
a) Illustrate the following name reactions giving suitable example in each case:
i) Clemmensen reduction
ii) Hell-Volhard-Zelinsky reaction
b) How are the following conversions carried out?
(i) Ethyl cyanide to ethanoic acid
(ii) Butan-1-ol to butanoic acid
(iii)Benzoic acid to m-bromobenzoic acid
Answer
232.8k+ views
Hint: As we know clemmensen reduction is based on the reduction of ketones, or aldehydes, and the reaction Hell-Volhard-Zelinsky reaction is related to the alpha position addition in the carboxylic acid. If we talk about the conversions, then these conversions will be done with the help of bromine, or potassium permanganate.
Complete step by step answer:
Now, we will talk about all the reactions step by step.
First, we will discuss part (a).
The first (i) reaction is Clemmensen reduction.
As mentioned this reaction includes the reduction of aldehydes or ketones using hydrochloric acid, and zinc amalgam, and it is reduced to the alkanes. So, let us take an example of acetophenone.
The chemical reaction is
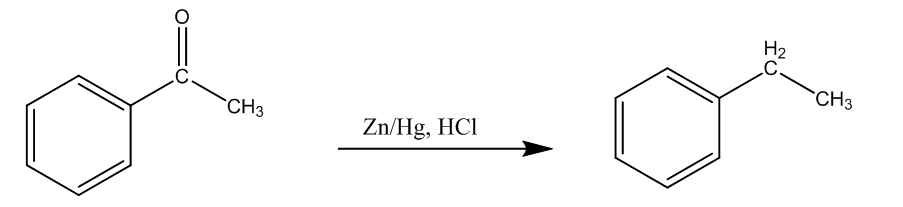
Here, we can see acetophenone is reduced to the Ethyl benzene, or 1-Phenyl Ethane
Now, the second (ii) reaction is Hell- Volhard-Zelinsky, it involves the halogenation of carboxylic acids at the alpha carbon position. This reaction is carried out with the catalytic amount of phosphorus tribromide, and the addition of diatomic bromine.
The chemical reaction is
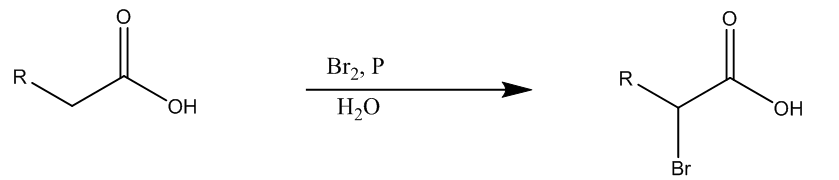
Here we can see the general carboxylic addition forms alpha-bromo carboxylic acid.
Now, we will discuss the part (b).
The first (i) is the conversion of Ethyl cyanide to ethanoic acid.
The conversion will take place thru hydrolysis, then diatomic bromine, and potassium permanganate. The chemical reaction is
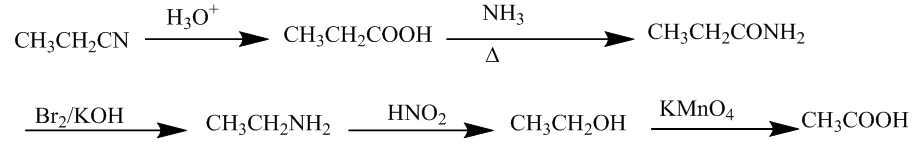
Now, the second (ii) is the conversion of butan -1- ol to butanoic acid. It is done by the use of potassium permanganate. The chemical reaction is
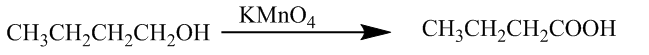
The third (iii) is the conversion of benzoic acid to m-bromobenzoic acid. It takes place through the bromination of benzoic acid. The chemical reaction is
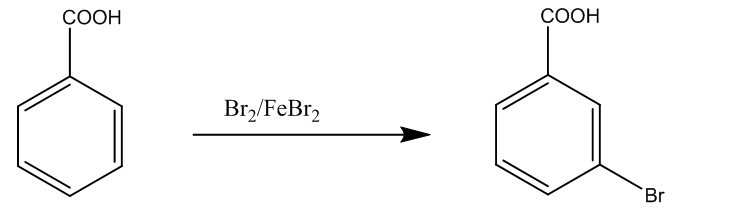
Now, in the end we can conclude that in part (a) (i) the product is alkane, and in (ii) the product is alpha – bromo carboxylic acid. In the part (b) we have shown the conversion reactions.
Note: It is important to know that in the Hell-Volhard- Zelinsky reaction, there is no fluorination, and iodination of carboxylic acids. So, we just consider the bromination of carboxylic acids.
Complete step by step answer:
Now, we will talk about all the reactions step by step.
First, we will discuss part (a).
The first (i) reaction is Clemmensen reduction.
As mentioned this reaction includes the reduction of aldehydes or ketones using hydrochloric acid, and zinc amalgam, and it is reduced to the alkanes. So, let us take an example of acetophenone.
The chemical reaction is

Here, we can see acetophenone is reduced to the Ethyl benzene, or 1-Phenyl Ethane
Now, the second (ii) reaction is Hell- Volhard-Zelinsky, it involves the halogenation of carboxylic acids at the alpha carbon position. This reaction is carried out with the catalytic amount of phosphorus tribromide, and the addition of diatomic bromine.
The chemical reaction is

Here we can see the general carboxylic addition forms alpha-bromo carboxylic acid.
Now, we will discuss the part (b).
The first (i) is the conversion of Ethyl cyanide to ethanoic acid.
The conversion will take place thru hydrolysis, then diatomic bromine, and potassium permanganate. The chemical reaction is

Now, the second (ii) is the conversion of butan -1- ol to butanoic acid. It is done by the use of potassium permanganate. The chemical reaction is
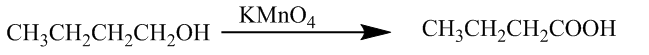
The third (iii) is the conversion of benzoic acid to m-bromobenzoic acid. It takes place through the bromination of benzoic acid. The chemical reaction is

Now, in the end we can conclude that in part (a) (i) the product is alkane, and in (ii) the product is alpha – bromo carboxylic acid. In the part (b) we have shown the conversion reactions.
Note: It is important to know that in the Hell-Volhard- Zelinsky reaction, there is no fluorination, and iodination of carboxylic acids. So, we just consider the bromination of carboxylic acids.
Recently Updated Pages
Types of Solutions in Chemistry: Explained Simply

JEE General Topics in Chemistry Important Concepts and Tips

JEE Extractive Metallurgy Important Concepts and Tips for Exam Preparation

JEE Amino Acids and Peptides Important Concepts and Tips for Exam Preparation

JEE Atomic Structure and Chemical Bonding important Concepts and Tips

Electricity and Magnetism Explained: Key Concepts & Applications

Trending doubts
JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding How a Current Loop Acts as a Magnetic Dipole

Understanding Average and RMS Value in Electrical Circuits

Understanding Collisions: Types and Examples for Students

Ideal and Non-Ideal Solutions Explained for Class 12 Chemistry

Other Pages
JEE Advanced Weightage 2025 Chapter-Wise for Physics, Maths and Chemistry

JEE Main Participating Colleges 2026 - A Complete List of Top Colleges

Understanding Atomic Structure for Beginners

NCERT Solutions For Class 12 Chemistry Chapter 2 Solutions Hindi Medium (2025-26)

AssertionIn electrolytic refining of metal impure metal class 12 chemistry JEE_Main

CBSE Class 12 Chemistry Set 1 56/2/1 2025: Question Paper, Answers & Analysis




