
Which of the following is a non-linear molecule?
(A) $C{{O}_{3}}^{2-}$
(B) $C{{O}_{2}}$
(C) $C{{S}_{2}}$
(D) $BeC{{l}_{2}}$
Answer
233.1k+ views
Hint: Firstly, the hybridization of each of the molecules in the options is determined. The hybridization is calculated by calculating the number of bond pairs and lone pairs in a molecule. Hybridization is the idea of combining the two atomic orbitals to form a new type of hybridised orbital. This mixing creates hybrid orbitals with entirely different energies and shapes.
Formula Used: Hybridization is calculated as:
Hybridization=$12(V+M-C+A)$
Where $V$= number of valence electrons of the central atom
$M$= number of monovalent atoms
$C$= positive charge
$A$= negative charge
Complete Step by Step Answer:
(A) $C{{O}_{3}}^{2-}$
Hybridization=$\frac{1}{2}(4+0-0+2)=3$
The hybridization of $C{{O}_{3}}^{2-}$is$s{{p}^{2}}$. Hence, $C{{O}_{3}}^{2-}$is non-linear in shape.
(B) $C{{O}_{2}}$
Hybridization=$\frac{1}{2}(4+0-0+0)=2$
The hybridization of $C{{O}_{2}}$is$sp$. Hence, $C{{O}_{2}}$is linear in shape.
(C) $C{{S}_{2}}$
Hybridization=$\frac{1}{2}(4+0-0+0)=2$
The hybridization of $C{{S}_{2}}$is$sp$. Hence, $C{{S}_{2}}$is linear in shape.
(D) $BeC{{l}_{2}}$
Hybridization=$\frac{1}{2}(2+2-0+0)=2$
The hybridization of $BeC{{l}_{2}}$is$sp$. Hence, $BeC{{l}_{2}}$is linear in shape.
All the other molecules in the options except $C{{O}_{3}}^{2-}$are $sp$ hybridised and thus, linear in shape.
The structures of all the molecules are shown below:
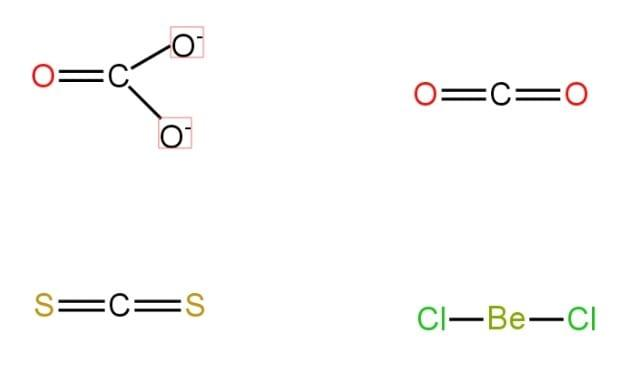
Thus, $C{{O}_{3}}^{2-}$is a non-linear molecule.
Correct Option: (A) $C{{O}_{3}}^{2-}$.
Note: The central atom in the geometry described by linear molecular geometry is bonded to two other atoms (or ligands) at a bond angle of${{180}^{o}}$. All the linear molecules must be $sp$ hybridized. Non-linear molecules have bond angles other than ${{180}^{o}}$, depending upon the shape of the molecule. The shape of the molecule may be bent, trigonal planar, tetrahedral, trigonal bipyramidal, etc.
Formula Used: Hybridization is calculated as:
Hybridization=$12(V+M-C+A)$
Where $V$= number of valence electrons of the central atom
$M$= number of monovalent atoms
$C$= positive charge
$A$= negative charge
Complete Step by Step Answer:
(A) $C{{O}_{3}}^{2-}$
Hybridization=$\frac{1}{2}(4+0-0+2)=3$
The hybridization of $C{{O}_{3}}^{2-}$is$s{{p}^{2}}$. Hence, $C{{O}_{3}}^{2-}$is non-linear in shape.
(B) $C{{O}_{2}}$
Hybridization=$\frac{1}{2}(4+0-0+0)=2$
The hybridization of $C{{O}_{2}}$is$sp$. Hence, $C{{O}_{2}}$is linear in shape.
(C) $C{{S}_{2}}$
Hybridization=$\frac{1}{2}(4+0-0+0)=2$
The hybridization of $C{{S}_{2}}$is$sp$. Hence, $C{{S}_{2}}$is linear in shape.
(D) $BeC{{l}_{2}}$
Hybridization=$\frac{1}{2}(2+2-0+0)=2$
The hybridization of $BeC{{l}_{2}}$is$sp$. Hence, $BeC{{l}_{2}}$is linear in shape.
All the other molecules in the options except $C{{O}_{3}}^{2-}$are $sp$ hybridised and thus, linear in shape.
The structures of all the molecules are shown below:

Thus, $C{{O}_{3}}^{2-}$is a non-linear molecule.
Correct Option: (A) $C{{O}_{3}}^{2-}$.
Note: The central atom in the geometry described by linear molecular geometry is bonded to two other atoms (or ligands) at a bond angle of${{180}^{o}}$. All the linear molecules must be $sp$ hybridized. Non-linear molecules have bond angles other than ${{180}^{o}}$, depending upon the shape of the molecule. The shape of the molecule may be bent, trigonal planar, tetrahedral, trigonal bipyramidal, etc.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




