
Plot a graph showing the variation of undecayed nuclei $N$ versus time $t$. From the graph, find out how one can determine the half-life and average life of the radioactive nuclei.
Answer
233.1k+ views
Hint: Radioactive decay is the process of slow disintegration of radioactive elements by releasing their energy in the form of radiation. The radioactive materials have unstable nuclei and therefore the nuclei breakdowns take place in the form of radiation. The radioactivity is given by the formula:
$A = - \dfrac{{dN}}{{dt}}$
where $A = $ total activity
$N = $ number of particles
$t = $ time
Complete step by step solution:
In radioactive decay, the rate of radioactive decay is proportional to the number of nuclei present. The number of nuclei in radioactive decay degrades and exponentially decreases with time. As per the radioactive decay law, the disintegration equation is given by:
$N = {N_0}{e^{ - \lambda t}}$
Where, $N = $ number of radioactive atoms at any random time.
${N_0} = $ number of radioactive atoms present initially.
$\lambda = $ decay constant
$t = $ time
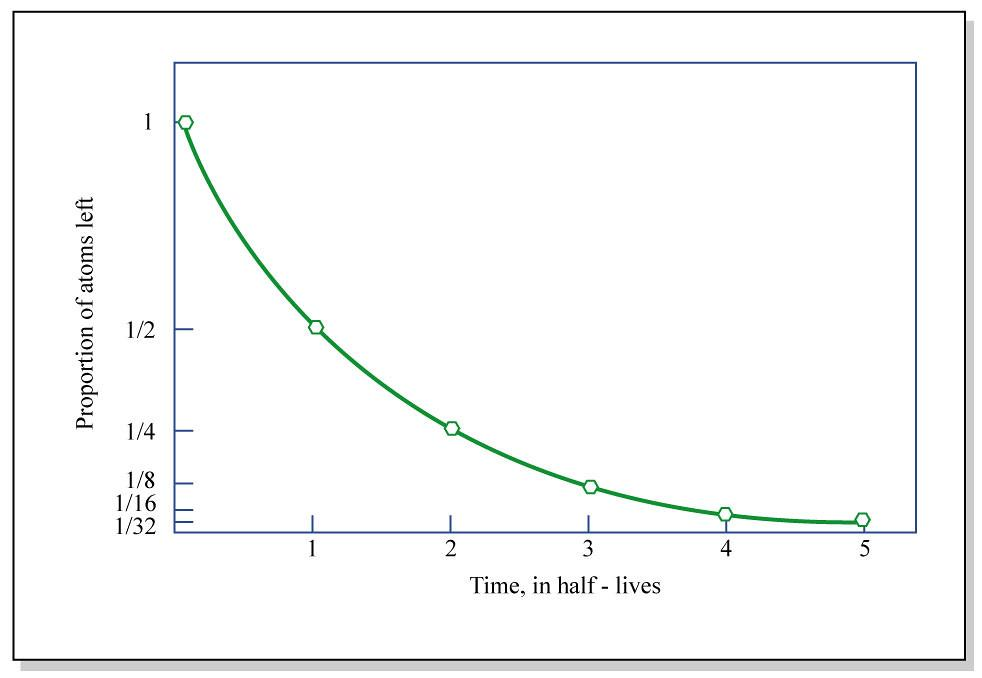
The above plot depicts the variation of the number of nuclei disintegrate with time. $N$ gradually decreases with increase in time and there comes a state where the nuclei are completely depleted at a given time. The rate of disintegration of nuclei is directly proportional to the nuclei available i.e. $ - \dfrac{{dN}}{{dt}} \propto N$
$ \Rightarrow - \dfrac{{dN}}{{dt}} = \lambda N$
The negative sign represents the gradual decrease of the number of nuclei with time.
The half-life of the radioactive element is the time required for its activity to decrease by one-half. So after one half-life, 50% of the initial activity remains and after two half-lives only 25% of the initial activity remains and after three half-lives, only 12.5% is present and so forth.
The half-life of a given nuclei can be derived as follows:
In half-life of a nuclei, the given lifespan of the nuclei is completed by 50% and it is denoted as ${t_{1/2}}$.
Therefore, the half-life of an atomic nuclei can be given by substituting $t = {t_{1/2}}$and $N = \dfrac{{{N_0}}}{2}$ in $N = {N_0}{e^{ - \lambda {t_{1/2}}}}$
Which gives, $\dfrac{{{N_0}}}{2} = {N_0}{e^{ - \lambda {t_{1/2}}}}$ $ \Rightarrow \dfrac{1}{2} = {e^{ - \lambda {t_{1/2}}}}$
Taking log on both side, we get ${\log _e}(2) = \lambda {t_{1/2}}$
$ \Rightarrow 0.693 = \lambda {t_{1/2}}$ $ \Rightarrow {t_{1/2}} = \dfrac{{0.693}}{\lambda }$
The average life of a nuclei atom is the amount of time required for the nuclei to decay to $36.8\% $ of the total amount. Therefore $N = 0.368{N_0}$ and $t$ changes as
$0.368{N_0} = {N_0}{e^{ - \lambda {t_{avg}}}}$
$ \Rightarrow 0.368 = {e^{ - \lambda {t_{avg}}}}$
Taking log on both sides,
${\log _e}(0.368) = - \lambda {t_{avg}}$
Which gives, $0.434 = \lambda {t_{avg}}$
$ \Rightarrow {t_{avg}} = \dfrac{{0.434}}{\lambda }$
Note: The unstable nuclei in radioactive samples do not decay simultaneously. Instead the decay of a given nucleus is an entirely random event. The rate of nuclei disintegration is insulated by surrounding electrons and hence the disintegration of the radioactive element is independent of temperature, pressure and doesn't follow any mass law or any other law that explains the physical and chemical change.
$A = - \dfrac{{dN}}{{dt}}$
where $A = $ total activity
$N = $ number of particles
$t = $ time
Complete step by step solution:
In radioactive decay, the rate of radioactive decay is proportional to the number of nuclei present. The number of nuclei in radioactive decay degrades and exponentially decreases with time. As per the radioactive decay law, the disintegration equation is given by:
$N = {N_0}{e^{ - \lambda t}}$
Where, $N = $ number of radioactive atoms at any random time.
${N_0} = $ number of radioactive atoms present initially.
$\lambda = $ decay constant
$t = $ time

The above plot depicts the variation of the number of nuclei disintegrate with time. $N$ gradually decreases with increase in time and there comes a state where the nuclei are completely depleted at a given time. The rate of disintegration of nuclei is directly proportional to the nuclei available i.e. $ - \dfrac{{dN}}{{dt}} \propto N$
$ \Rightarrow - \dfrac{{dN}}{{dt}} = \lambda N$
The negative sign represents the gradual decrease of the number of nuclei with time.
The half-life of the radioactive element is the time required for its activity to decrease by one-half. So after one half-life, 50% of the initial activity remains and after two half-lives only 25% of the initial activity remains and after three half-lives, only 12.5% is present and so forth.
The half-life of a given nuclei can be derived as follows:
In half-life of a nuclei, the given lifespan of the nuclei is completed by 50% and it is denoted as ${t_{1/2}}$.
Therefore, the half-life of an atomic nuclei can be given by substituting $t = {t_{1/2}}$and $N = \dfrac{{{N_0}}}{2}$ in $N = {N_0}{e^{ - \lambda {t_{1/2}}}}$
Which gives, $\dfrac{{{N_0}}}{2} = {N_0}{e^{ - \lambda {t_{1/2}}}}$ $ \Rightarrow \dfrac{1}{2} = {e^{ - \lambda {t_{1/2}}}}$
Taking log on both side, we get ${\log _e}(2) = \lambda {t_{1/2}}$
$ \Rightarrow 0.693 = \lambda {t_{1/2}}$ $ \Rightarrow {t_{1/2}} = \dfrac{{0.693}}{\lambda }$
The average life of a nuclei atom is the amount of time required for the nuclei to decay to $36.8\% $ of the total amount. Therefore $N = 0.368{N_0}$ and $t$ changes as
$0.368{N_0} = {N_0}{e^{ - \lambda {t_{avg}}}}$
$ \Rightarrow 0.368 = {e^{ - \lambda {t_{avg}}}}$
Taking log on both sides,
${\log _e}(0.368) = - \lambda {t_{avg}}$
Which gives, $0.434 = \lambda {t_{avg}}$
$ \Rightarrow {t_{avg}} = \dfrac{{0.434}}{\lambda }$
Note: The unstable nuclei in radioactive samples do not decay simultaneously. Instead the decay of a given nucleus is an entirely random event. The rate of nuclei disintegration is insulated by surrounding electrons and hence the disintegration of the radioactive element is independent of temperature, pressure and doesn't follow any mass law or any other law that explains the physical and chemical change.
Recently Updated Pages
JEE Main 2026 Session 2 Registration Open, Exam Dates, Syllabus & Eligibility

JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

Trending doubts
JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Why does capacitor block DC and allow AC class 12 physics JEE_Main

Understanding Average and RMS Value in Electrical Circuits

Understanding Collisions: Types and Examples for Students

Ideal and Non-Ideal Solutions Explained for Class 12 Chemistry

Understanding Atomic Structure for Beginners

Other Pages
JEE Advanced Weightage 2025 Chapter-Wise for Physics, Maths and Chemistry

CBSE Class 12 Physics Set 2 (55/2/2) 2025 Question Paper & Solutions

Inductive Effect and Its Role in Acidic Strength

Degree of Dissociation: Meaning, Formula, Calculation & Uses

Units and Measurements Mock Test for JEE Main 2025-26 Preparation

Chemistry Question Papers for JEE Main, NEET & Boards (PDFs)




