
Which of the following are examples of nucleophilic addition reaction in the case of ethyne
A. Addition of water
B. Addition of HCN
C. Addition of \[AsC{l_3}\]
D. All of these
Answer
232.8k+ views
Hint: A nucleophilic addition reaction in which a nucleophile forms a sigma bond with an electron-deficient species. In acetylene, the carbon-carbon pi bond is broken, constructing an intermediate that later forms the product.
Complete Step by Step Solution:
Let us know about acetylene.
It is the chemical compound with the formula.
It is a hydrocarbon and the first alkyne. This colourless gas is mostly utilised as a fuel and a chemical building block.
It is also known as ethyne.
In this question, we have to find out that one is an example of a nucleophilic addition reaction in the case of acetylene.
In nucleophilic addition, the substrate is originally attacked by a nucleophile, and then one or more very simple molecules get added across multiple bonds.
A. Addition of water
Alkynes are not hydrated readily in aqueous acid so a mercuric salt is used as a catalyst.
The addition of one molecule of water to ethyne probably provides vinyl alcohol which is unstable and fragrances to form ethanal.
The reaction happens as follows:
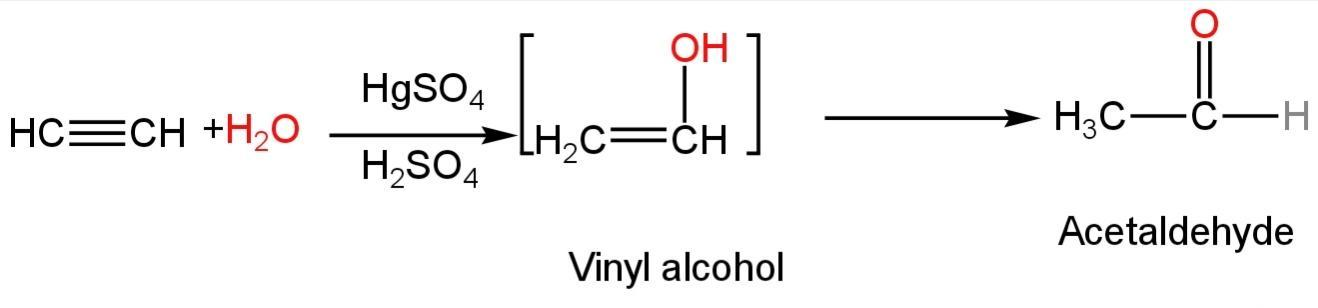
Image: Reaction of water with acetylene
In this reaction, the hydroxyl ion i.e., the nucleophile attacks the double bond first.
So, it is a nucleophilic reaction.
So, A is correct.
B. Addition of HCN
Addition with HCN in the presence of\[Ba{\left( {CN} \right)_2}\] forms vinyl cyanide.
The reaction happens as follows:-
Image:
In this reaction, the cyanide ion i.e., the nucleophile attacks the double bond first.
So, it is a nucleophilic reaction.
So, B is correct.
C. Addition of \[AsC{l_3}\]
The addition of \[AsC{l_3}\]to acetylene gives lewisite.
\[AsC{l_3} + {C_2}{H_2} \to ClCHCHAsC{l_2}\]
The product created is named Lewisite.
It is an organoarsenic compound i.e., consisting of organic component ethene and arsenic component.
In this reaction the chloride ion attacks first which is a nucleophile.
So, it is a nucleophilic reaction.
So, C is correct.
As all the given options are nucleophilic. So, D is correct.
So, option D is correct.
Note: Lewisite is an organoarsenic compound. It was manufactured for its usage as a chemical weapon, acting as a vesicant (blister agent) and lung irritant. This substance is colourless and odourless in its purest feature, impure species of lewisite are a yellow, brown, violet-black, green, or amber oily liquid with a unique odour.
Complete Step by Step Solution:
Let us know about acetylene.
It is the chemical compound with the formula.
It is a hydrocarbon and the first alkyne. This colourless gas is mostly utilised as a fuel and a chemical building block.
It is also known as ethyne.
In this question, we have to find out that one is an example of a nucleophilic addition reaction in the case of acetylene.
In nucleophilic addition, the substrate is originally attacked by a nucleophile, and then one or more very simple molecules get added across multiple bonds.
A. Addition of water
Alkynes are not hydrated readily in aqueous acid so a mercuric salt is used as a catalyst.
The addition of one molecule of water to ethyne probably provides vinyl alcohol which is unstable and fragrances to form ethanal.
The reaction happens as follows:

Image: Reaction of water with acetylene
In this reaction, the hydroxyl ion i.e., the nucleophile attacks the double bond first.
So, it is a nucleophilic reaction.
So, A is correct.
B. Addition of HCN
Addition with HCN in the presence of\[Ba{\left( {CN} \right)_2}\] forms vinyl cyanide.
The reaction happens as follows:-
Image:
In this reaction, the cyanide ion i.e., the nucleophile attacks the double bond first.
So, it is a nucleophilic reaction.
So, B is correct.
C. Addition of \[AsC{l_3}\]
The addition of \[AsC{l_3}\]to acetylene gives lewisite.
\[AsC{l_3} + {C_2}{H_2} \to ClCHCHAsC{l_2}\]
The product created is named Lewisite.
It is an organoarsenic compound i.e., consisting of organic component ethene and arsenic component.
In this reaction the chloride ion attacks first which is a nucleophile.
So, it is a nucleophilic reaction.
So, C is correct.
As all the given options are nucleophilic. So, D is correct.
So, option D is correct.
Note: Lewisite is an organoarsenic compound. It was manufactured for its usage as a chemical weapon, acting as a vesicant (blister agent) and lung irritant. This substance is colourless and odourless in its purest feature, impure species of lewisite are a yellow, brown, violet-black, green, or amber oily liquid with a unique odour.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




