




Introduction to Isomerism in Organic Compounds
The term ‘isomerism’ was given by Berzelius, and it represents the existence of two or more compounds with the same molecular formula but different structure and properties (physical, chemical or both). Compounds exhibiting this isomerism are called isomers. The difference in properties of two isomers is due to the difference in (bond connectivity or spatial arrangement) the arrangement of atoms within their molecules. Isomerism is broadly classified into two types viz., constitutional isomerism and stereoisomerism.
Types of Isomerism
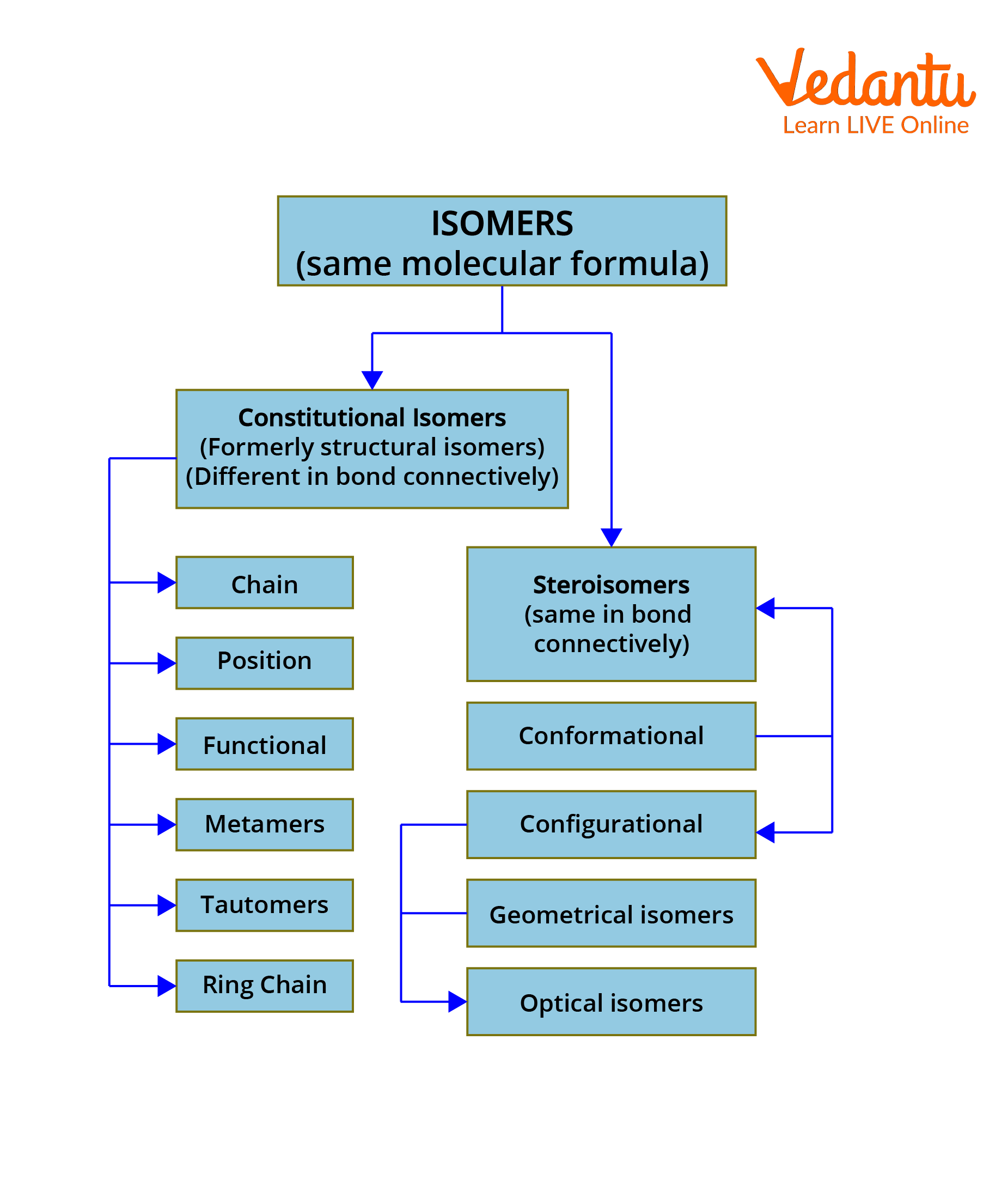
Types of Isomers
Isomers are generally classified into structural and stereoisomers. The structural or constitutional isomers are further divided into chain, position, functional, metamers, tautomers and ring chain isomerism. The stereoisomers are divided into conformational and configurational isomers. Configurational isomers are further subdivided into geometrical isomers and optical isomers.
Constitutional Isomers (Formerly Structural Isomers)
This type of isomers have the same molecular formula but differ in their bonding sequence. Example: Isopropyl bromide structure and Propyl bromide. This is further classified into chain and position isomerism, functional isomerism, metamerism and ring chain isomerism.
Chain or Nuclear or Skeletal Isomerism:
The chain or skeletal isomers differ in the way in which the carbon atoms are bonded to each other in a carbon chain. Or in other words we can say that, the isomers have similar molecular formula but differ in the nature of the carbon skeleton (ie. straight or branched) exhibit chain isomerism.
Examples:
CH3 – CH2 – CH2 – CH2 – CH3
n – pentane

Structure of 2-Methyl Butane/Isopentane

Structure of 2,2-Dimethylpropane/Neopentane
Position Isomerism:
Different compounds belonging to the same homologous series with the same molecular formula and carbon skeleton, but differ in the position of substituent or functional group or an unsaturated linkage are said to exhibit position isomerism. The compounds that exhibit this type of isomerism are commonly referred to as position isomers.
Position Isomerism Example:
(i) Molecular Formula C5H10
CH3 – CH2 – CH2 – CH = CH2
Pent-1-ene and
CH3 – CH2 – CH = CH – CH3
Pent-2-ene
(ii) Molecular Formula C4H9Cl

Structure of 1-Chlorobutane
And

Structure of 2-Chlorobutane
(iii) Molecular Formula C5H10O

Structure of Pentan-2-one
And

Structure of Pentan-3-one
Functional Isomerism:
Functional group isomerism is exhibited in different compounds that have the same molecular formula but different functional groups.
Example:
(i) Molecular formulaC3H6O
CH3 – CH2 –CHO
Propanal - Aldehyde Group

Structure of Propanone
Propanone - Ketone group
(ii) Molecular FormulaC3H6O2
CH4 – CH2– COOH
Propanoic Acid - Acid Group
CH3 – COOCH3
Methyl Acetate - Ester Group
Metamerism:
This type of isomerism is a special kind of structural isomerism arising due to the unequal distribution of carbon atoms on either side of the functional group or different alkyl groups attached to the either side of the same functional group and having the same molecular formula. This isomerism is shown by compounds with functional groups such as ethers, ketones, esters and secondary amines between two alkyl groups.
Example:
(i) Molecular FormulaC4H10O
CH3 – O – C3H7
Methyl Propyl Ether
1-Methoxypropane
C2H5 – O – C2H5
Diethyl Ether
Ethoxyethane

Structure of 2-Methoxypropane/Methyl iso - Propyl Ether
(ii) Molecular FormulaC5H10O

Structure of Diethyl Ketone/ Pentan-3-One

Structure of Methyl Propyl Ketone/Pentan-2-One

Structure of Methyl isopropyl Ketone/3-Methylbutan-2-one
Tautomerism
It is a special type of functional isomerism in which a single compound exists in two readily interchangeable structures that differ markedly in the relative position of at least one atomic nucleus, generally hydrogen. The two different structures are known as tautomers. For example, keto-enol tautomerism, where ketone and and enol are in isomerism.
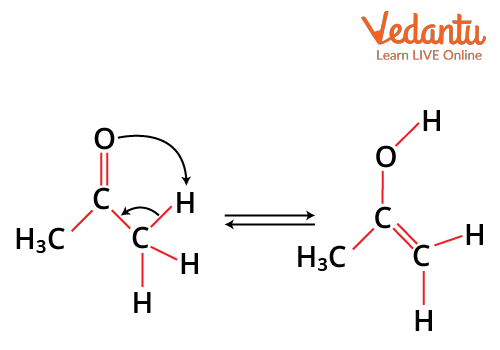
keto-enol tautomerism
Ring Chain Isomerism
In this type of isomerism, compounds have the same molecular formula but differ in terms of bonding of carbon atoms to form open chain and cyclic structures. For example:
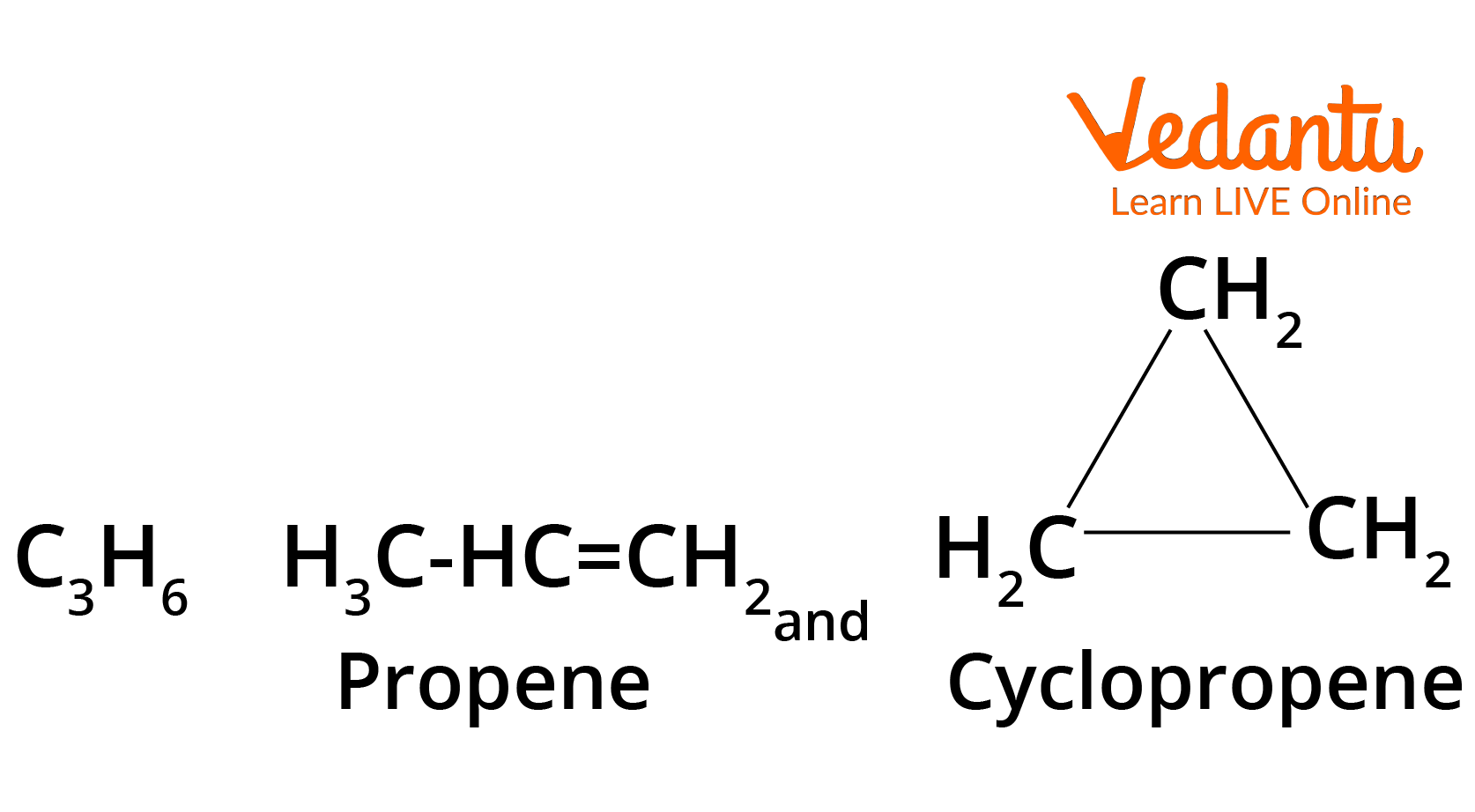
Structure of Propene and Cyclopropane
Stereoisomerism
The type of isomers that have the same bond connectivity but a different arrangement of atoms or group of atoms in space are referred as stereoisomers. This branch of chemistry dealing with the study of three-dimensional nature, that is the spatial arrangement of molecules, is known as stereochemistry. The metabolic activities in all living organisms, synthesis that is occurring naturally and drug synthesis involve various stereoisomers.
Geometrical Isomerism:
Geometrical isomers are the type of stereoisomers that have different arrangements of atoms or a group of atoms around a rigid framework of double bonds (or ring). This type of isomerism generally occurs due to restricted rotation of the double bonds present in the skeleton, or about single bonds in cyclic compounds. In the case of alkenes, the carbon-carbon double bond is sp2 hybridised. The C = C double bond consists of a sigma ($\sigma$) bond and a single pi ($\pi$) bond. The sigma ($\sigma$) bond is formed by the head-on overlap of sp2 hybrid orbitals. The pi ($\pi$) bond is formed by the sidewise overlap of ‘p’ orbitals. The presence of the pi bond locks the molecule in a particular position. Hence, rotation around the carbon – carbon double bond is not possible. This restriction of rotation about C=C double bond is evidently responsible for geometrical isomerism in alkenes.

Geometric Isomers of Butene
These two compounds (e.g. cis-2-butene and trans-2-butene) are termed as geometrical isomers and are distinguished from each other by the terms cis and trans. The cis isomer is the type of isomer in which two similar groups are on the same side of the double bond. The trans isomer is the type in which the two similar groups are on the opposite side of the double bond. Hence, this type of isomerism is often described as cis-trans isomerism.
Conclusion
The molecular formula of an organic compound represents only the number of different atoms present in a molecule. However, the molecular formula does not tell about the arrangement of atoms. A given molecular formula may lead to more than one arrangement of atoms such that there are many compounds that may have the same molecular formula but with different physical and chemical properties. This phenomenon in which the same molecular formula may exhibit some other different structural arrangement is called isomerism. It is divided into structural and stereoisomerism.
FAQs on Chain and Position Isomerism for JEE
1. Distinguish between position isomerism and functional isomerism.
Position isomers are compounds that have the same formula, carbon skeleton and functional groups but have the functional groups located at some other different positions along the carbon skeleton. Example: n-butyl alcohol and sec-butyl alcohol, Isobutyl alcohol and tert-butyl alcohol.
Functional isomers are the compounds that have the same molecular formula but have different functional groups. Examples of this type are Ethyl Alcohol CH3 – CH2 – OH and Dimethyl Ether CH3 – O – CH3, Propanoic Acid CH3 – CH2 – COOH and Methyl Acetate CH3 – COO – CH3.
2. Illustrate the optical isomerism shown by the organic compounds.
Compounds having the same physical and chemical properties but differ only in the rotation of the plane of the polarised light are known as optical isomers. The phenomenon is known as optical isomerism.
Some organic compounds such as glucose have the ability to rotate the plane of the plane polarised light and are said to be optically active compounds. This property of a compound is called optical activity. The optical isomer, which rotates the plane of the plane polarised light to the right or in clockwise direction is said to be dextrorotatory (dexter means right) denoted by the sign (+). The optical isomer, which rotates the plane of polarised light to the left or in anticlockwise direction is said to be levorotatory denoted by the sign (-).


































