D) is correct.
Note: Terylene is not a chain growth polymer as they synthesised from the linkage of monomers with double and triple bonds. They are also called addition polymers. As it is synthesised by the condensation of two monomer units, so terylene is not a chain growth polymer.
Complete Step by Step Solution:
Terylene is a form of condensation polymer arised from the condensation of ethylene glycol and terephthalic acid. The condensation has an outcome in the form of loss of water molecules. The condensation is written as:

Terylene is synthesised by the condensation of an alcohol i.e. ethylene glycol and an acid i.e. terephthalic acid. The reaction between alcohol and acid results in the development of an ester bond. As the monomers of ethylene glycol and terephthalic acid are associated by ester linkages and the ester is used for preparing clothes. So we call terylene a polyester fibre.
Terylene is a fibre , and not a thermosetting plastic because when we heat them they melt and do not show plastic property.
Terylene is made by the condensation of repeating units of bifunctional monomers to form long-chain polymers. In this whole process, the monomers merged to form dimmers then trimers then oligomers, and finally polymers. So terylene is a step-growth polymer.
Thus, Option (D) is correct.
Note: Terylene is not a chain growth polymer as they synthesised from the linkage of monomers with double and triple bonds. They are also called addition polymers. As it is synthesised by the condensation of two monomer units, so terylene is not a chain growth polymer.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




