




Reduction of Alkynes: A Brief Introduction
Alkene formation is frequently caused by reactions involving alkynes and catalysts. Since alkynes differ from alkenes in that they have two procurable bonds, they are more prone to addition reactions. These catalysts have an effect on the arrangement of substituents on the newly produced alkene molecules in addition to turning them into alkenes.
Depending on which one is used, the catalysts cause anti- or syn-addition of hydrogens. Due to the presence of two bonds, alkynes can easily undergo addition. By using a Lindlar catalyst and by birch reduction, alkynes can be converted to alkenes. The stereochemistry is determined by the reaction's mechanism, which determines whether a cis- or trans-alkene is generated since the partial reduction of an alkyne yields an alkene.
What is Lindlar's Catalyst?
A catalyst is a material that alters or accelerates the rate of a chemical reaction without causing the reaction to change on its own. A catalyst is normally used in lesser proportions in comparison to the reactants or reaction participants.
Lindlar is a heterogeneous catalyst composed of palladium and calcium carbonate that has been treated with several forms of lead. A Lindlar catalyst is used to reduce alkynes to alkenes. A heterogeneous catalyst is one that is always in a different phase or condition than the reactant solution (solid, liquid, or gas solution). The fact that the hydrogenation of an alkyne can be stopped at the alkene stage if the reaction mixture contains a catalyst poison (a chemical that impairs the function of a catalyst) significantly increases the value of catalytic hydrogenation.
Different chemical pollutants, such as lead acetate and lead oxide, are employed to poison palladium. Lindlar catalyst reaction (H2 Lindlar catalyst).
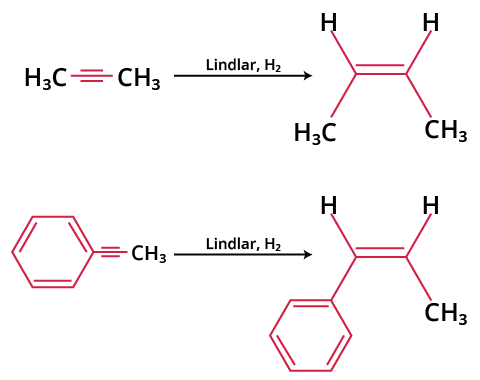
H2 Lindlar Catalyst Reaction
It is usually made by reducing palladium chloride in a calcium carbonate solution and then adding lead acetate. Finally, a large-surface-area catalyst with increased reactivity develops. The use of quinoline precludes further reduction to alkanes if the catalyst is employed to reduce alkynes to alkenes. Quinoline acts as a deactivator to improve the selectivity of the Lindlar’s catalyst reagent. The Lindlar’s catalyst formula is given as 5% Pd-CaCO3 + Pb(OCOCH3)2 + Quinoline.
Lindlar Catalyst Mechanism
The alkyne and hydrogen get adsorbed on the surface of the catalyst in the initial step. The hydrogen atom is moved to the same side (cis) of the alkyne in the hydrogenation reaction, resulting in cis alkenes by adding syn (addition of two substituents on the same side of a double or triple bond, resulting in a decrease in bond number). All hydrogen and alkyne molecules are tightly linked to the catalyst's broad surface, where the hydrogen atoms slowly bind to the triple bond of the alkyne.
As a result, alkyne hydrogenation is now stereoselective and takes place via syn addition. Stereoselectivity causes an uneven mixture of stereoisomers to form. Furthermore, the process is exothermic.
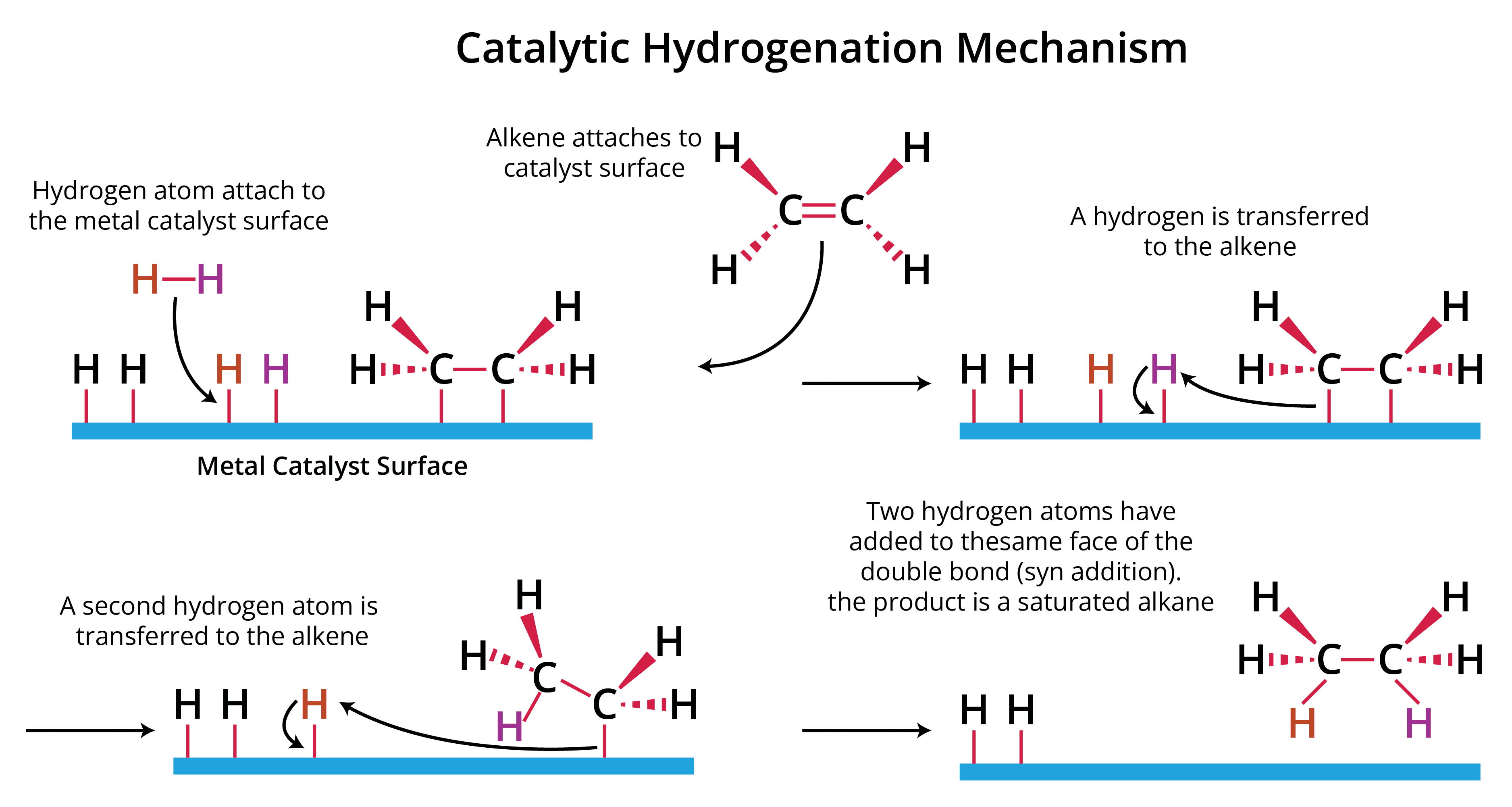
Mechanism of Lindlar Catalyst
Stereochemistry:- As hydrogen is added simultaneously from one side of alkyne, this addition is syn addition reaction and gives cis alkene as a product.
Birch Reduction of Alkynes
When an alkyne pi bond is treated with sodium or lithium metal dissolved in ammonia, the anti-addition of hydrogen occurs. The process of this reaction, which is known as dissolving metal reduction or birch reduction, involves a radical mechanism and yields a trans-alkene as a result. In birch reduction, liquid ammonia with sodium, lithium, or potassium and alcohol is used to reduce the alkynes to alkenes via one electron transfer mechanism. The medium is reducing as solvated electrons are present in ammonia and the reagent is reducing in nature. The process of reduction stops at alkynes and does not reduce alkenes to alkanes.
Birch Reduction Mechanism
A blue solution is formed when alkali metals dissolve in liquid ammonia. Since it has a 3s1 electron in its valence shell, sodium metal is a powerful reducing agent. This electron is easily given up by sodium metal, resulting in Na+. The mechanism begins with a sodium atom giving an electron to the alkyne, resulting in a radical anion and a negatively charged intermediate with an unpaired electron. This intermediate quickly switches between cis and trans conformations, determining the reaction's stereochemistry.
The anion is then protonated by the amine solvent, resulting in the formation of a vinyl radical. The radical is subsequently paired with a second sodium atom, turning it into a vinyl anion. Due to the lower steric crowding, the trans-vinyl anion is produced preferentially. Finally, the trans-vinyl anion protonation produces the trans-alkene product.
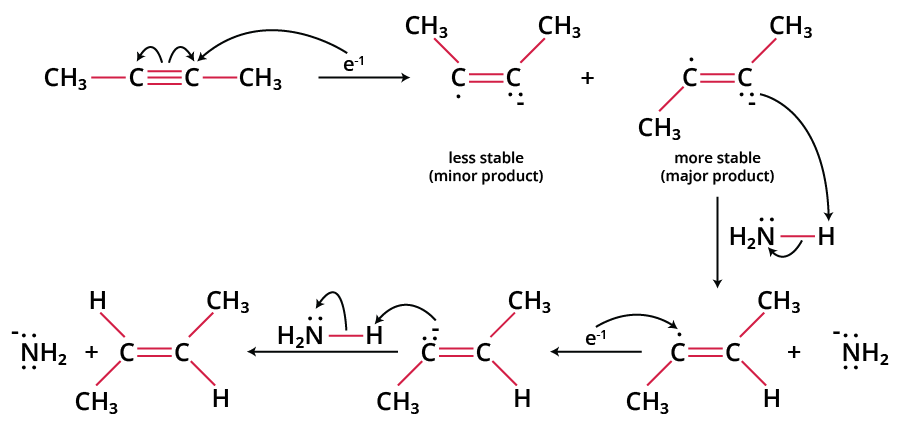
Mechanism of Birch Reduction
Conclusion
Alkene formation is frequently caused by reactions involving alkynes and catalysts. Since alkynes differ from alkenes in that they have two procurable bonds, they are more prone to additions. Lindlar is a heterogeneous catalyst composed of palladium and calcium carbonate that has been treated with several forms of lead. Lindlar catalyst is used to reduce alkynes to alkenes, giving cis alkenes as a product. The Lindlar’s catalyst formula is given as 5% Pd-CaCO3 + Pb(OCOCH3)2 + Quinoline. When an alkyne pi bond is treated with sodium or lithium metal dissolved in ammonia, the anti-addition of hydrogen occurs. The process of this reaction is known as dissolving metal reduction or birch reduction, which involves a radical mechanism and yields a trans-alkene as a result.
FAQs on Birch Reduction and Lindlar Catalyst - JEE Important Topic
1. What is Rosenmund reduction?
The Rosenmund Reduction mechanism explains how acyl chlorides are converted to aldehydes selectively. The Rosenmund reaction is a hydrogenation reaction in which molecular hydrogen combines with acyl chloride in the presence of a palladium-on-barium-sulphate catalyst. Due to its limited surface area, barium sulphate limits the activity of palladium, preventing excessive reduction. If palladium activity is needed further, the reduction is required (for example, in the case of more reactive acyl chlorides). A poison can be added to completely deactivate the palladium catalyst. In the Rosenmund approach, thioquinanthrene and thiourea are common toxins employed to restrict palladium action.
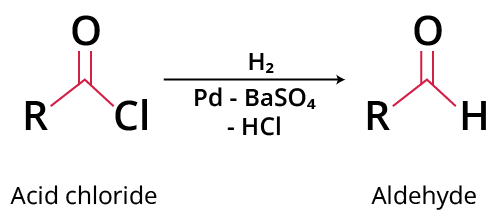
Rosenmund Reduction Reaction
2. Define Clemmensen reduction reaction.
The Clemmensen Reduction permits aldehydes or ketones to be deoxygenated and the corresponding hydrocarbon to be produced. The Clemmensen reduction is a reaction that uses hydrochloric acid and zinc amalgam to convert aldehydes or ketones to alkanes. The Clemmensen reduction is named after Erik Christian Clemmensen, a Danish scientist. The underlying substance must be unaffected by the acidic environment. In the Wolff-Kishner reduction, which contains a strong base, the acid-sensitive base material interacts. Acid-sensitive compounds must not be used in this reaction as the reaction is not for compounds that are sensitive to acid.
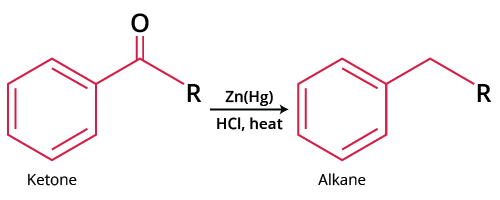
Clemmensen Reduction Reaction


































