
Structural formula for lewisite is
A.
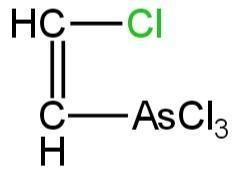
B.
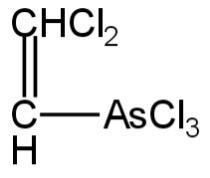
C.
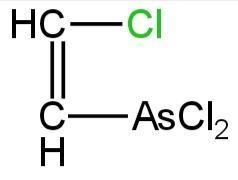
D. None of these
Answer
233.4k+ views
Hint: A molecule of AsCl3 gets added to acetylene giving lewisite. It is an organoarsenic compound i.e., including organic units, ethene, and arsenic. It was employed by many countries for its usage as a chemical weapon, behaving as a blister agent and lung irritant.
Complete Step by Step Solution:
Lewisite is a compound that is readied by the addition of a molecule of arsenic trichloride to acetylene in the presence of a desirable catalyst.
This catalyst mainly is aluminium chloride.
\[AsC{l_3} + {C_2}{H_2} \to ClCHCHAsC{l_2}\]
Lewisite undergoes hydrolysis in water to form hydrochloric acid and chlorovinyl arsenious oxide which is a less strong blister agent.
\[{\rm{ClCHCHAsC}}{{\rm{l}}_{\rm{2}}}{\rm{ + 2}}{{\rm{H}}_{\rm{2}}}{\rm{O}} \to {\rm{ClCHCHAs}}{\left( {{\rm{OH}}} \right)_{\rm{2}}}{\rm{ + 2 HCl}}\]
This reaction is quickened by alkaline solutions and forms acetylene and trisodium arsenate.
Lewisite reacts with metals to yield hydrogen gas. It is combustible, but difficult to ignite.
It is an arsenic-based compound that was formulated to be a powerful chemical to be used in the war.
Susceptibility to lewisite commonly influences the eyes, skin, and respiratory tract which happen nearly immediately after the following contact.
As a chemical combat agent, it can be circulated as a liquid, aerosol, or vapour and can be utilised to provoke fatalities and for area denial.
So, the chemical formula of lewisite is\[ClCHCHAsC{l_2}\]. So, its structure is C.
So, option C is correct.
Note: Lewisite was made in 1904 by Julius Arthur Nieuwland during research for his Ph.D. In his thesis, he depicted a reaction between acetylene and arsenic trichloride, which guided the formation of lewisite. Revelation to the ensuing compound made Nieuwland so sick he was in the hospital for many days.
Complete Step by Step Solution:
Lewisite is a compound that is readied by the addition of a molecule of arsenic trichloride to acetylene in the presence of a desirable catalyst.
This catalyst mainly is aluminium chloride.
\[AsC{l_3} + {C_2}{H_2} \to ClCHCHAsC{l_2}\]
Lewisite undergoes hydrolysis in water to form hydrochloric acid and chlorovinyl arsenious oxide which is a less strong blister agent.
\[{\rm{ClCHCHAsC}}{{\rm{l}}_{\rm{2}}}{\rm{ + 2}}{{\rm{H}}_{\rm{2}}}{\rm{O}} \to {\rm{ClCHCHAs}}{\left( {{\rm{OH}}} \right)_{\rm{2}}}{\rm{ + 2 HCl}}\]
This reaction is quickened by alkaline solutions and forms acetylene and trisodium arsenate.
Lewisite reacts with metals to yield hydrogen gas. It is combustible, but difficult to ignite.
It is an arsenic-based compound that was formulated to be a powerful chemical to be used in the war.
Susceptibility to lewisite commonly influences the eyes, skin, and respiratory tract which happen nearly immediately after the following contact.
As a chemical combat agent, it can be circulated as a liquid, aerosol, or vapour and can be utilised to provoke fatalities and for area denial.
So, the chemical formula of lewisite is\[ClCHCHAsC{l_2}\]. So, its structure is C.
So, option C is correct.
Note: Lewisite was made in 1904 by Julius Arthur Nieuwland during research for his Ph.D. In his thesis, he depicted a reaction between acetylene and arsenic trichloride, which guided the formation of lewisite. Revelation to the ensuing compound made Nieuwland so sick he was in the hospital for many days.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




