




Concepts of Organic Compound Contains Oxygen for JEE Main Chemistry
In JEE Main Chemistry, the topic Organic Compounds containing Oxygen is very essential. Under the wide category of General Organic Chemistry, it plays a critical role. From the Organic Compounds containing Oxygen chapter, at least one or two questions are asked, totalling roughly four marks.
As a result, the total weightage of this chapter in the JEE Main exams is up to 1-2 percent. If you thoroughly understand the theoretical section of this chapter, you will have no trouble answering the chapter's questions. Ethers, Alcohols, and the Cannizzaro Reaction are a few of the concepts investigated under the Organic Compounds containing Oxygen heading.
JEE Main Chemistry Chapters 2026
Important Topics of Oxygen Containing Compound Chapter
Alcohols
Aldehydes
Ester
Phenol
ketone
Acyl group
Ether
Carboxylic acid
Amide
Organic Compound Contains Oxygen Important Concept for JEE Main
Oxygen Containing Organic Compounds
There are four major oxygen containing compounds in organic chemistry: A carboxylic acid is an organic molecule with a carbon (C) atom double linked to an oxygen (O) atom and a single attached hydroxyl group (OH).
Important Reactions
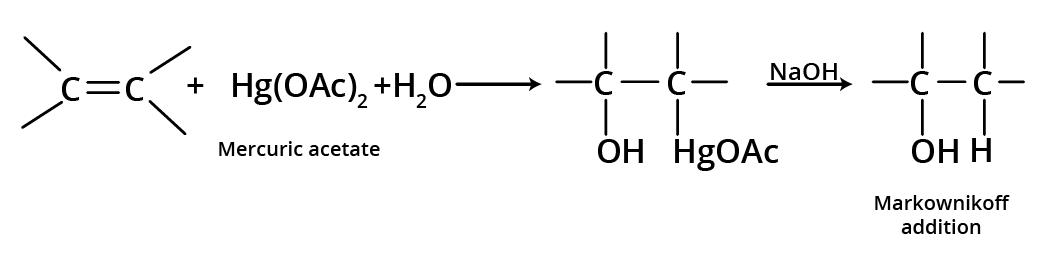
1. Oxymercuration-Demercuration
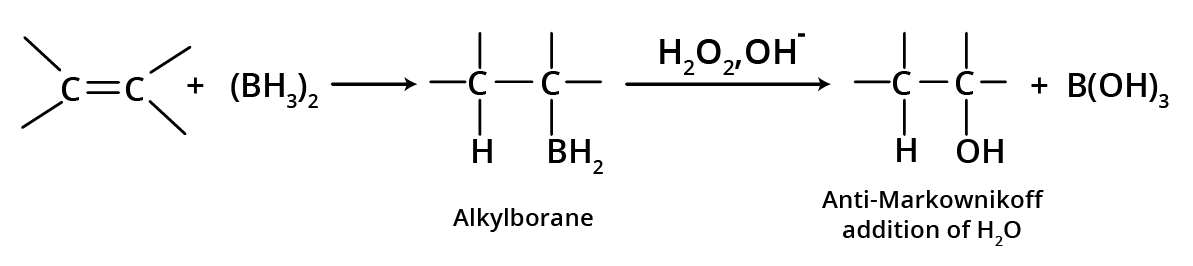
2. Hydroboration Oxidation
Alkene combines with diborane to generate trialkyl boranes, which then react with alkaline H2O2 to form alcohols, resulting in anti-water Markovnikov's addition.
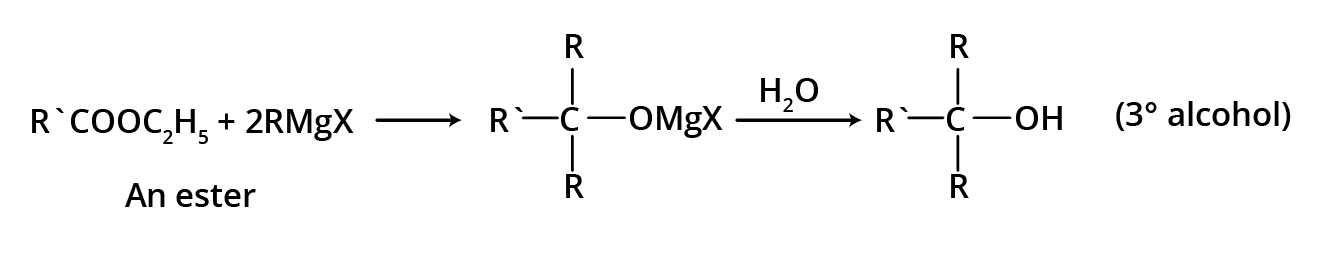
3. By using esters
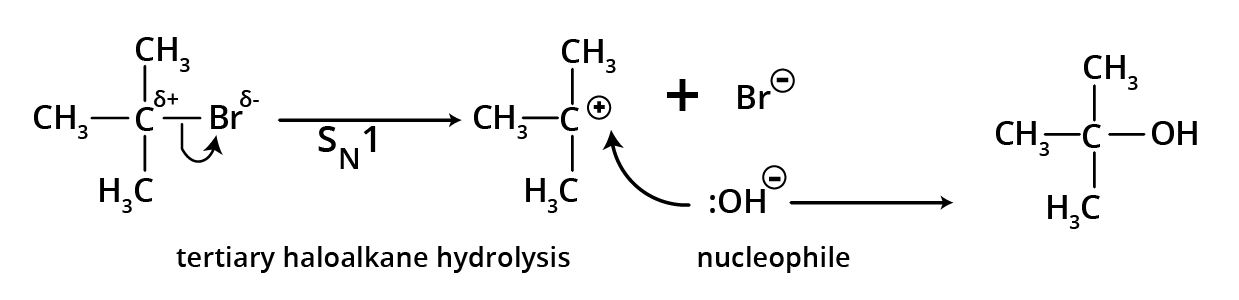
4. Hydrolysis of alkyl halides
Physical Properties
The OH group has a big influence on the alcohol's characteristics.
The boiling points of the lower members, such as methanol, ethanol, and 1propanol, are greater.
Alcohols with smaller alkyl groups are more water soluble, but as the size of the alkyl group grows larger, the solubility declines.
Chemical Properties of the Alcohols
1. Reaction with Hydrogen Halides
Substitution occurs when alcohols react with a hydrogen halide, resulting in an alkyl halide and water.
R—OH + HX → R—X + H2O
2. Reaction with PX3 and PX5
Alkyl halides are formed when alcohols combine with PX3 and PX5.
3 ROH + PBr3 → 3RBr + H3PO3
(1° or 2° alcohol)
ROH + PCl5 → RCl + POCl3 + HCl
3. Reaction with SOCl2
ROH + SOCl2 → R — Cl + SO2 + HCl
4. Esterification
The creation of an ester is the third method of replacing hydroxyl hydrogen with an acyl group. Acid chloride, acid anhydride, or carboxylic acid are all frequent sources of acyl groups (RCO). Esters form when they react with alcohol.
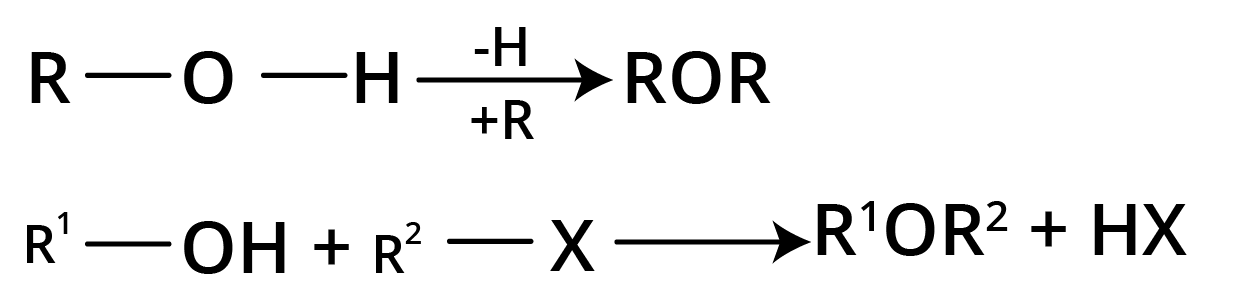
5. Reaction with RMgX
Alcohol is a non-acidic substance. A Grignard reagent, which is a strong base, can remove hydrogen from an alcohol's (OH) group.
CH3OH + CH3MgI → CH3OMgI + CH4
6. Reaction with Ceric Ammonium Solution
With ceric ammonium nitrate solution, an organic molecule containing one oxygen produces a red hue. Any monohydric alcohol, including phenol, can give off a red colour when it contains one oxygen.
Methods of Preparation of Phenols
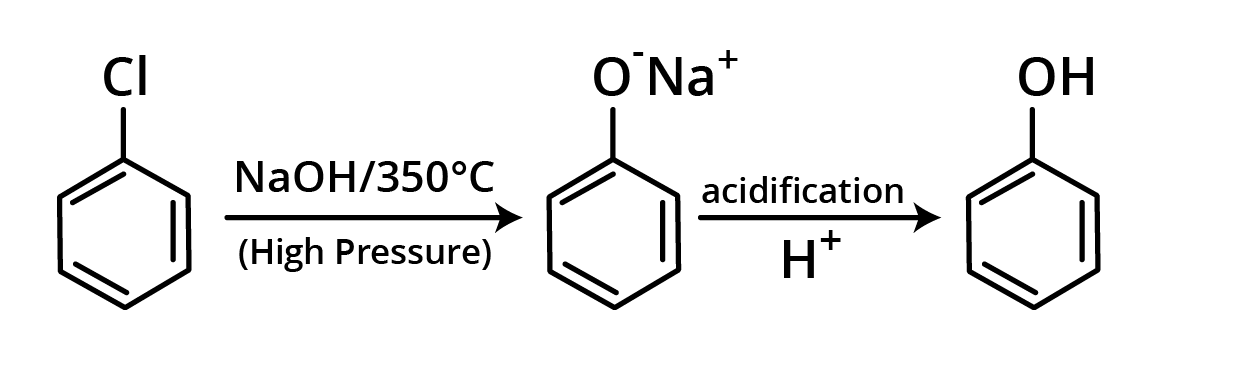
1. Hydrolysis of Chlorobenzene
Chlorobenzene is heated at 350°C under high pressure with aqueous NaOH in this procedure. The reaction creates C6H5ONa+ (sodium phenoxide), which is converted to phenol by acidification. Dow's method is the name for this reaction.
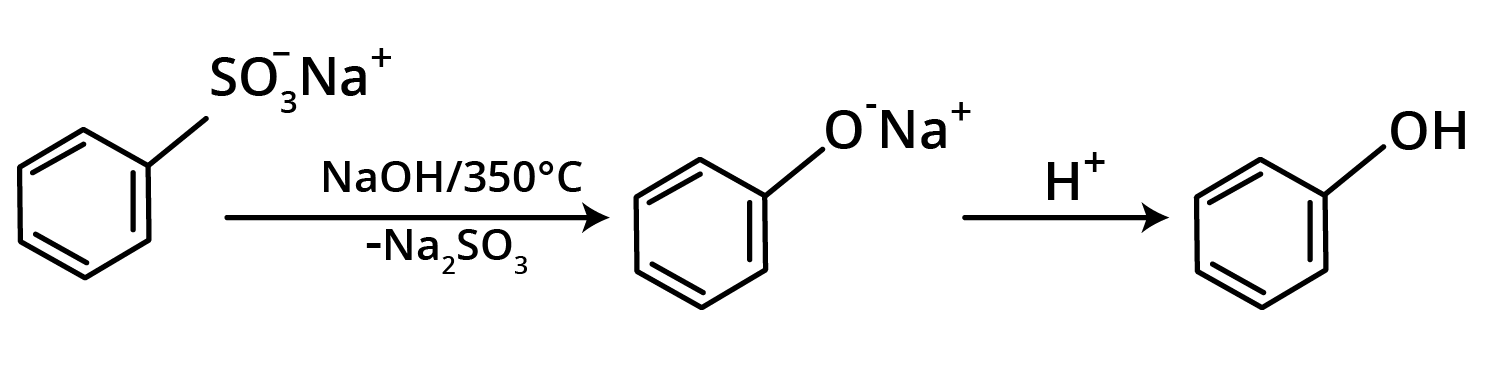
2. Alkali Fusion of Sodium Benzene Sulphonate
This process involves melting sodium benzene sulphonate with sodium hydroxide to make sodium phenoxide, which is then acidified to produce phenol.
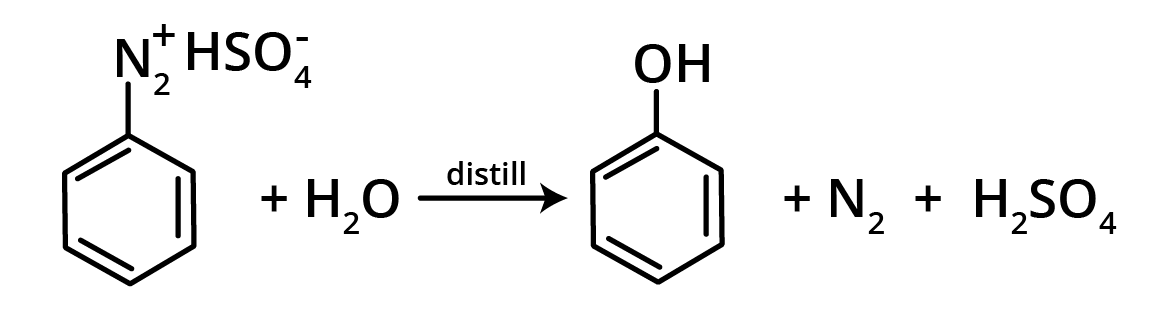
3. Hydrolysis of Diazonium Salts
Phenol is created when a diazonium sulphate solution is steam distilled.
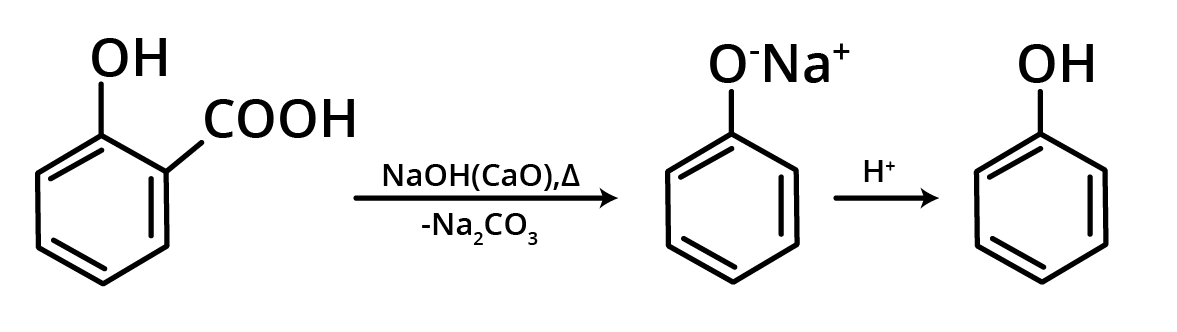
4. By Distillation of Phenolic Acid
Decarboxylation occurs when phenolic acids are heated with soda lime, resulting in phenols.
Physical Properties of the Phenols
Phenol (C6H5OH) is a colourless solid.
The m.p. is 41°C.
The b.p. 182°C.
It becomes coloured on exposure to air.
It is fairly soluble in water.
Chemical Properties of the Phenols
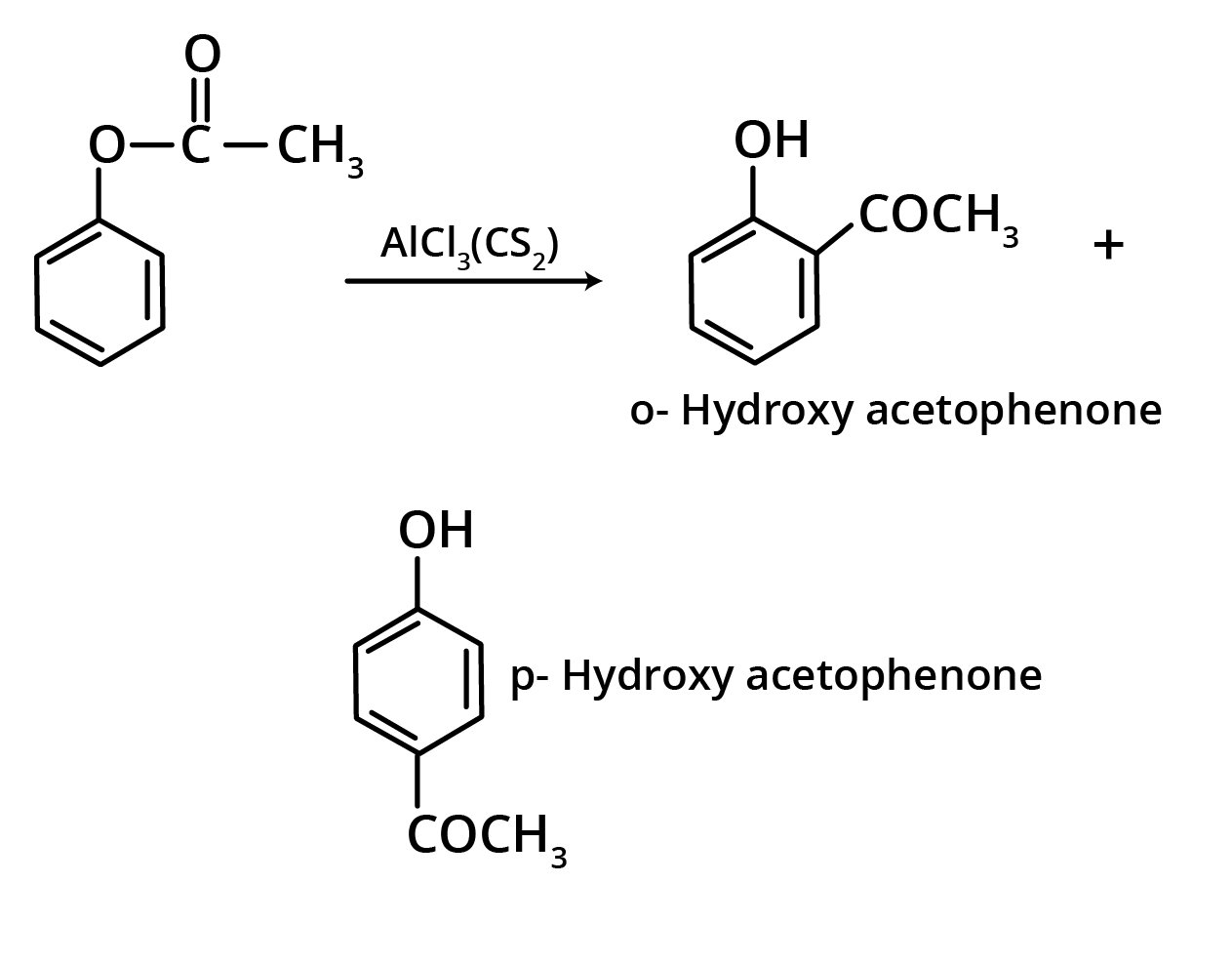
1. Fries Rearrangement
The ortho and para hydroxy acetophenones are produced by the Fries rearrangement of phenyl acetate with AlCl3. The ortho isomer is removed from the mixture using steam distillation.
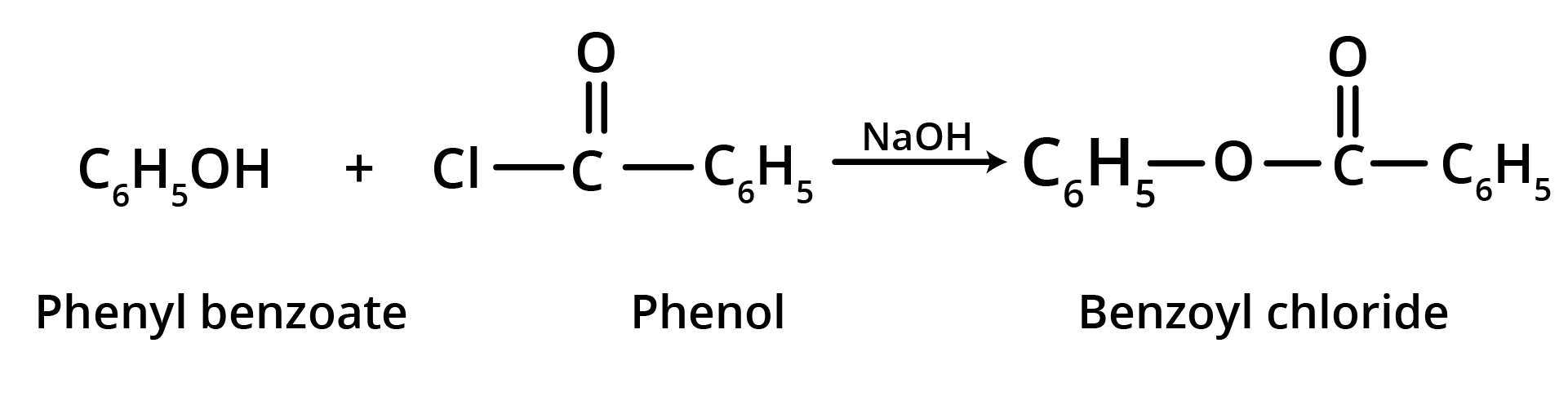
2. Schotten Baumann Reaction
When an alkaline phenol solution is forcefully shaken with benzoyl chloride, phenyl benzoate is produced. It's known as the Schotten Baumann response.
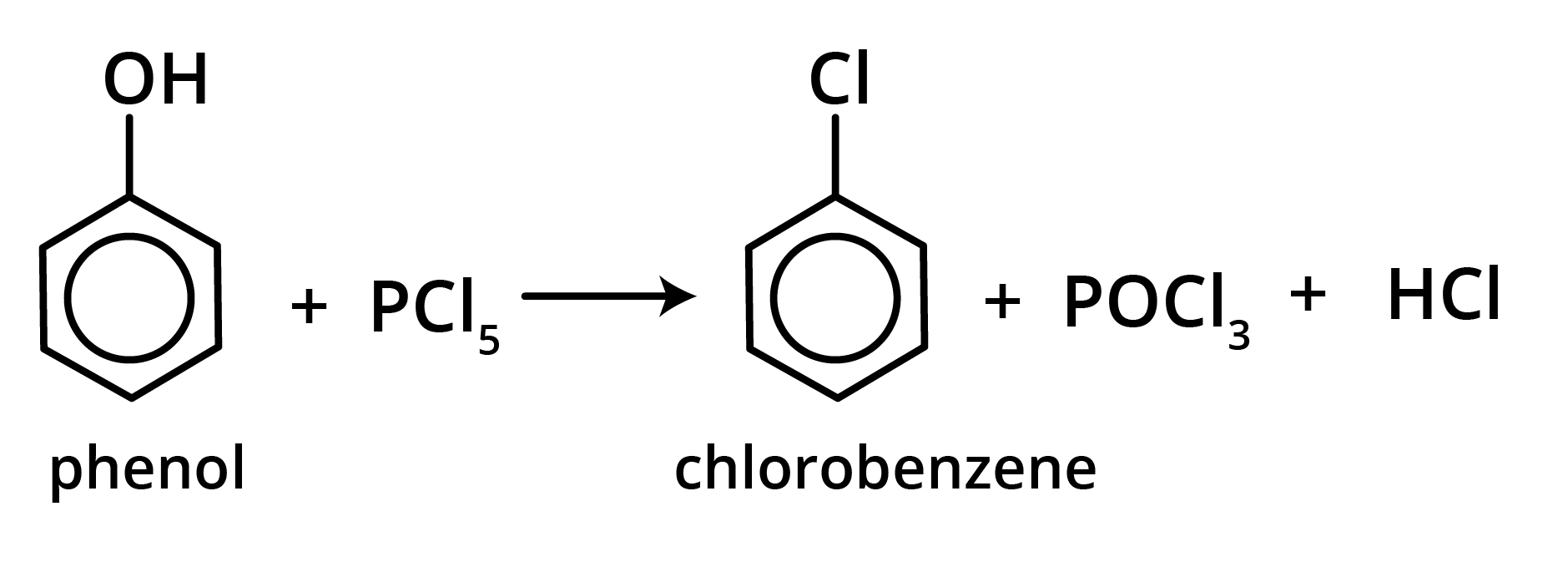
3. Reaction with PCl5
Aryl chloride is generated when phenol is treated with PCl5 (poor yield). Triphenyl phosphate is the major product of this process.
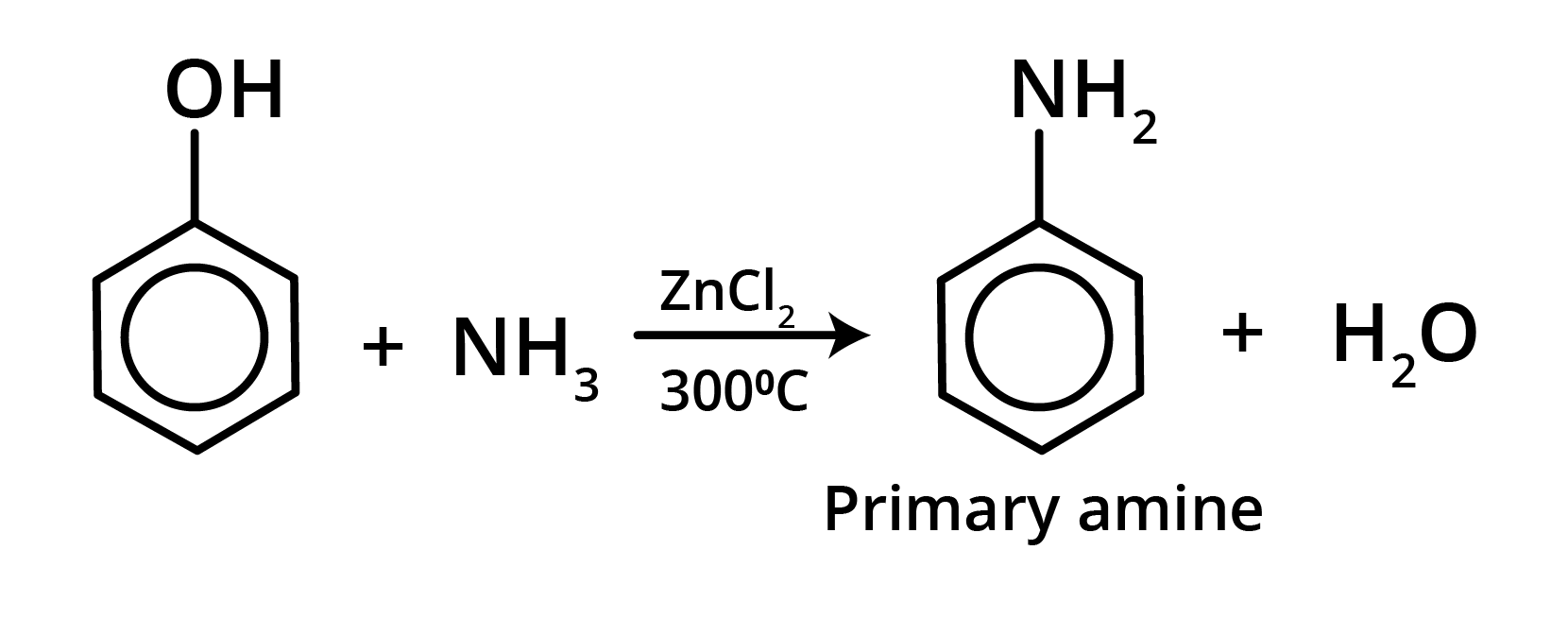
4. Reaction with NH3
When phenol is treated with ammonia at 300°C under high pressure in the presence of anhydrous AlCl3, ZnCl2, or CaCl2, aniline is produced.
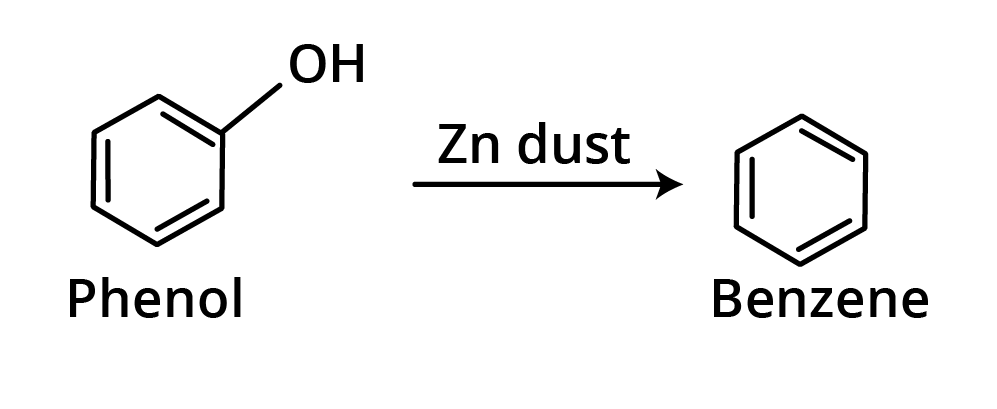
5. Reaction with Zn dust
When phenols are heated with Zn dust, aromatic hydrocarbons are formed.
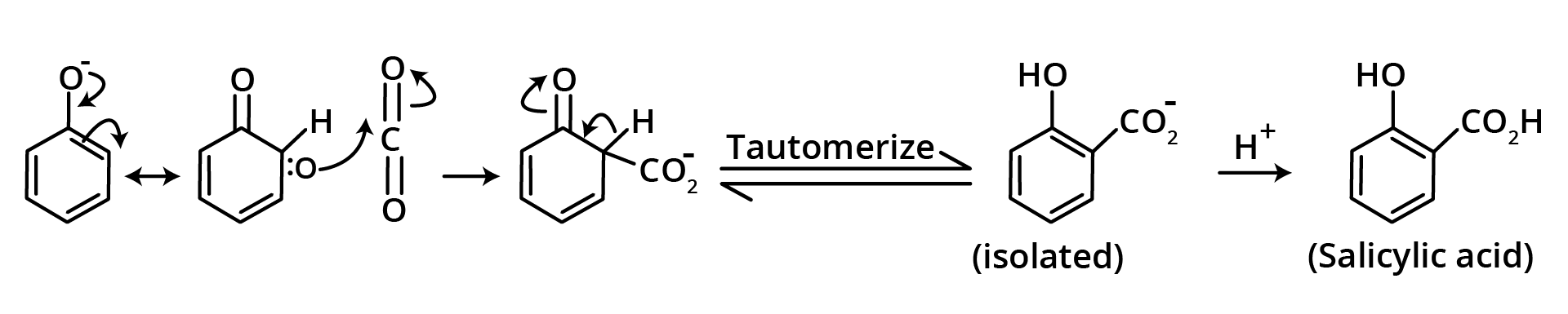
6. Kolbe's Schmidt Reaction
When carbon dioxide gas is passed through sodium phenoxide at 400 degrees Celsius and 67 pounds per square inch of air pressure, sodium salicylate is created, which when acidified yields salicylic acid, but some para isomer is also produced.
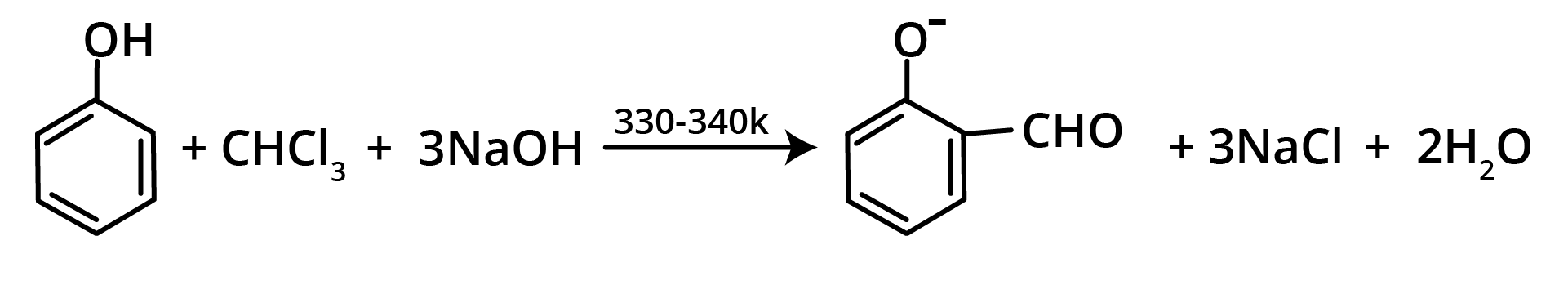
7. Reimer Tiemann Reaction
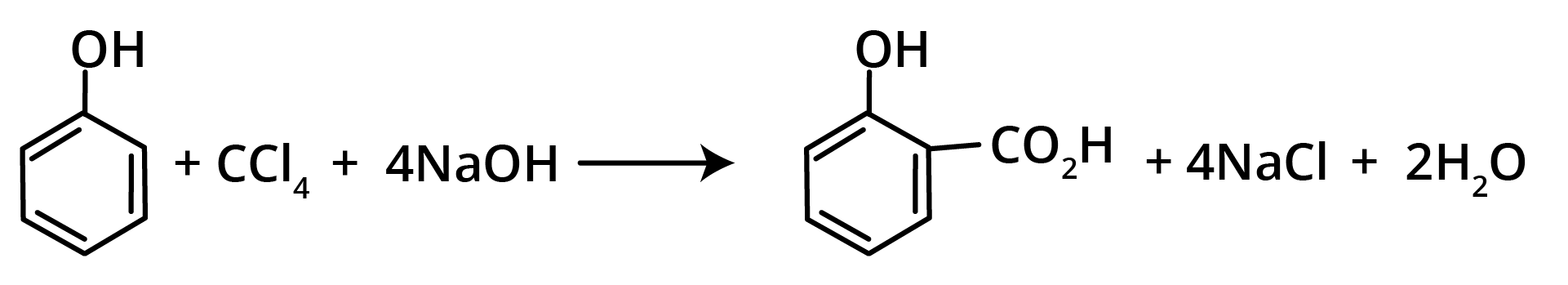
Methods of Preparation of Ethers
1. Williamson's Continuous Etherification Process
2ROH + 2Na → 2RO-Na+ + H2
RO-Na+ + R’X → R-O-R’ + NaX
It involves sodium alkoxide reacting with an alkyl halide. The action of Na on alcohol produces sodium alkoxide.
2. Intermolecular Dehydration of Alcohols
ROH + HOR → R-O-R + H2O (at 140 degrees celsius temperature)
Physical Properties of the Ethers
Ethers boil at a substantially lower temperature than the alcohols from which they are formed or similar molecular weight alcohols.
The boiling points are very similar to those of similar molecular complexity substituted alkanes.
Chemical Properties of the Ethers
1. Salt formation
Though ethers are neutral compounds, these form oxonium salts with inorganic acids.
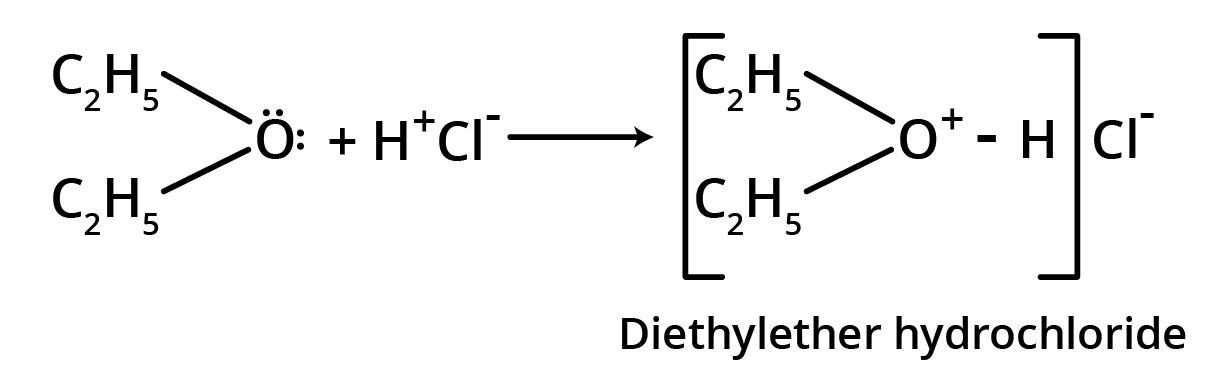
This reaction is due to lone pairs of electrons on oxygen of the ether functional group.
2. Cleavage by Acids
R-O-R + HX → RX + R’OH + R’X + H2O
Reactivity of HX : HI > HBr > HCl
Cleavage takes place only under vigorous conditions, i.e., concentrated acids (usually HI or HBr) and at high temperatures.
Action of HI
(a) At room temperature
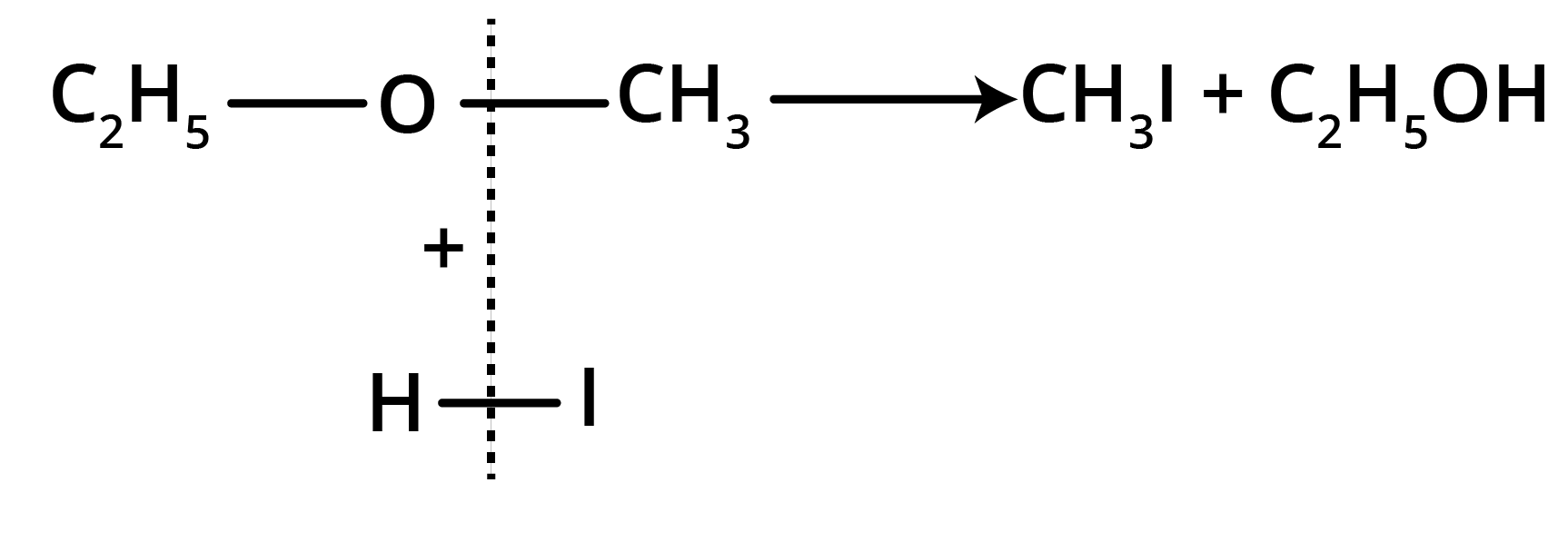
(b) At 100° C (excess of HI)
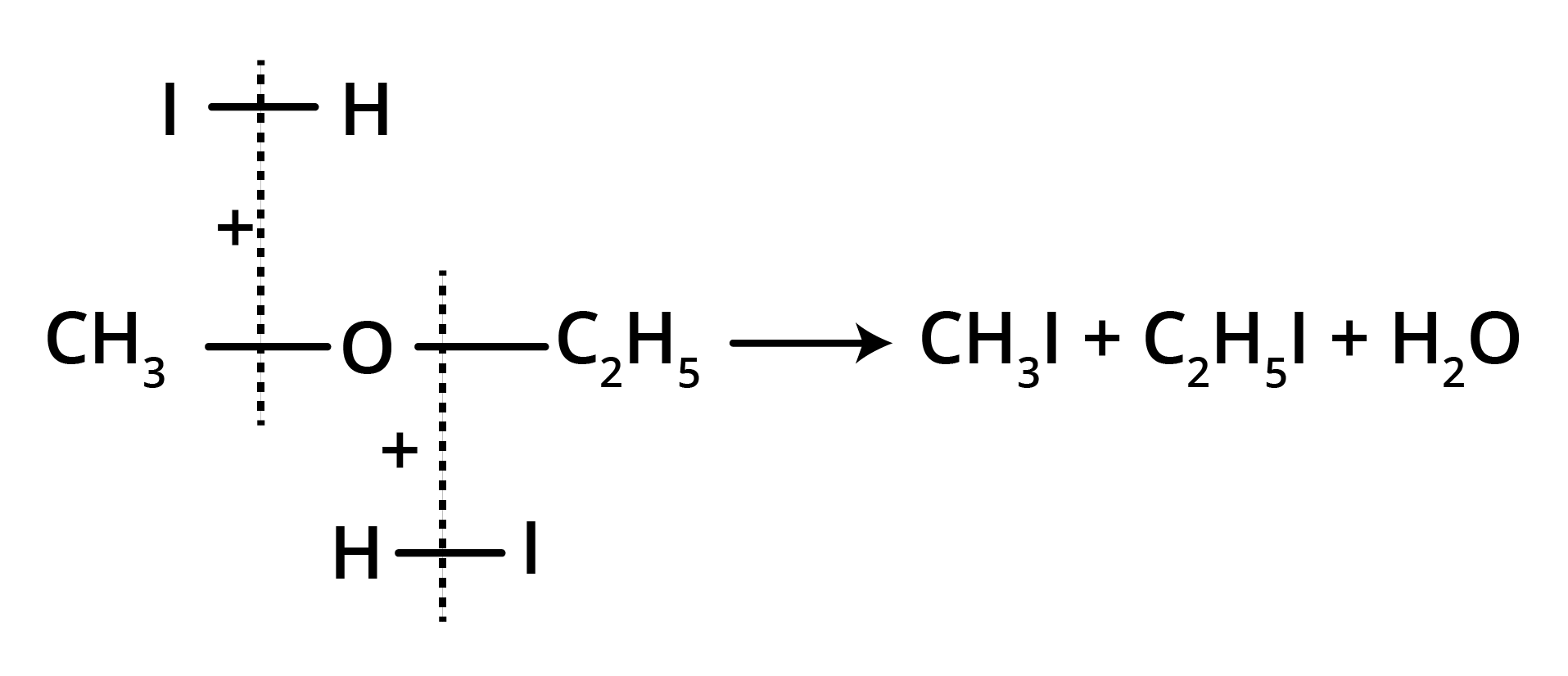
Thus, the metameric ethers can be easily identified by the cleavage with HI.
3. Action of PCl5 or SOCl2
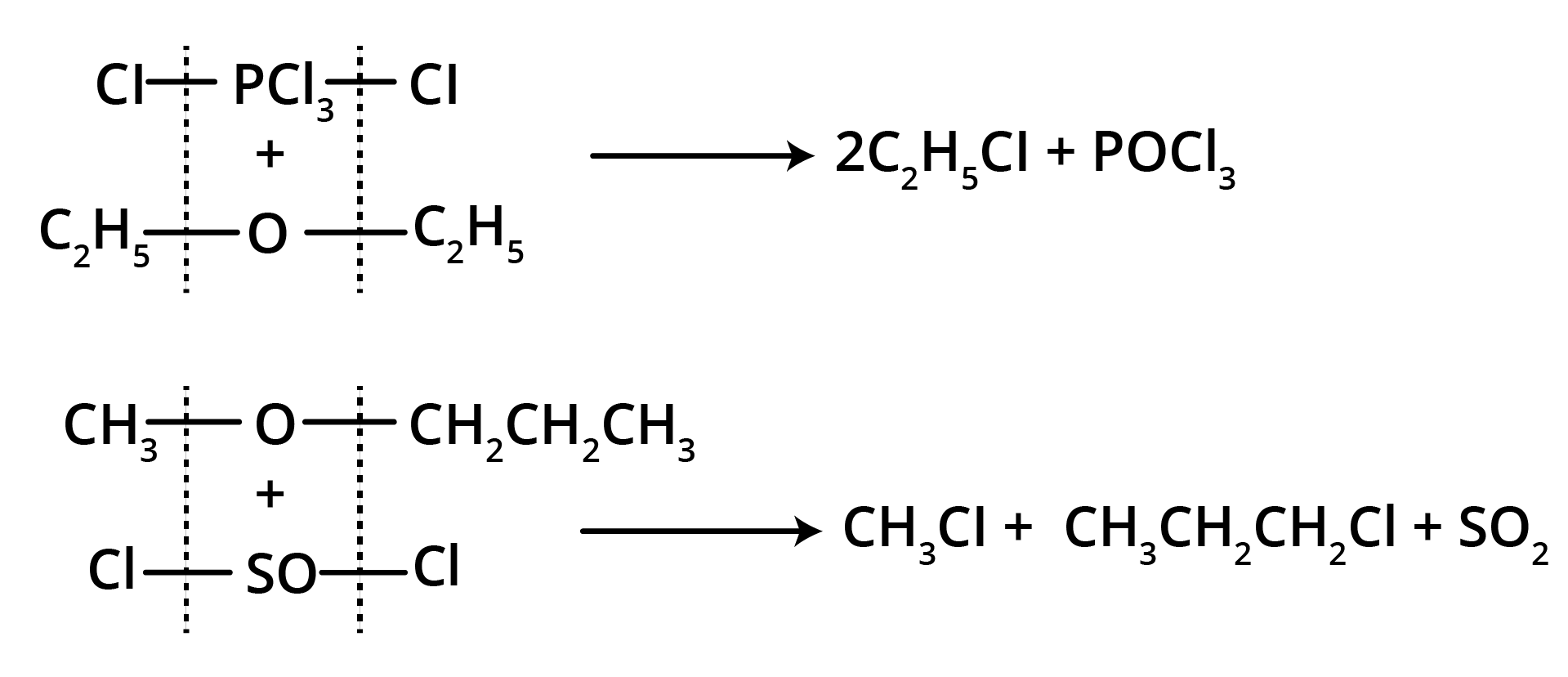
Thus, the reaction of PCl5 or SOCl2 can be used to identify the metameric ethers.
Aldehydes & Ketones
Aldehydes and ketones belong to a class of compounds having general formula CnH2nO and are represented as RCHO and RR'CO, respectively.
Methods of Preparation of Aldehydes
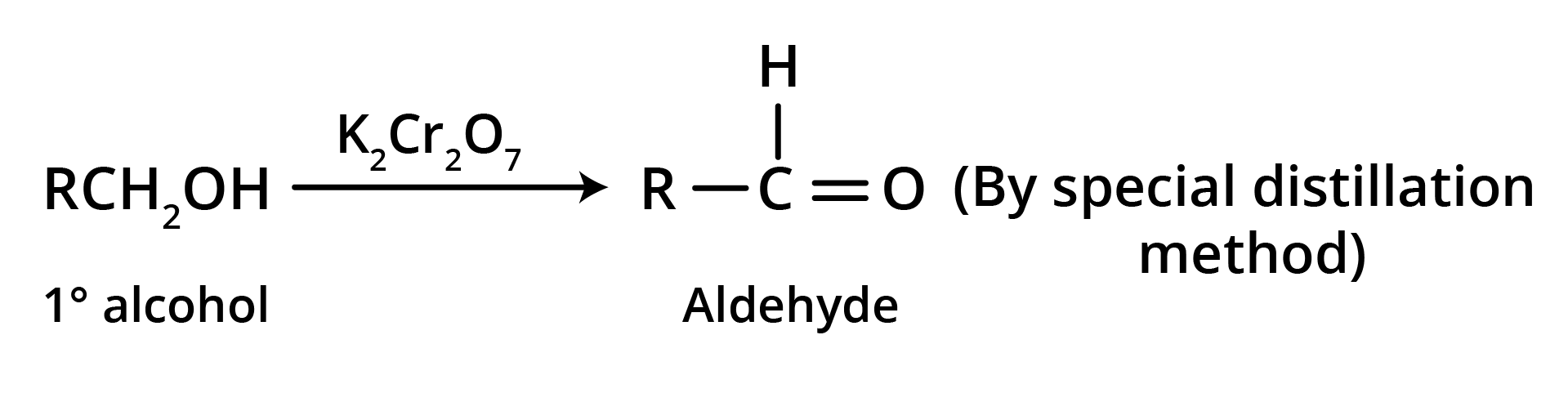
1. Oxidation of primary alcohols
Oxidation can be affected with acidic K2Cr2O7 or with PCC (Pyridine Chlorochromate, mild oxidising agent) in CH2Cl2.
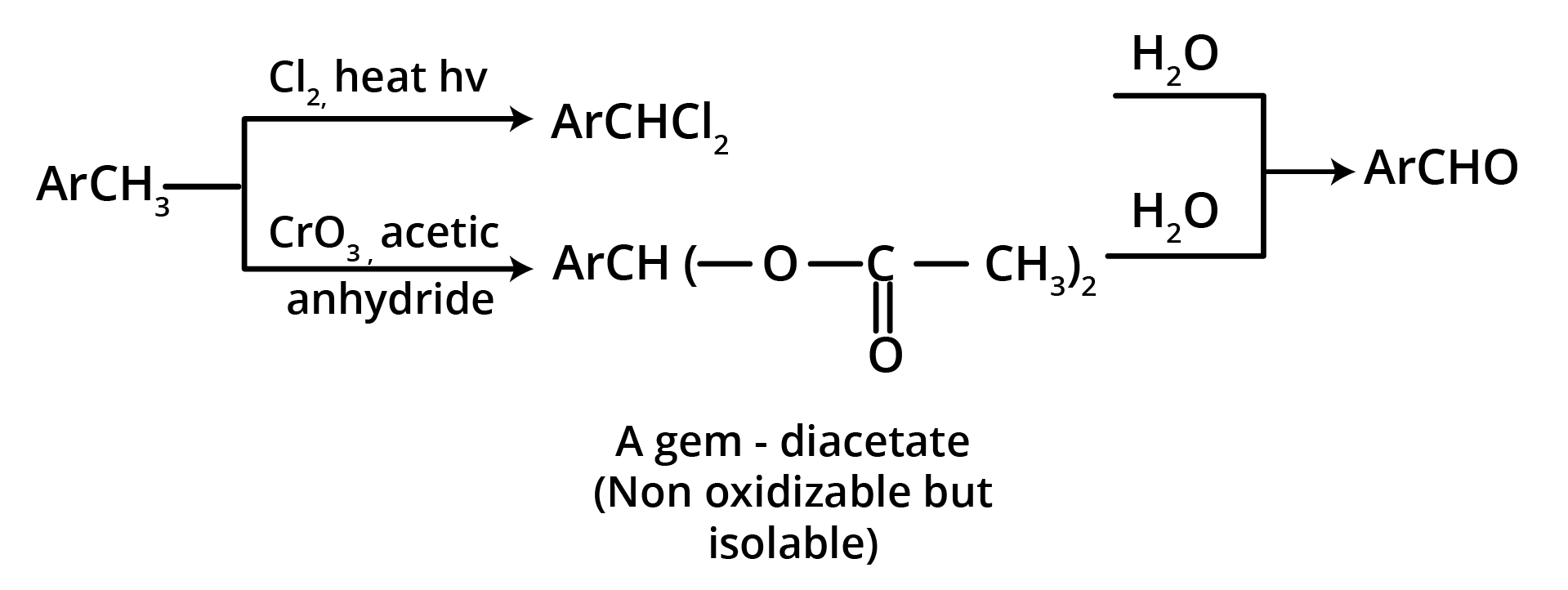
2. Oxidation of Methylbenzenes
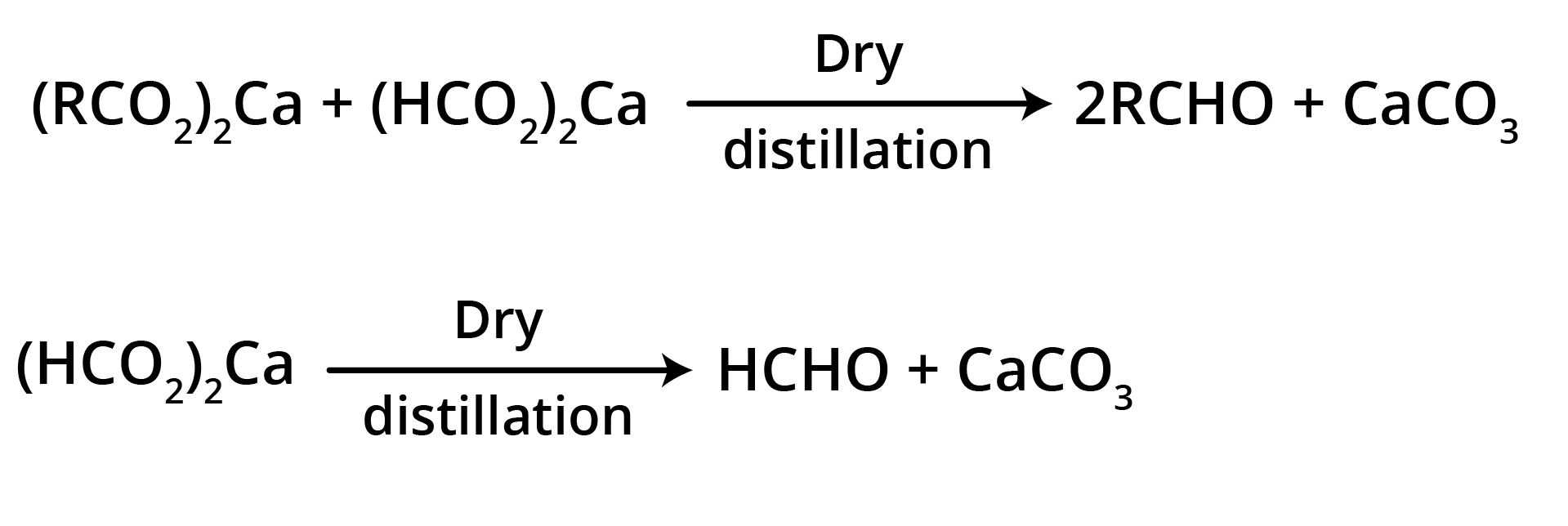
3. By Heating a Mixture of the Calcium Salts of Formic Acid and any one of its Homologues
Methods of Preparation of Ketones
1. Oxidation of Secondary Alcohols
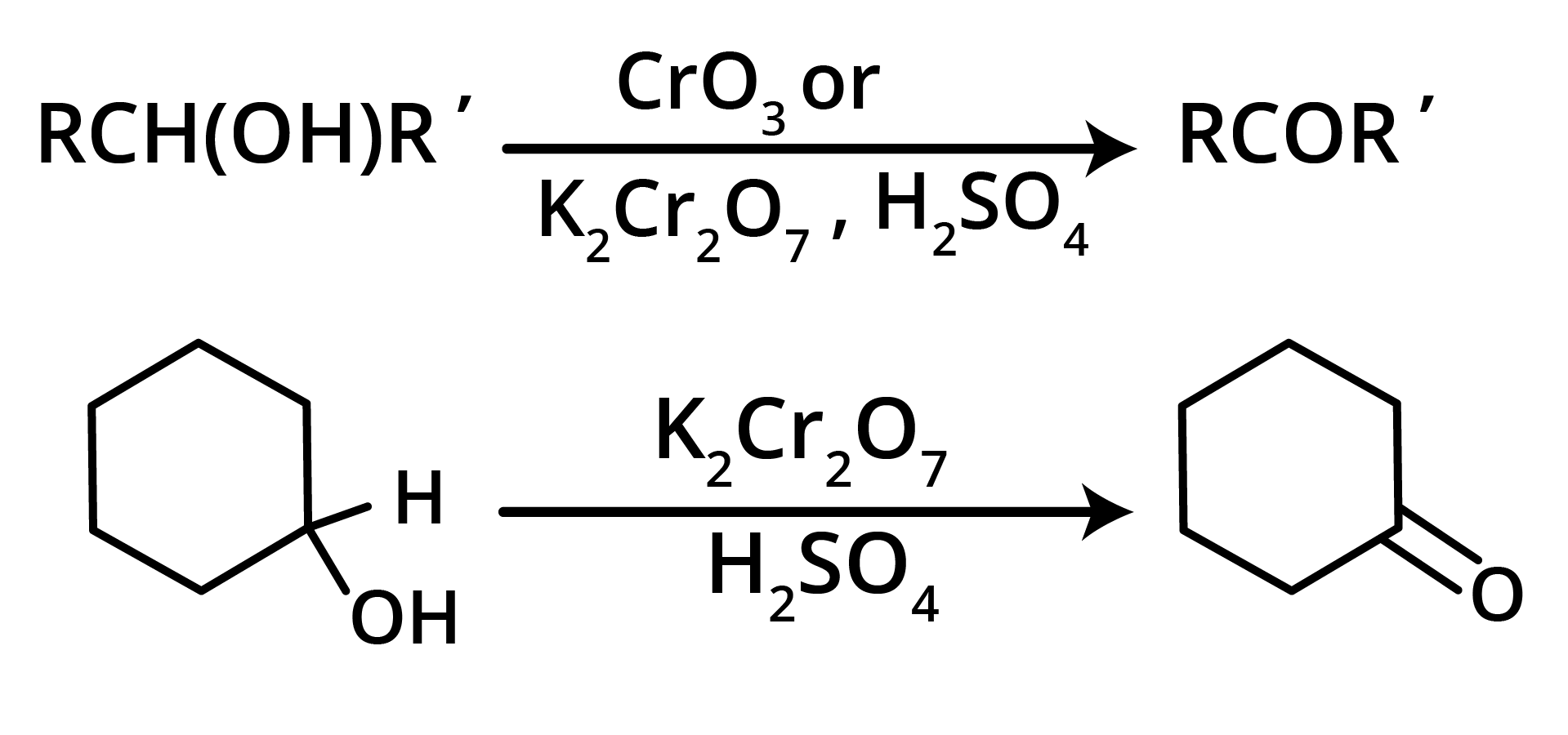
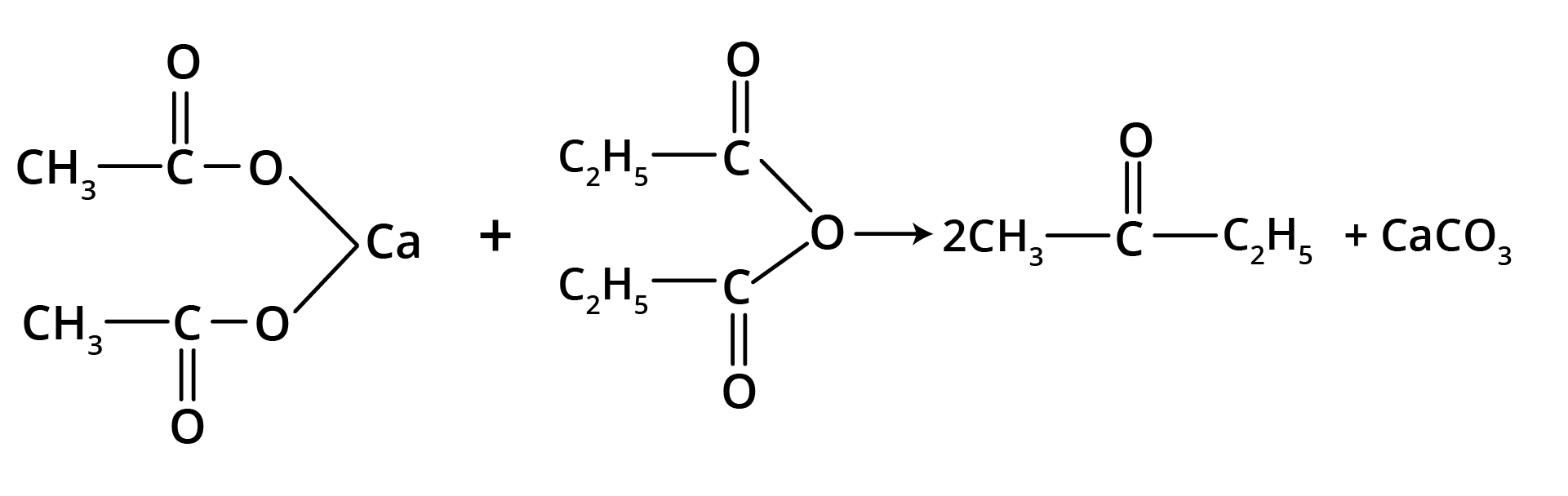
2. By Heating the Calcium Salt of any Monocarboxylic Acid other than Formic Acid
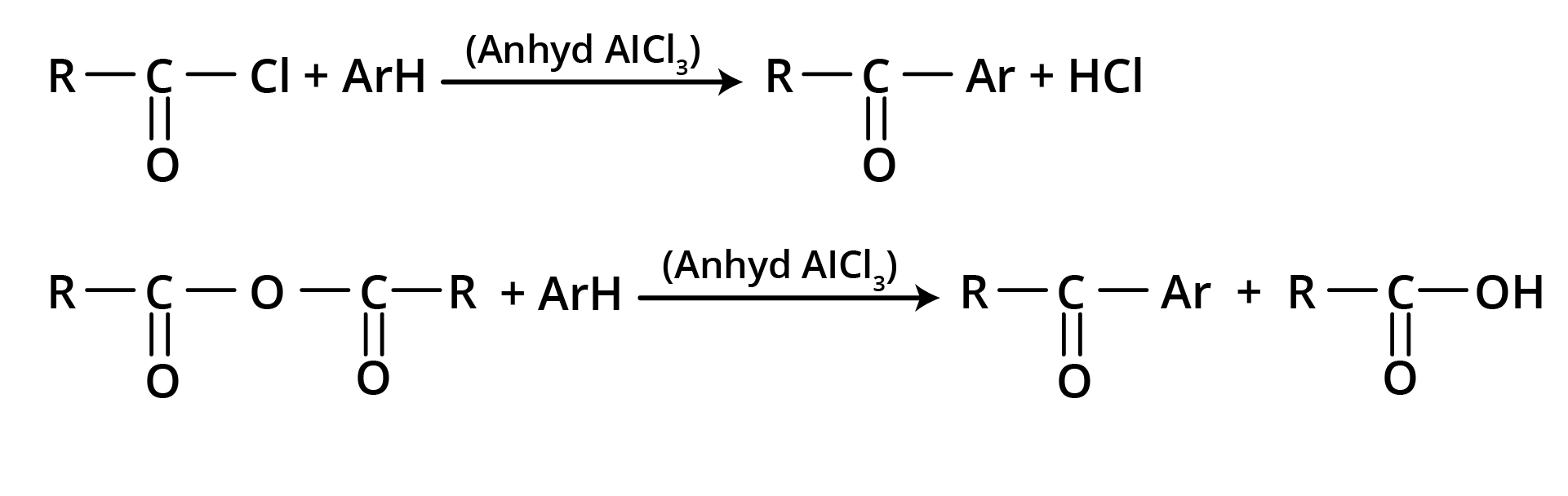
3. Friedel – Crafts Acylation
Used for the preparation of aliphatic aromatic ketones or aromatic ketones.
Physical Properties of Aldehydes and Ketones
The polar carbonyl group makes aldehydes and ketones polar compounds and hence they have higher boiling points than non-polar compounds of comparable molecular weights.
Chemical Properties of Aldehydes and Ketones
1. Oxidation
(a)
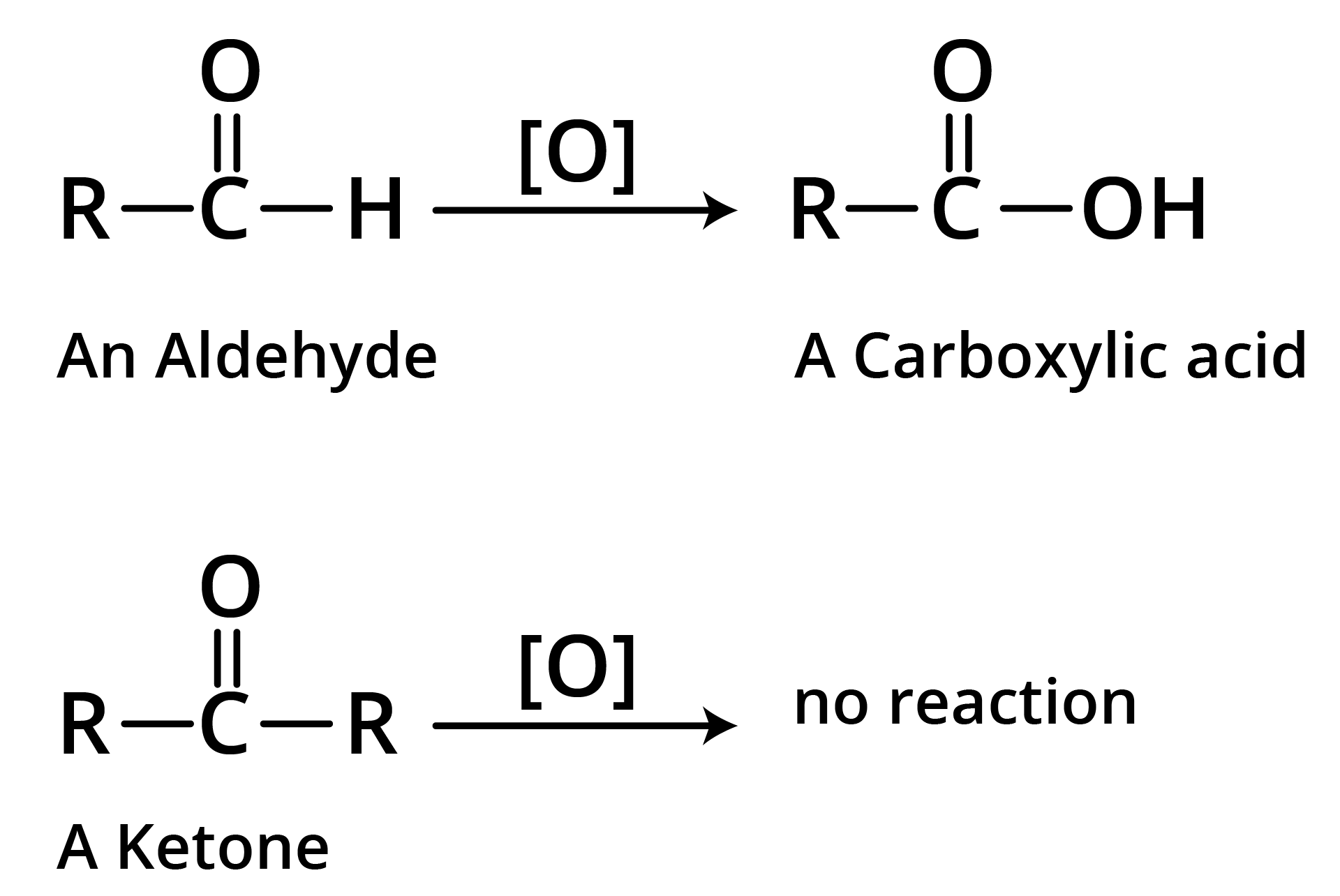
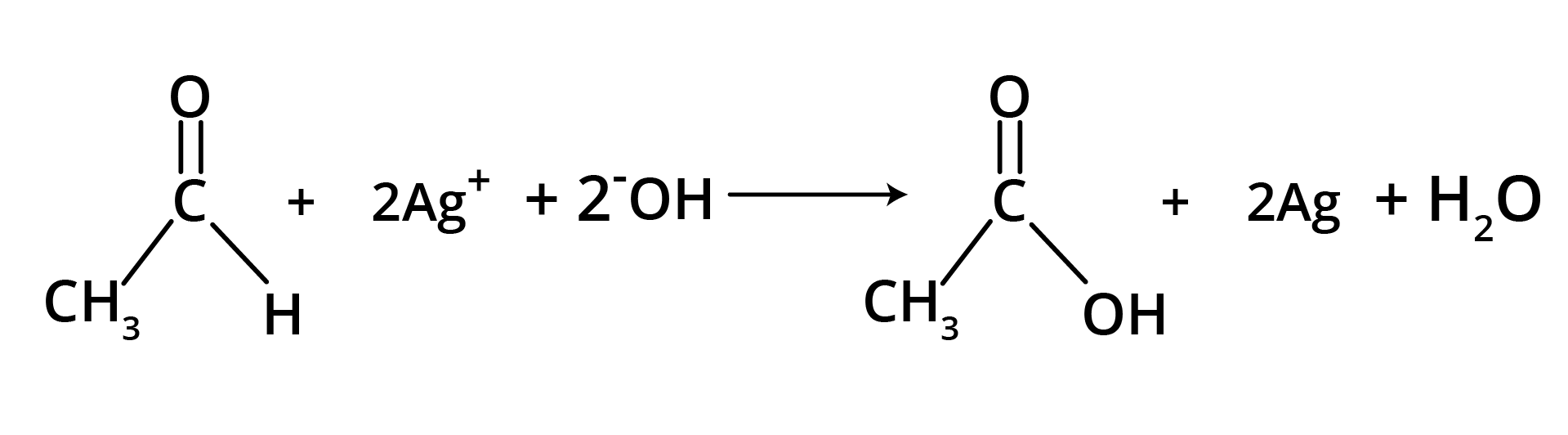
(b) Tollen's Reagent
A specific oxidant for RCHO is Ag(NH3)2+. Ketones do not give this reaction.
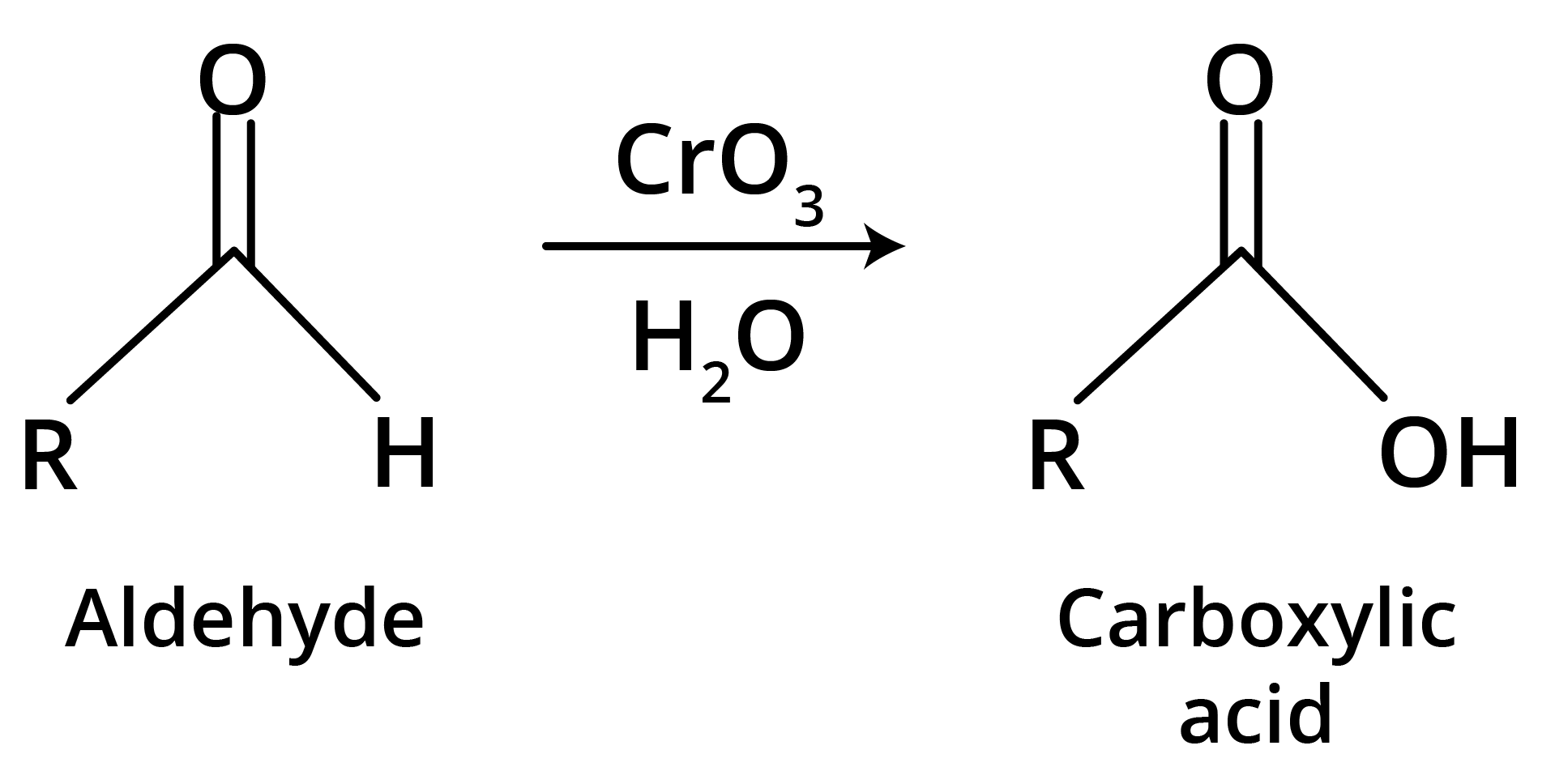
(c) Strong Oxidants
Ketones resist mild oxidation, but with strong oxidants at high temperature, they undergo cleavage of C-C bond on either side of the carbonyl group.
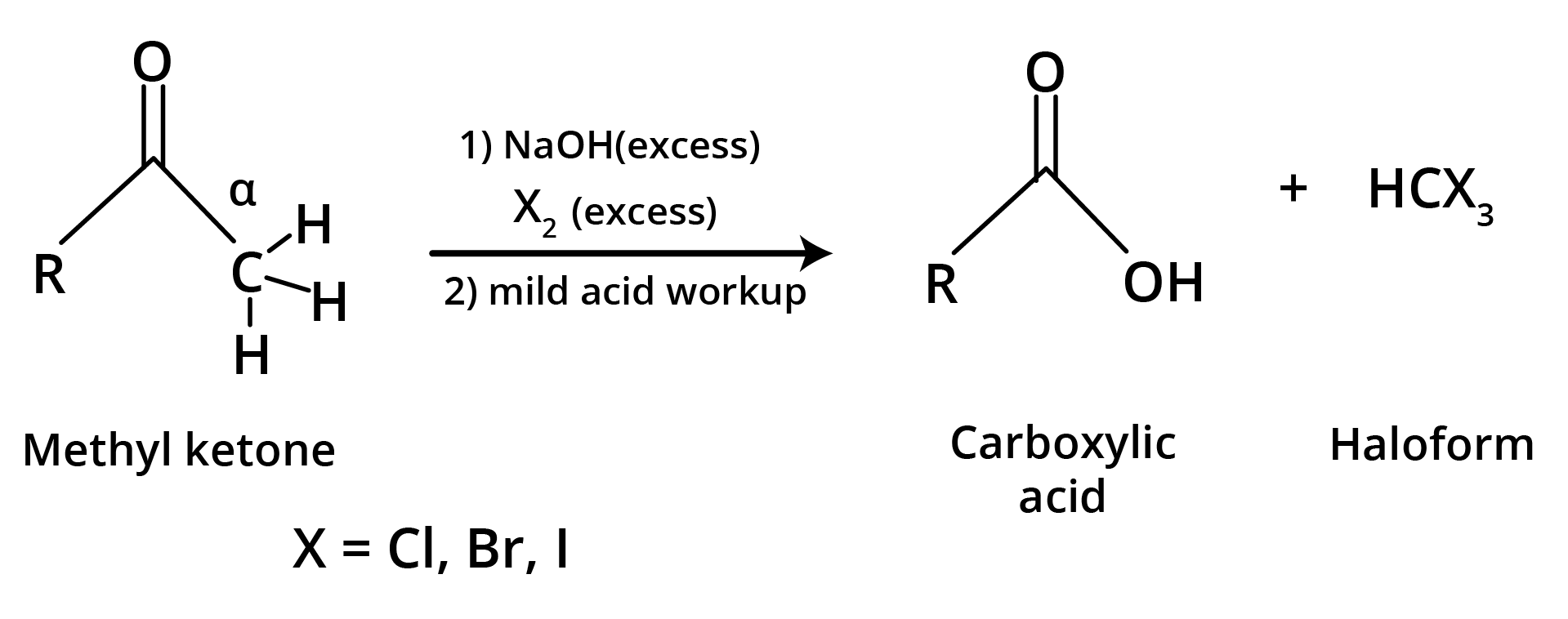
(d) Haloform Reaction is shown by methyl ketones.
CH3COR are readily oxidised by NaOI (NaOH + I2) to iodoform, CHI3, and RCO2Na.
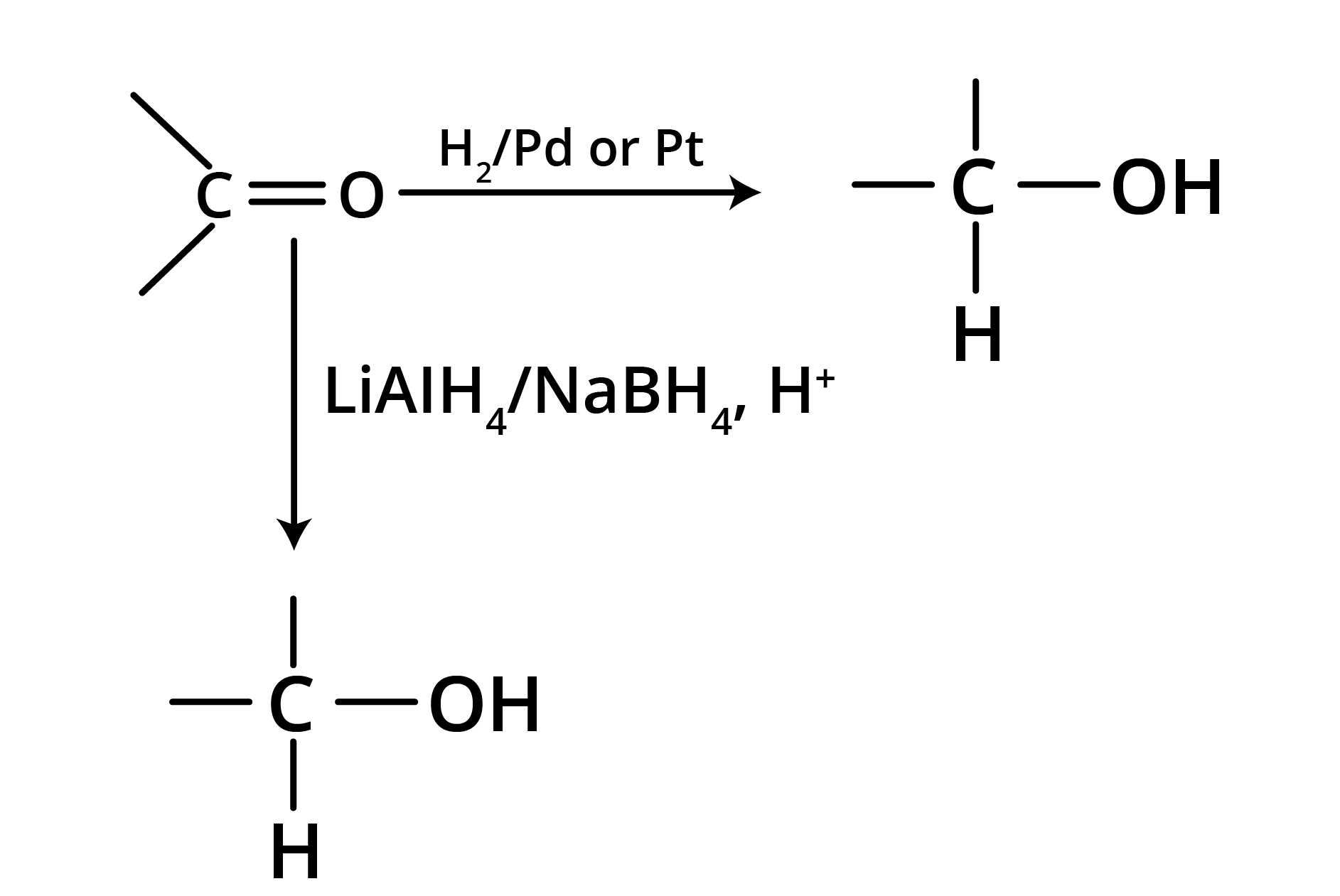
2. Reduction
(a) Reduction to alcohols
Reduction to alcohols can be achieved either by hydrogenation in the presence of Pt or Pd or by LiAlH4/NaBH4.
(b) Reduction to Hydrocarbons
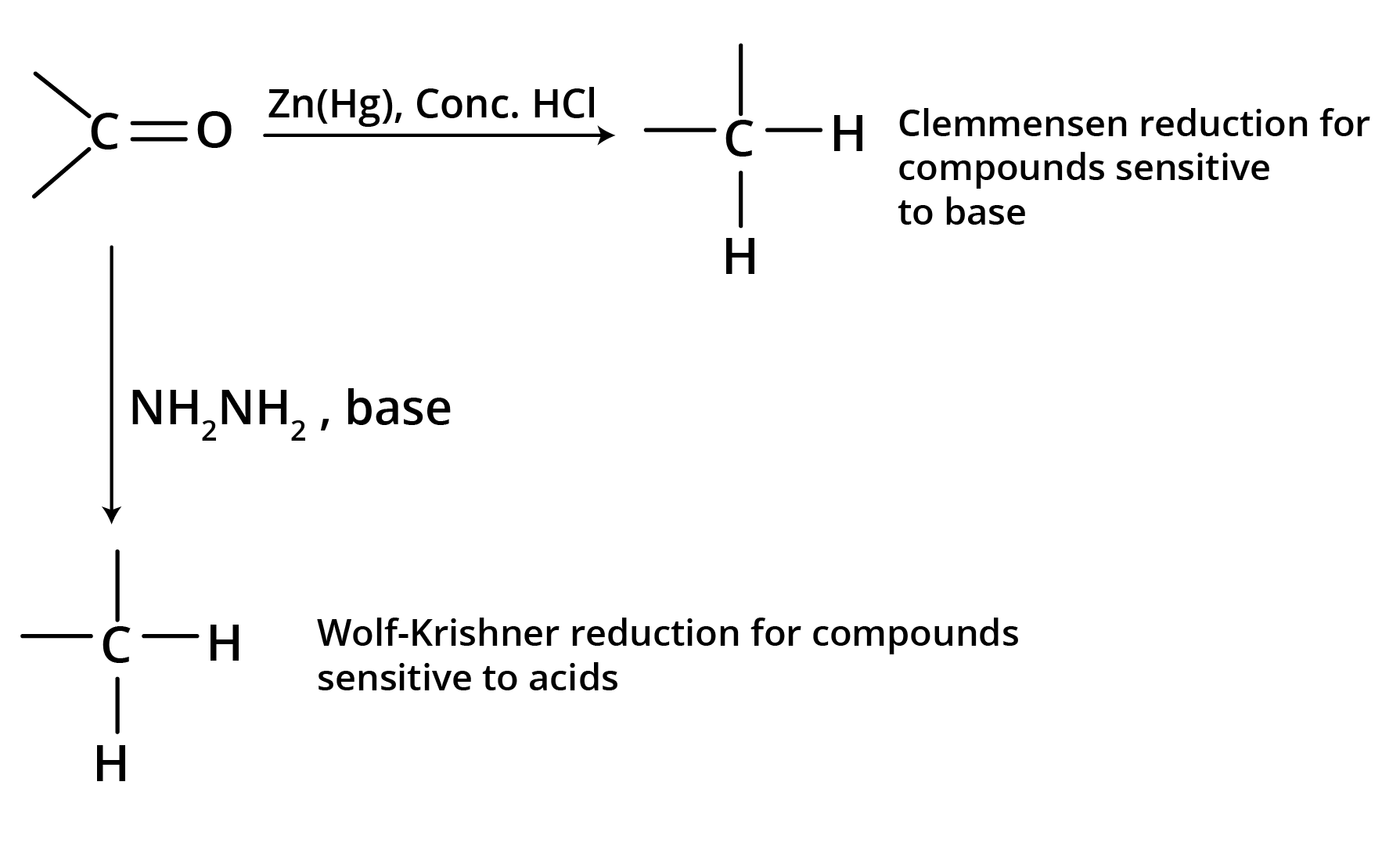
3. Addition Reactions of Nucleophiles
The carbon of the carbonyl group is electrophilic and thus initially attacked by a nucleophile.
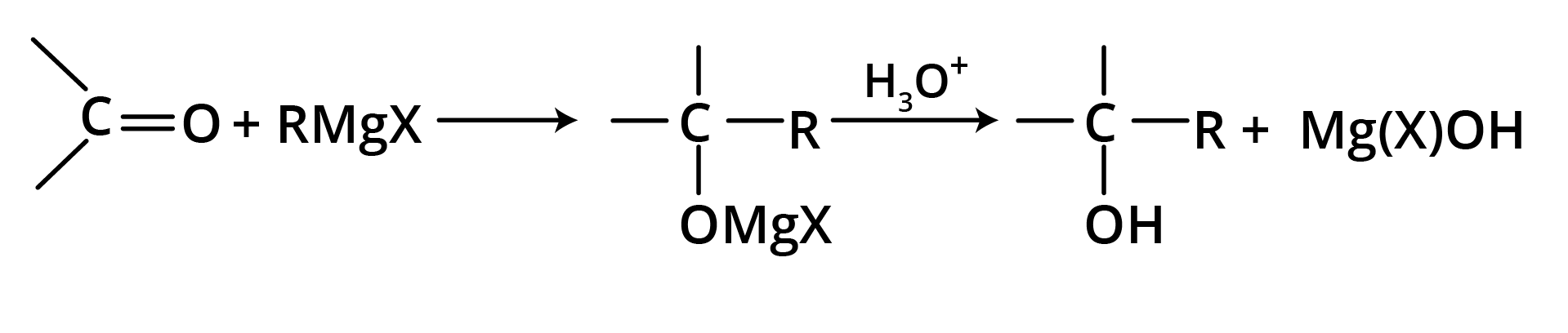
(a) Addition of Grignard Reagent
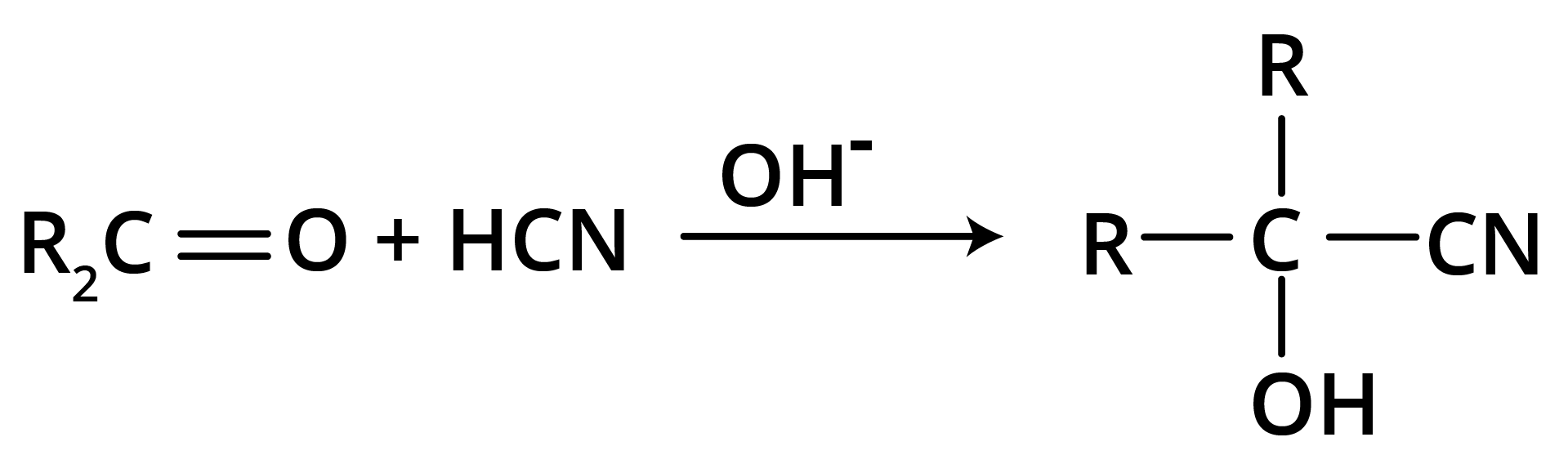
(b) Addition of Hydrogen Cyanide
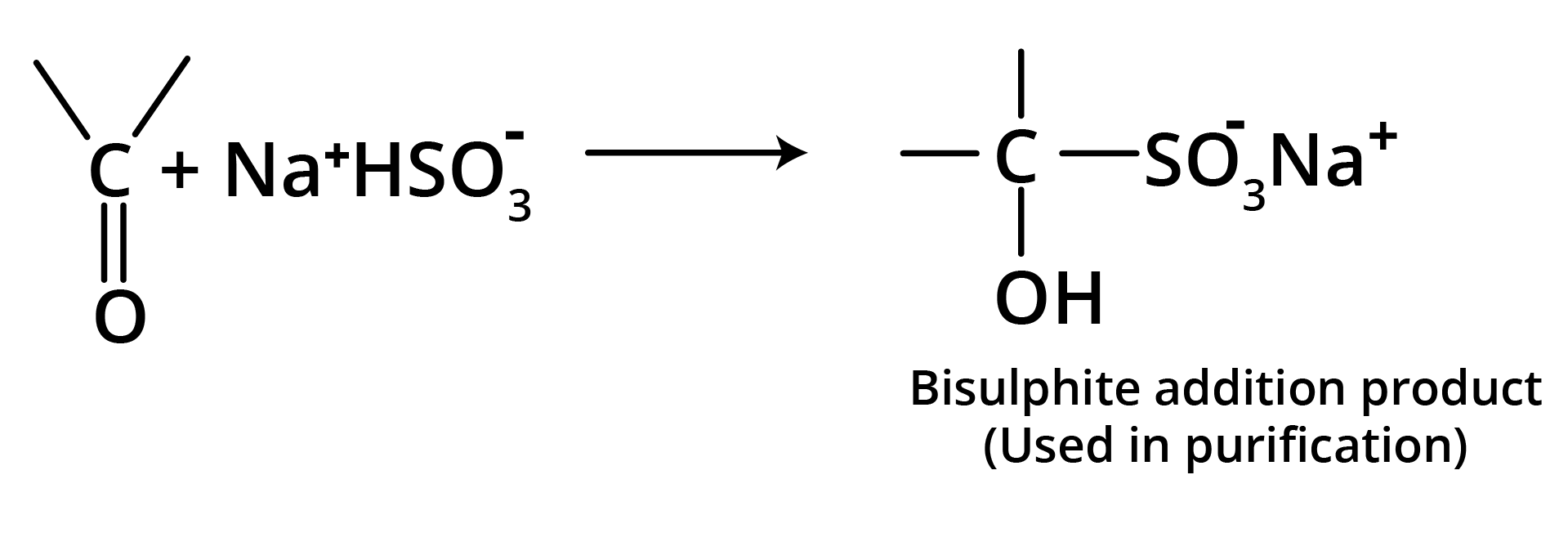
(c) Addition of Sodium Bisulfite
Bisulfite compound formation is confined to aldehydes, methyl ketones, and some cyclic ketones.
(d) Nucleophilic Addition of Derivatives of Ammonia
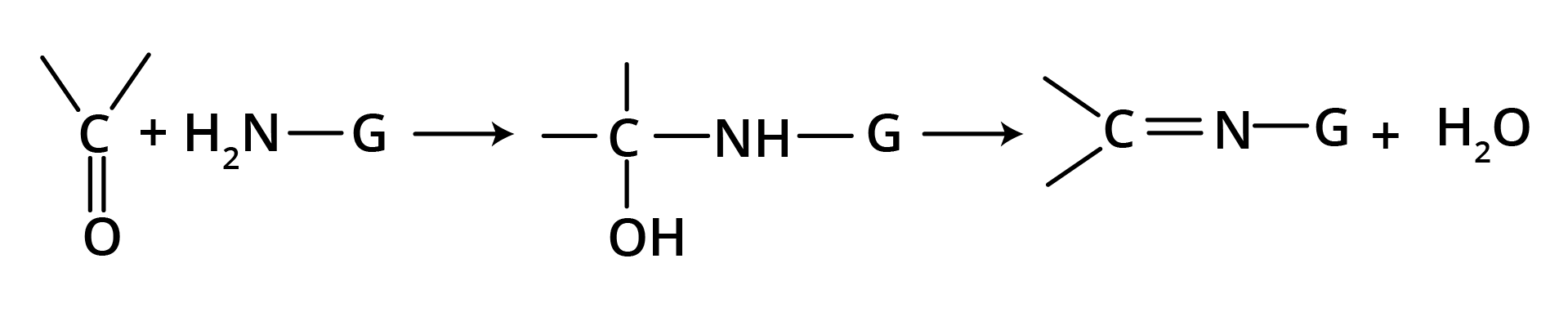
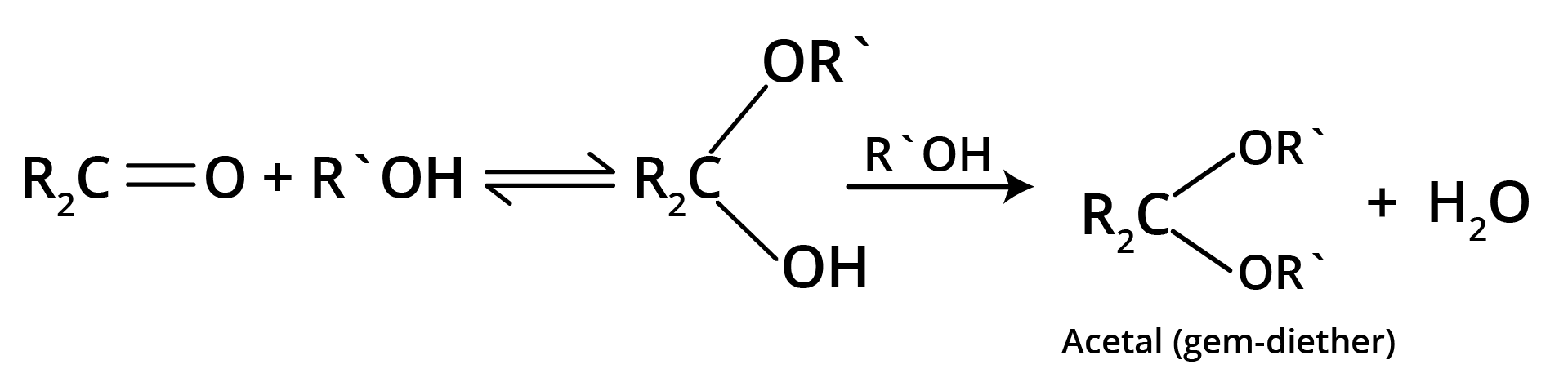
4. Addition of Alcohols
The carbonyl compounds react with alcohols, R'OH, to yield hemiacetals.
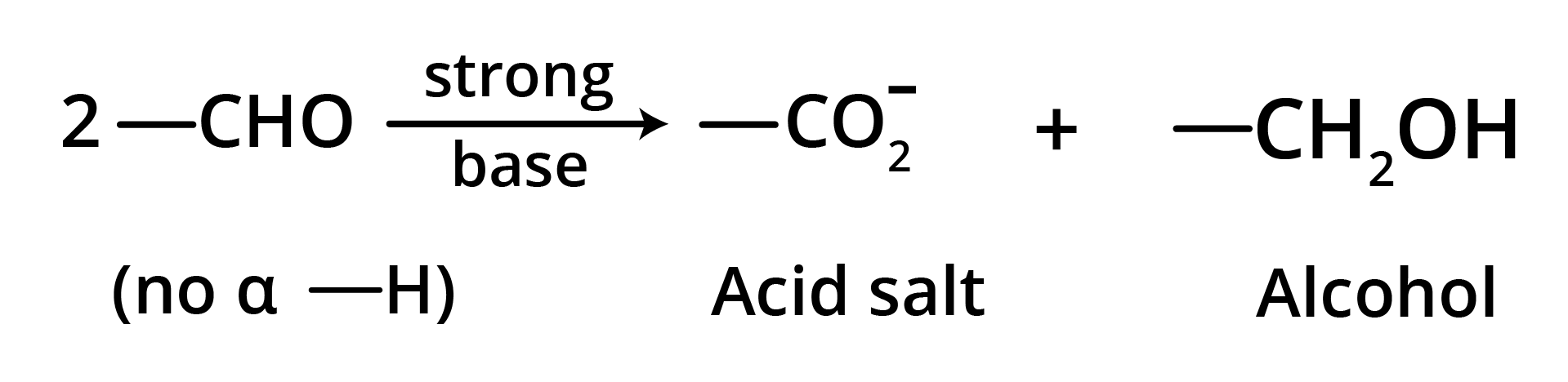
5. Cannizzaro Reaction
6. Aldol Condensation
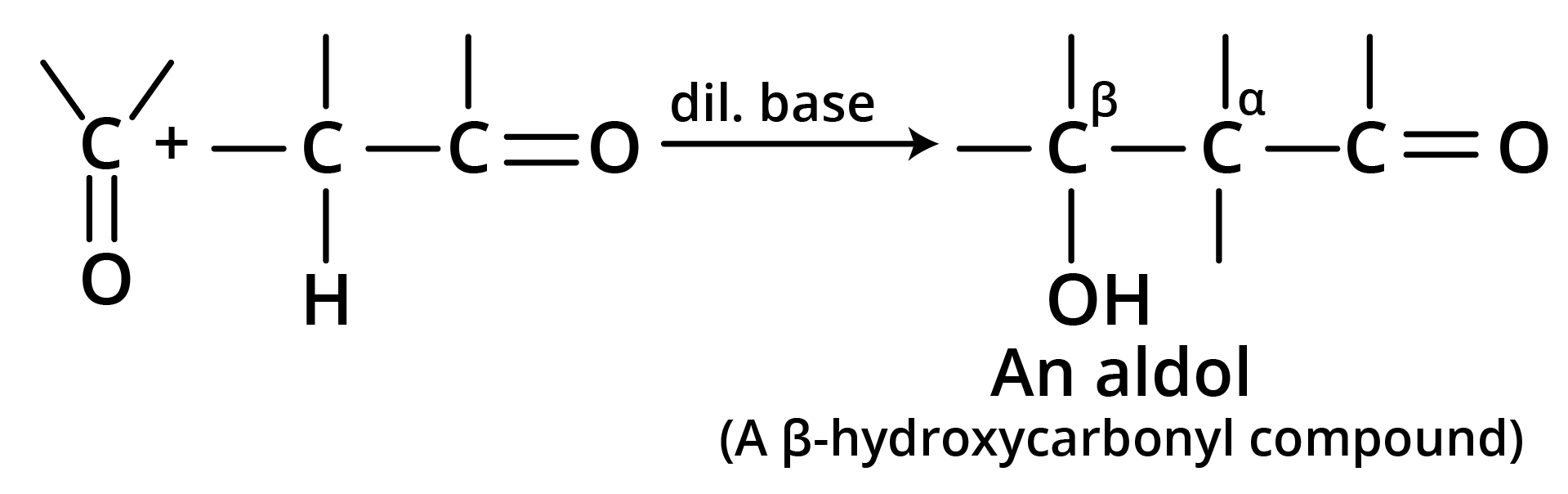
7. Perkin Condensation
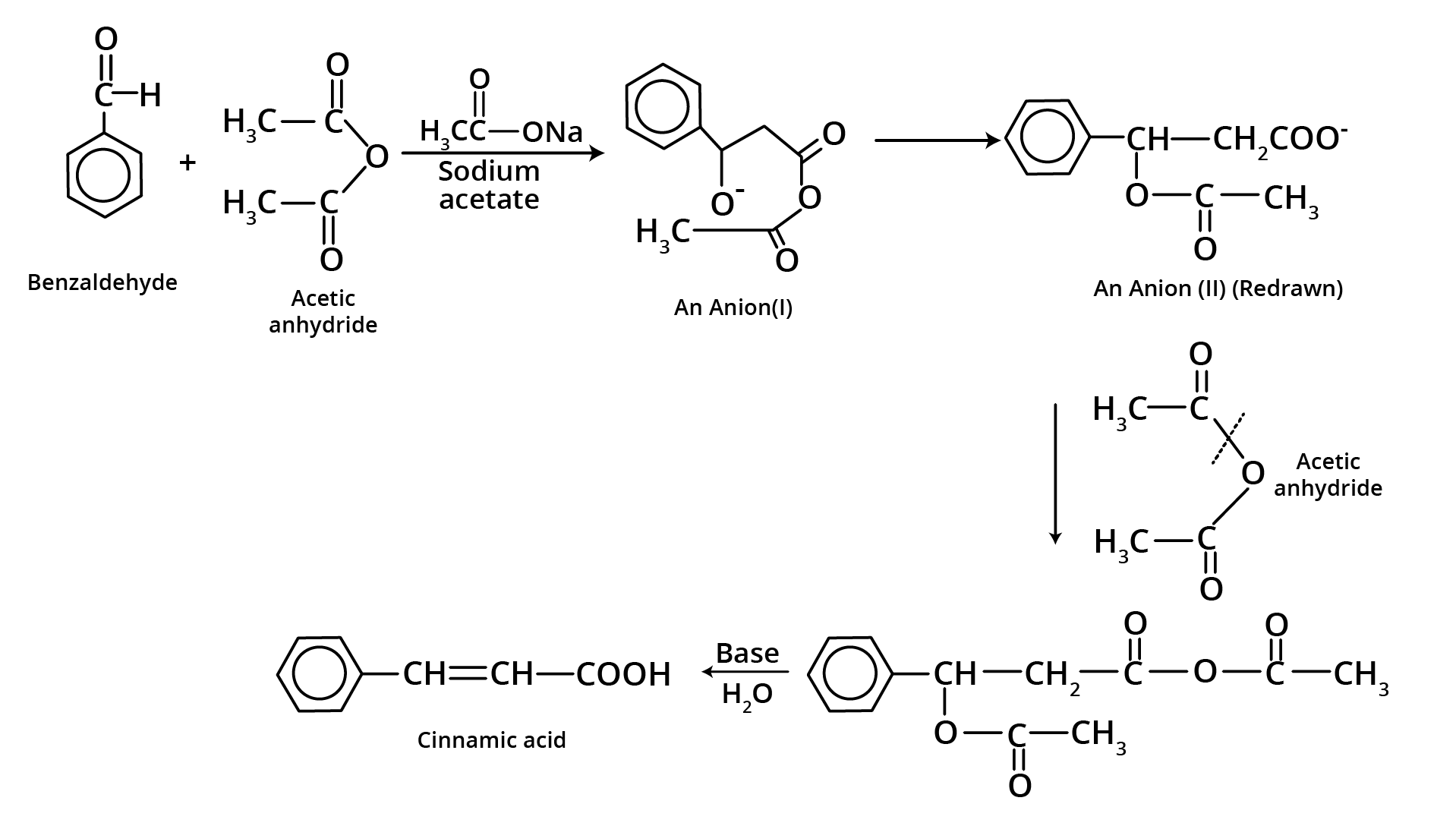
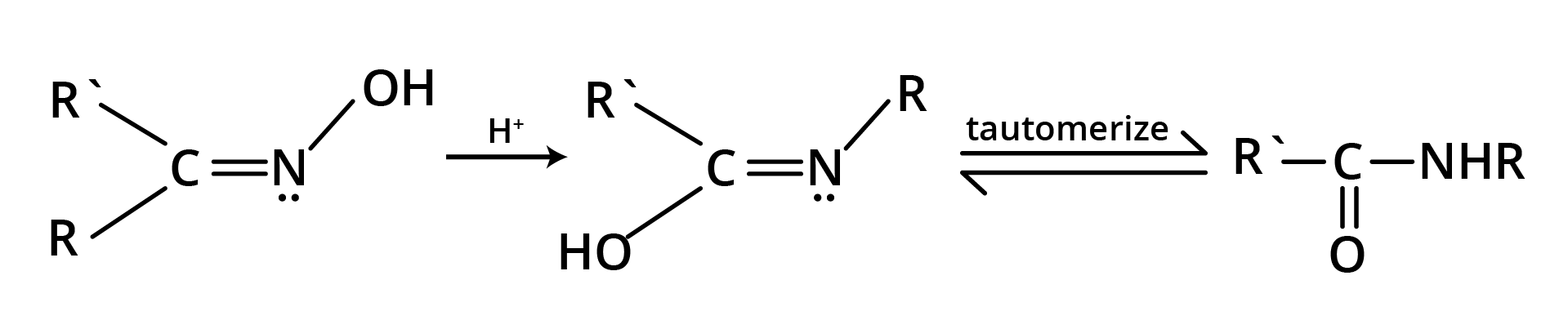
8. Beckmann Rearrangement
The acid catalysed conversion of ketoximes to N substituted amides is known as Beckmann rearrangement.
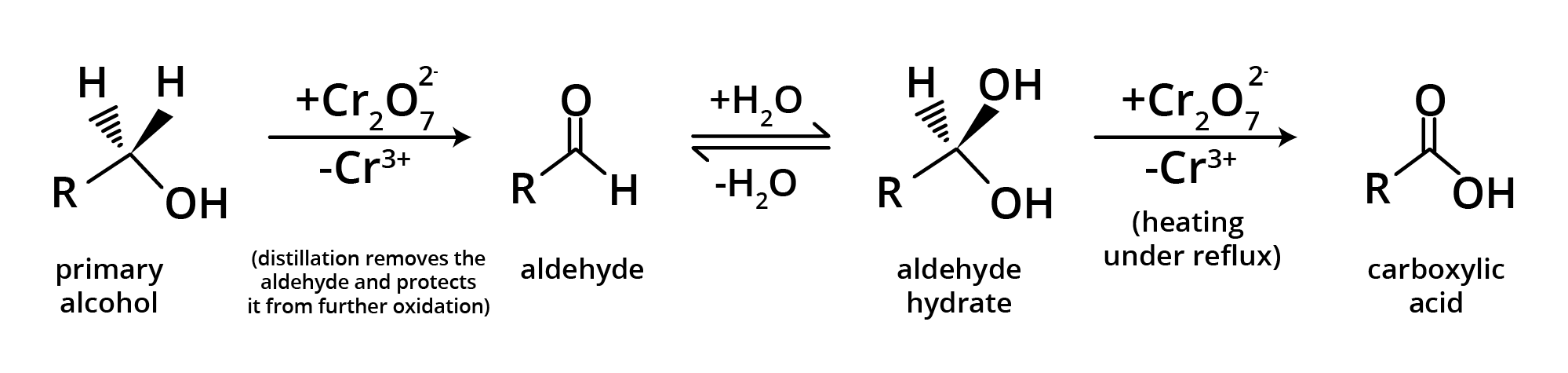
Methods of Preparation of Carboxylic Acids
1. By Oxidation of Primary Alcohol
2. By Hydrolysis of Cyanides
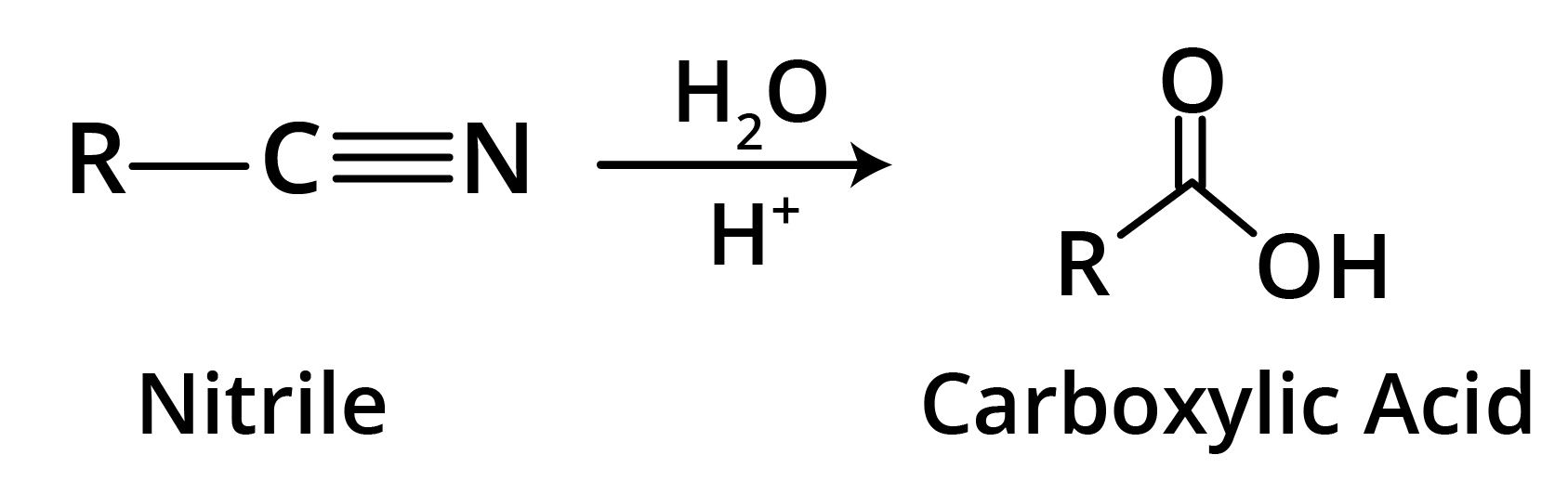
General Physical Properties of the Carboxylic Acids
The molecules of carboxylic acids are polar and exhibit hydrogen bonding.
The first four members are miscible with water. The higher acids are virtually insoluble. The simplest aromatic acid, benzoic acid, contains too many carbon atoms to show appreciable solubility in water.
General Chemical Properties of the Carboxylic Acids
The carboxylic acids react with metal to liberate hydrogen and are soluble in NaOH and NaHCO3 solutions.
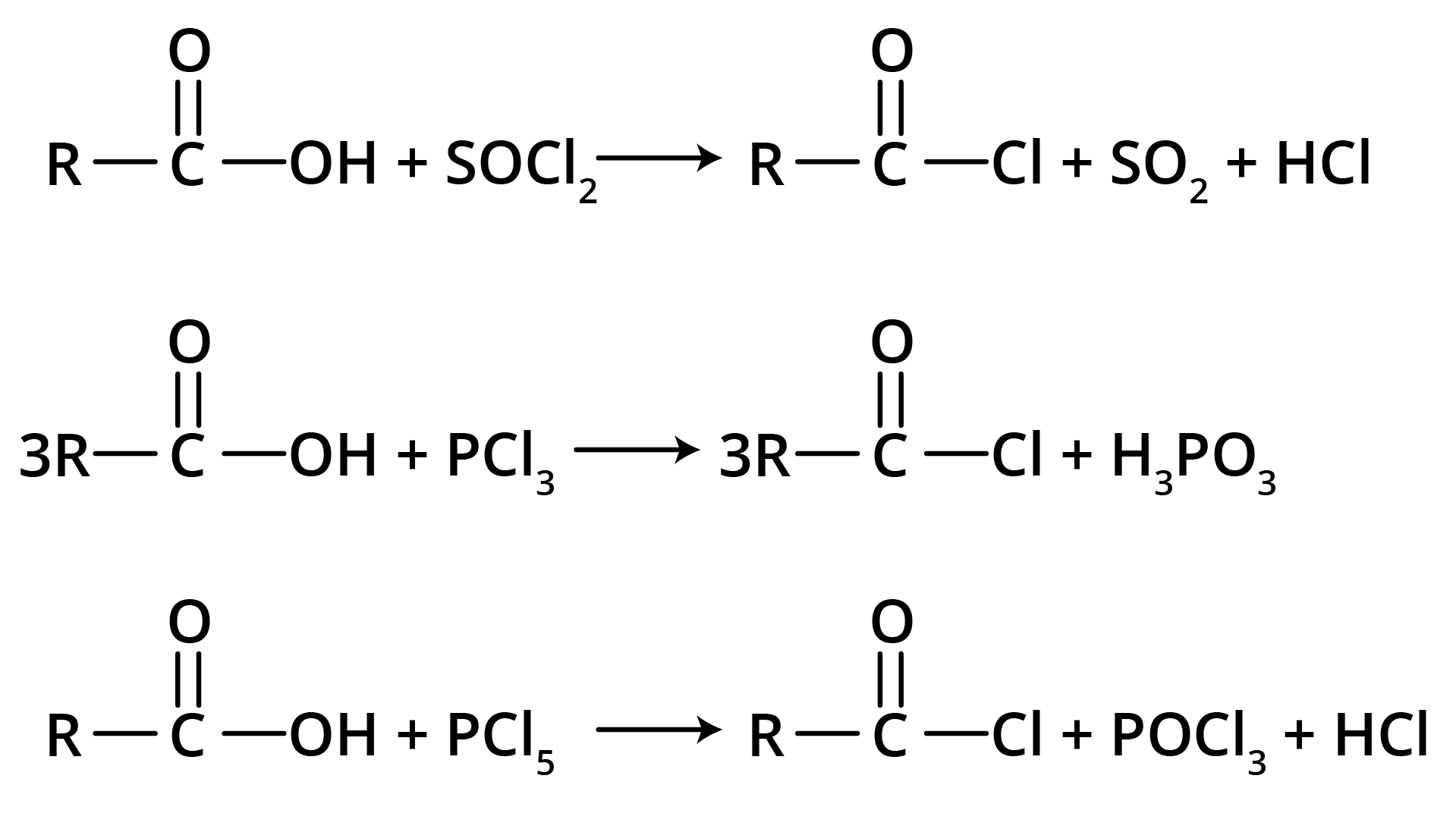
Carboxylic acid reacts with halogenated compounds and forms acid halide.
Carboxylic acid gets reduced to alcohols.
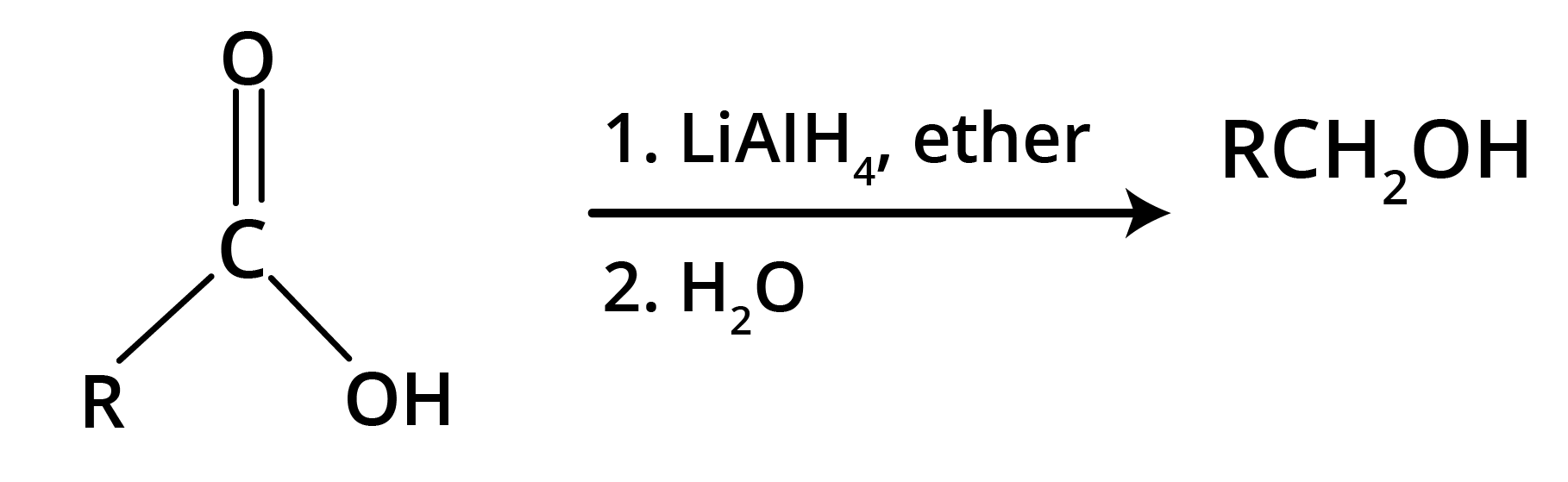
Hell Volhard Zelinsky Reaction: Aliphatic carboxylic acids react with Br2 or Cl2 in presence of phosphorus (or phosphorus halides) to give haloacids.
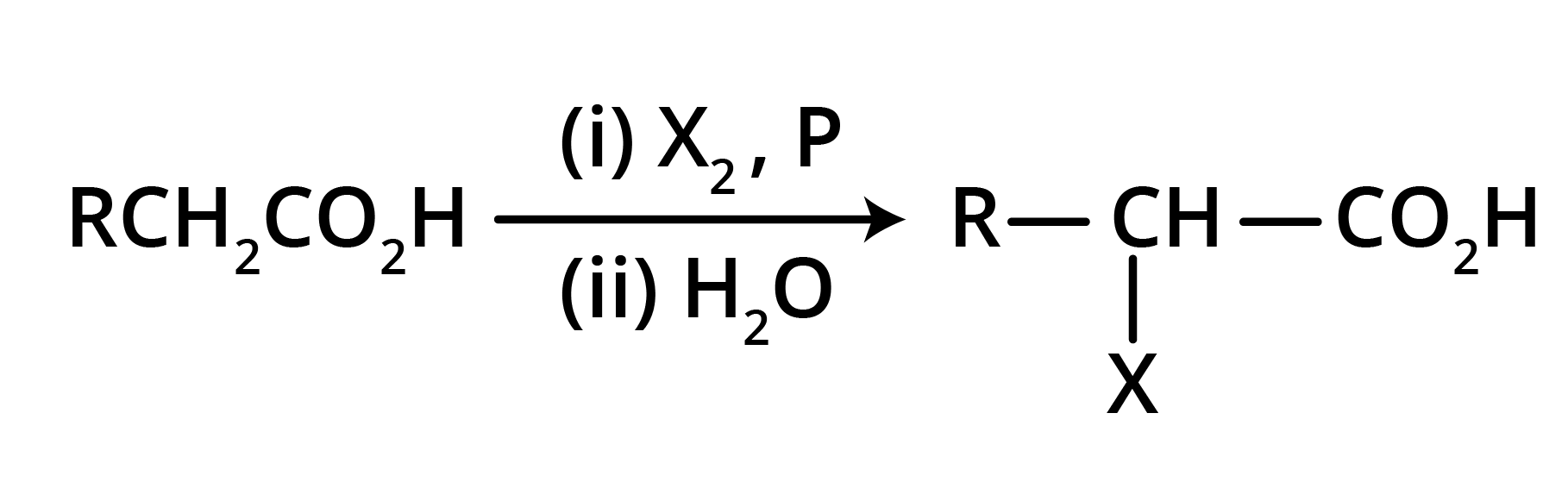
Schmidt Reaction: Carboxylic acids react with hydrazoic acid in presence of concentrated H2SO4 to give amine.
Benzoic acid is attacked by the usual electrophilic reagents Chlorine, Bromine, Nitric and sulphuric acids to give m-derivatives.
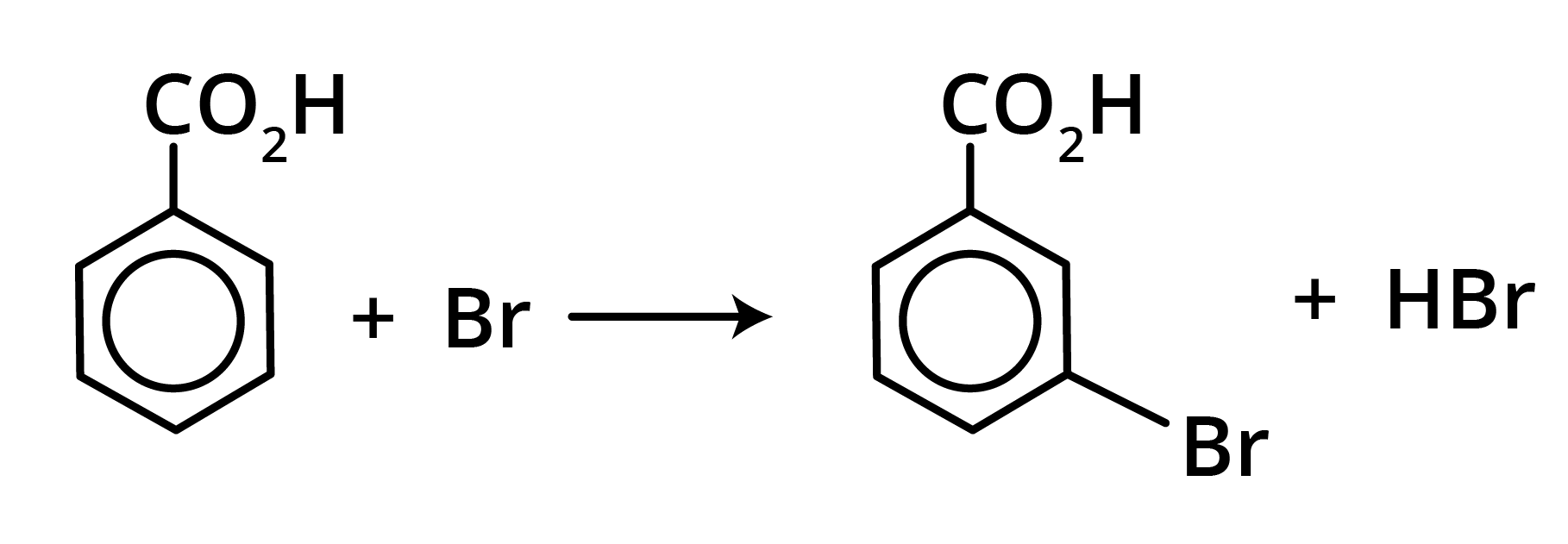
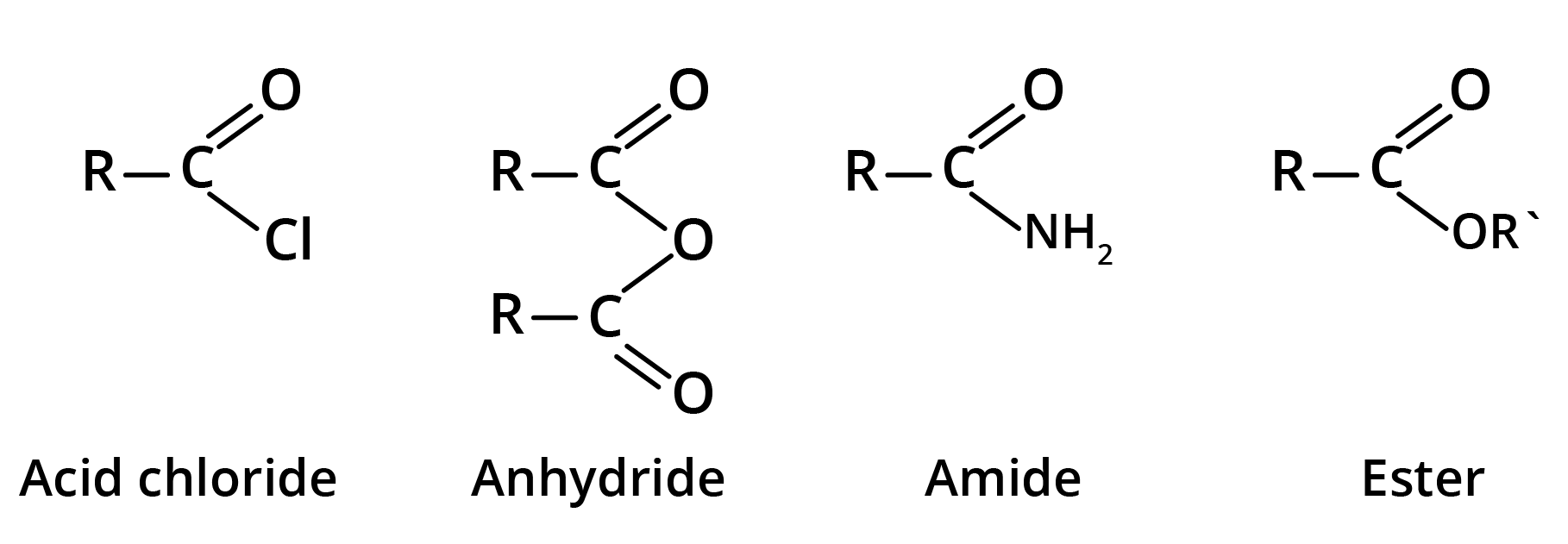
Derivatives of Carboxylic Acids
Acid derivatives are the compounds in which OH group of carboxyl group has been replaced by Cl, COR, NH2, or OR'.
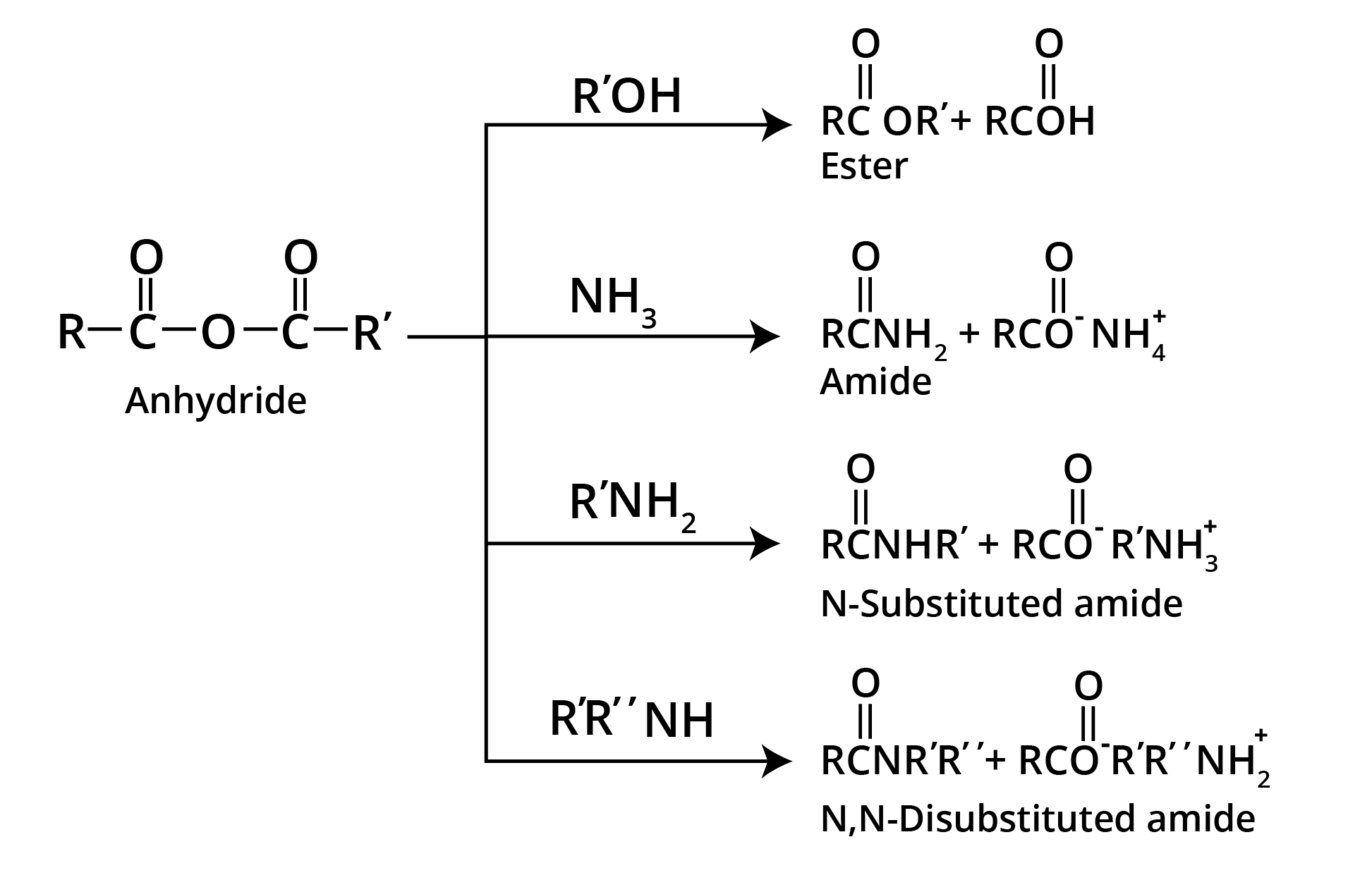
Reactions of Acyl Chloride

Reactions of Carboxylic Acid Anhydrides
Carboxylic Acids - Acidic Strength and Factors Affecting It
Carboxylic acids are a class of organic compounds that contain the carboxyl functional group (-COOH). They are known for their acidic properties due to the presence of the hydrogen atom attached to the oxygen atom of the hydroxyl group (-OH) within the carboxyl group. The acidic strength of carboxylic acids can vary, and it is influenced by several factors:
Electronegativity of the Atoms: The electronegativity of the atoms directly involved in the acidic hydrogen's bonding plays a crucial role. The greater the electronegativity of the atoms, the more acidic the compound. For example, in a series of carboxylic acids, the one with a more electronegative atom in the carboxyl group will be stronger. For instance, acetic acid ($CH_3COOH$) is less acidic than chloroacetic acid ($ClCH_2COOH$).
Resonance Stabilization: Carboxylic acids can exhibit resonance stabilization due to the delocalization of electrons in the carboxyl group. This resonance results in the dispersal of negative charge and enhances the stability of the conjugate base (carboxylate ion). The more stable the conjugate base, the stronger the acid. For example, benzoic acid ($C_6H_5COOH$) is stronger than acetic acid.
Inductive Effect: The inductive effect involves the polarization of sigma (σ) bonds, leading to the withdrawal or donation of electron density. In carboxylic acids, electronegative substituents that pull electron density away from the carboxyl group enhance acidity, while electron-donating groups decrease acidity. For example, chloroacetic acid is more acidic than methoxyacetic acid.
Substituent Effect: Substituents on the carbon adjacent to the carboxyl group can influence acidity. Electron-withdrawing groups like halogens increase acidity, while electron-donating groups decrease it. For example, trichloroacetic acid ($CCl_3COOH$) is stronger than acetic acid.
Hydrogen Bonding: The ability to form hydrogen bonds can enhance acidity. Carboxylic acids are capable of forming intermolecular hydrogen bonds due to the presence of the hydroxyl group. The stronger the hydrogen bonding, the more acidic the compound. Formic acid (HCOOH) and acetic acid ($CH_3COOH$) exhibit strong hydrogen bonding and are relatively strong acids.
Solvent Effects: The solvent in which the acid is dissolved can influence its acidity. In a highly polar solvent, acidic strength may increase because of enhanced ionization of the carboxylic acid.
Concentration: The concentration of the carboxylic acid in a solution affects its acidity. Higher concentrations typically result in stronger acidity.
Temperature: Temperature can influence the ionization of carboxylic acids. Higher temperatures can promote ionization and, therefore, increase acidity.
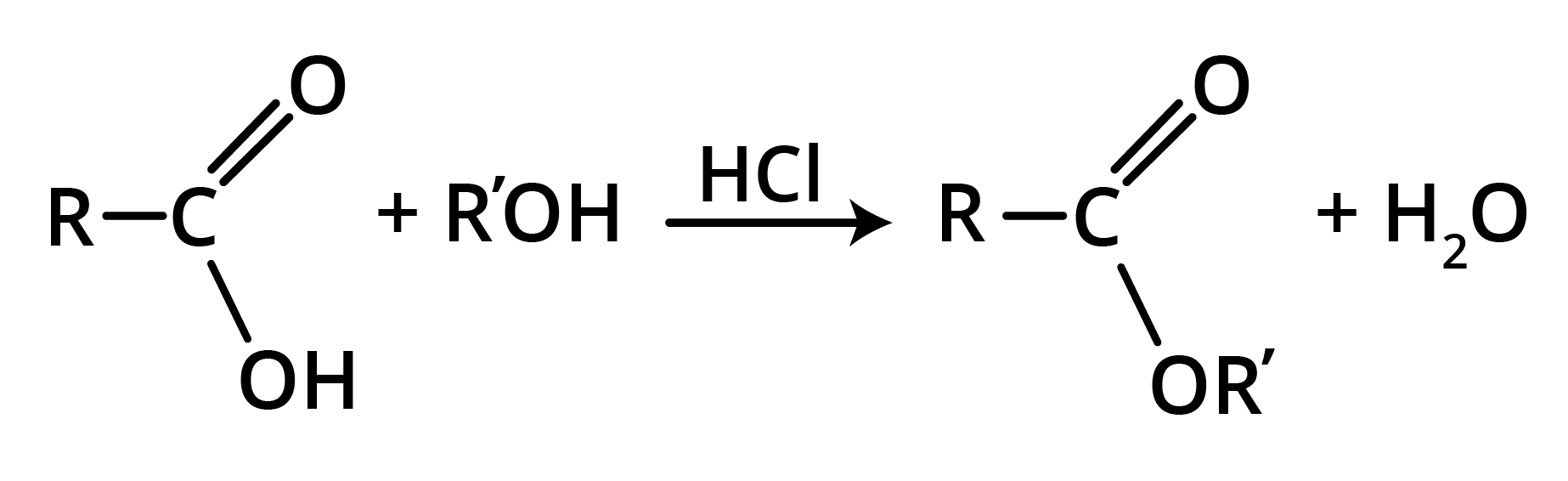
Preparation of Esters
1. Esters from Acyl Chlorides
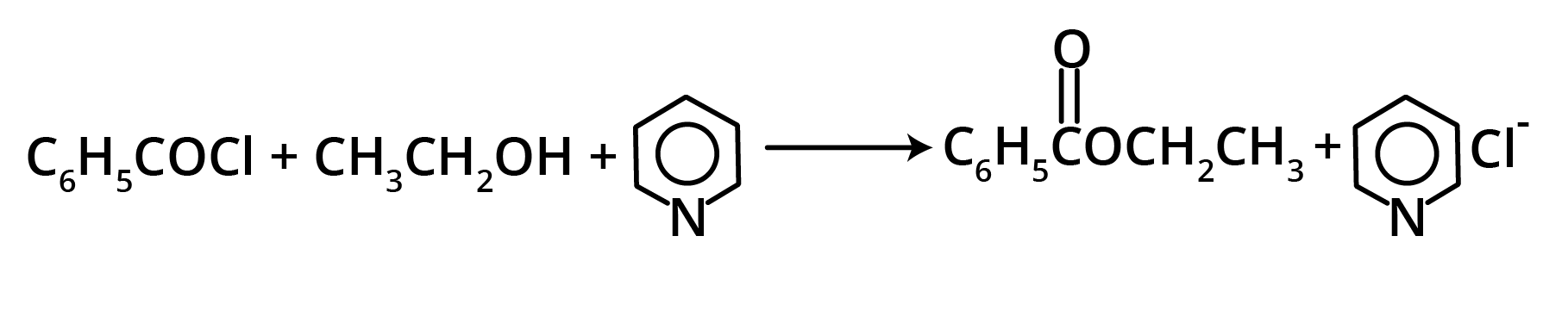
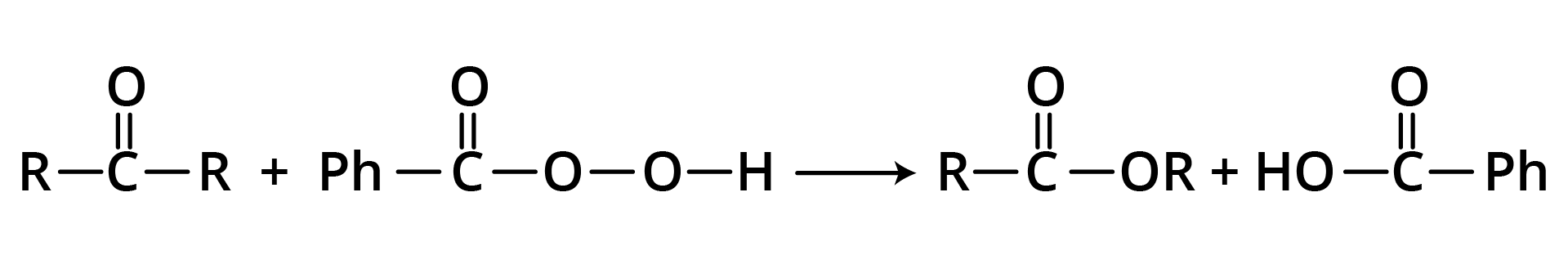
2. From ketones (Baeyer-Villiger Oxidation)
Reactions of Esters
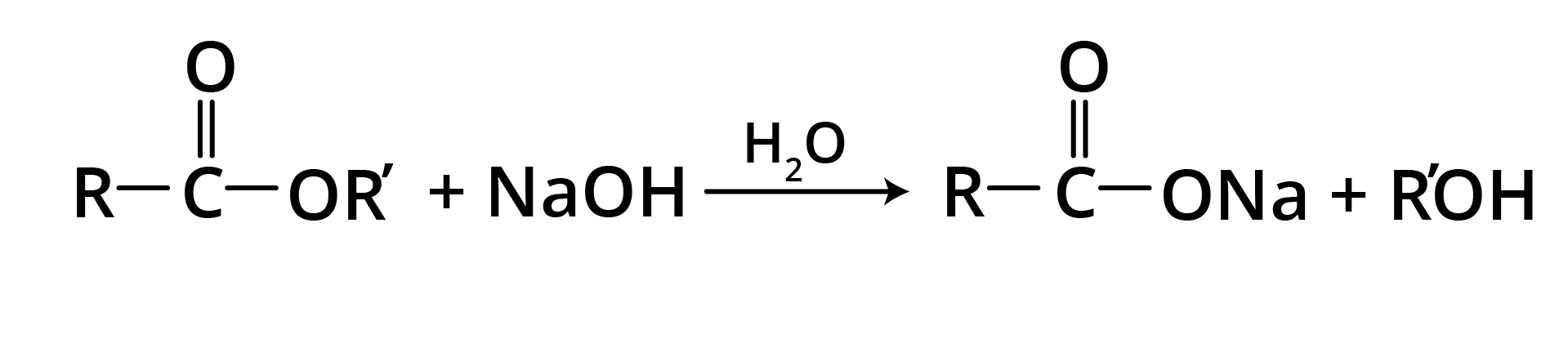
1. Base Promoted Hydrolysis of Esters: Saponification
The carboxylate ion is very unreactive towards nucleophilic substitution because it is negatively charged, hence the reaction is essentially irreversible.
2. Reaction with Grignard Reagent
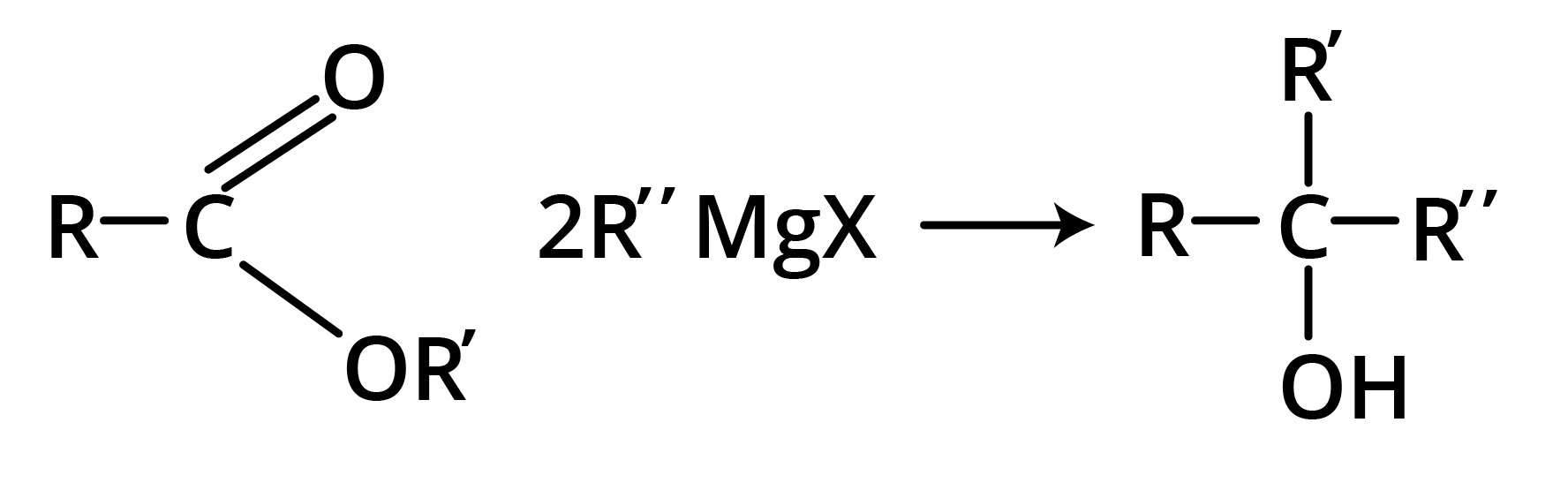
Preparation of Acid Amides
1. By Heating Ammonium Carboxylate
RCOONH4 → RCONH2 + H2O (on heating)
Reactions of Acid Amides
1. Hydrolysis
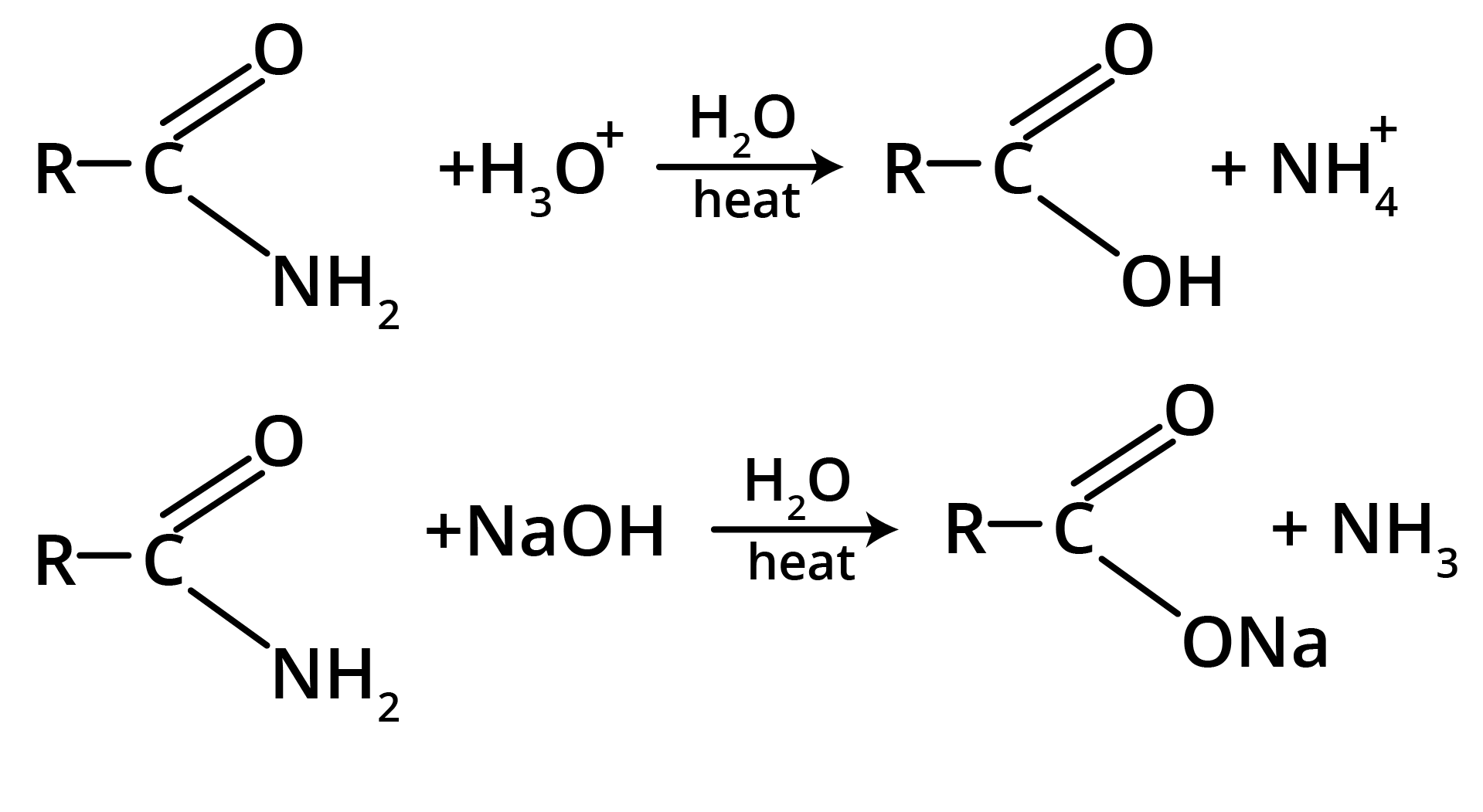
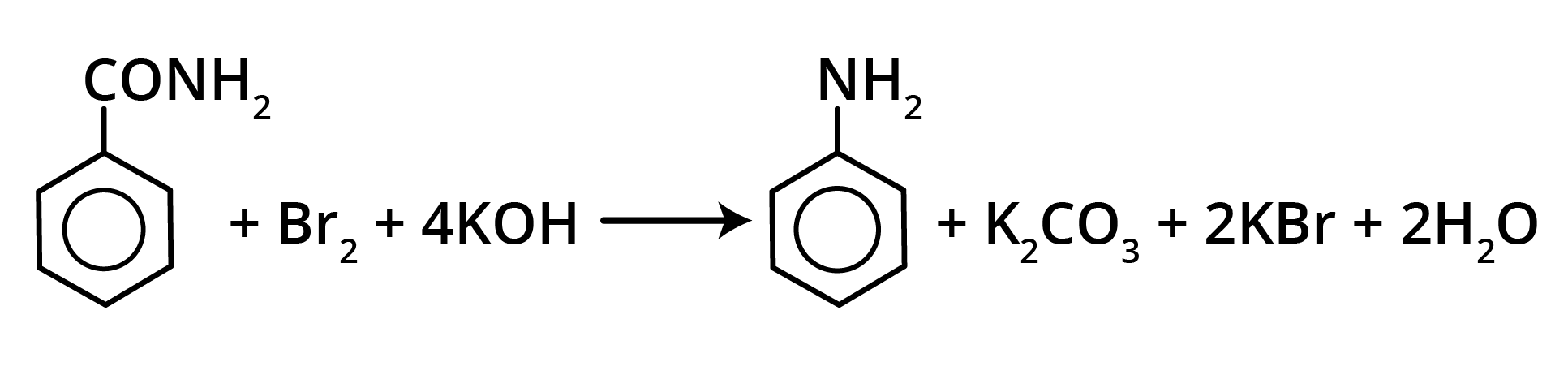
2. Hoffmann’s Bromamide Reaction
Amides (having a primary nitrogen atom) react with bromine in the presence of alkali to form a primary amine having one carbon atom less than the parent amide.
Solved Examples/Problems from the Chapter: Organic Compound Containing Oxygen
1. Give the structures and IUPAC names of the products expected from the given reaction: catalytic reduction of butanal.
Solution: The catalytic reduction of butanal takes in the presence of a reducing agent. This reaction of butanal will lead to the formation of butanol.
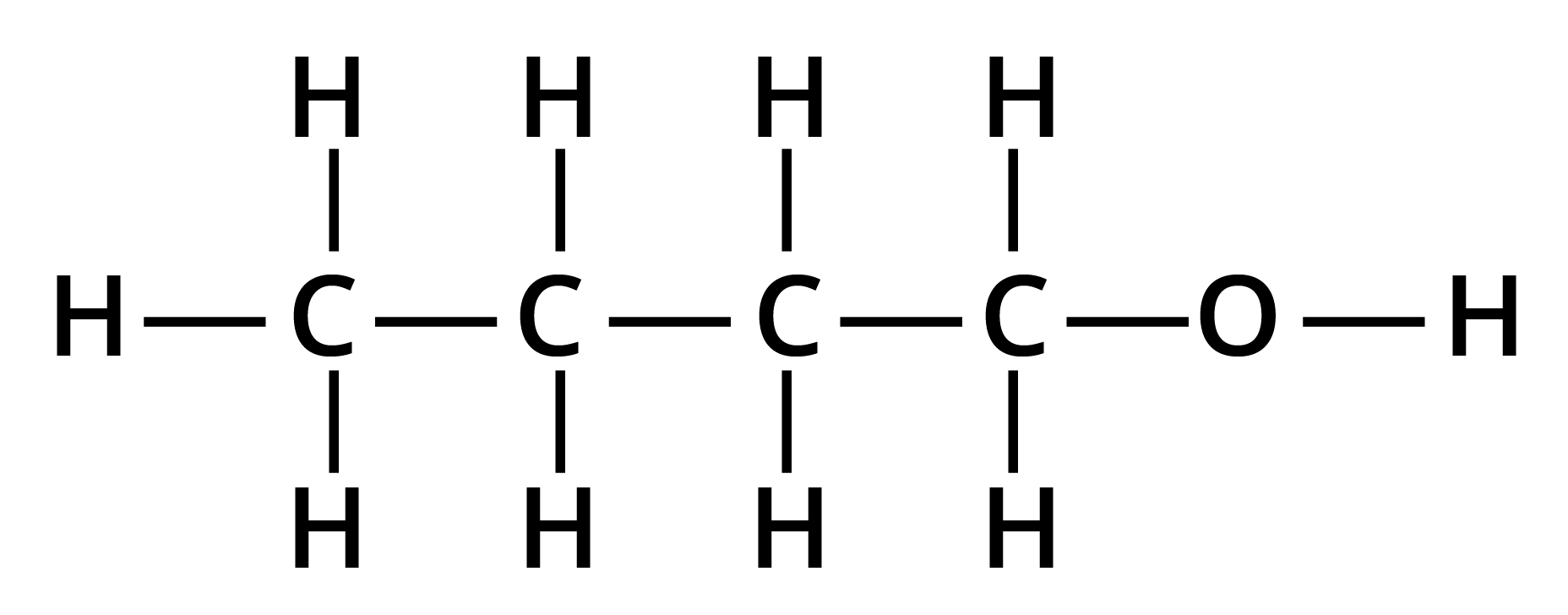
Key Points: Aldehyde is reduced to give alcohols.
2. Write structures of the products of the given reaction.
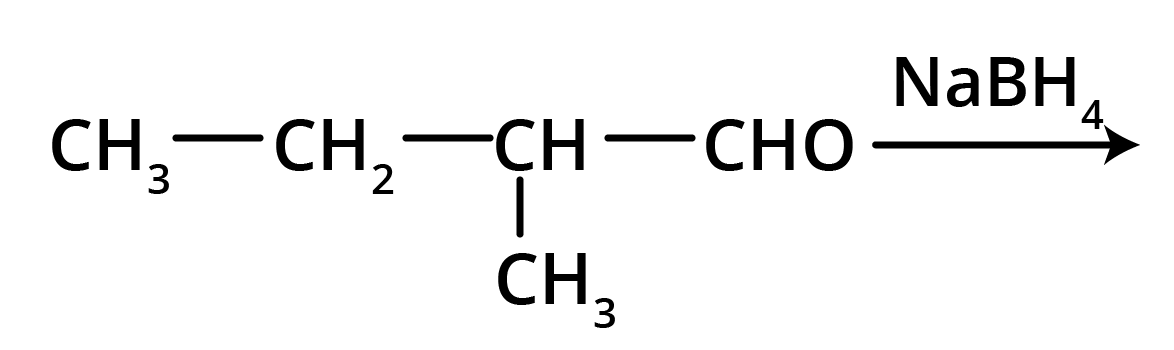
Solution: The product formed in the above reaction will be 2-Methylbutanol. The structure of this compound is given below.
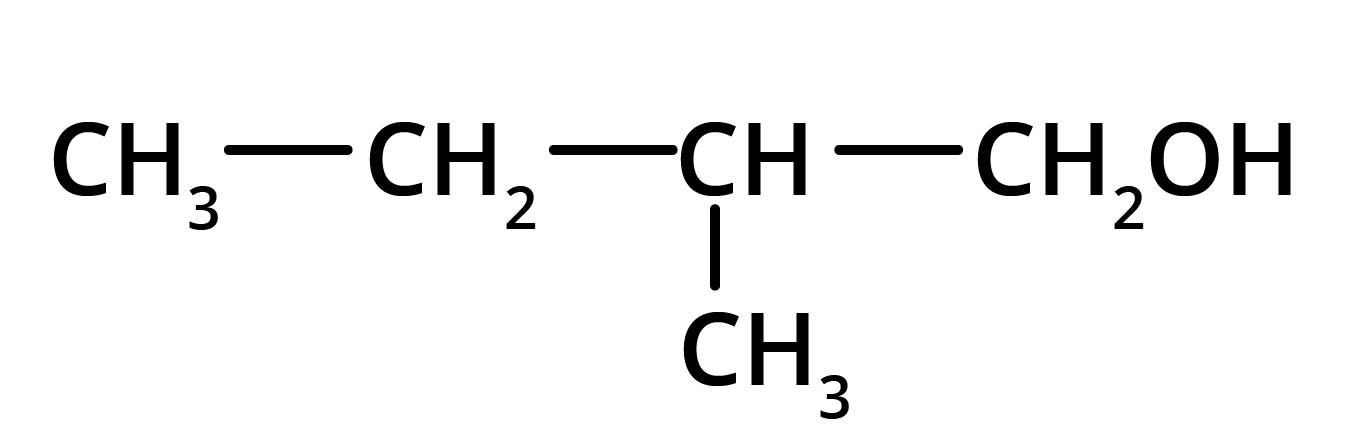
Key Points: Aldehyde group is reduced to alcohols. The chain with which the functional group is attached is considered as the parent chain.
Solved Problems of Previous Year Question from the Organic Compound Contains Oxygen
1.
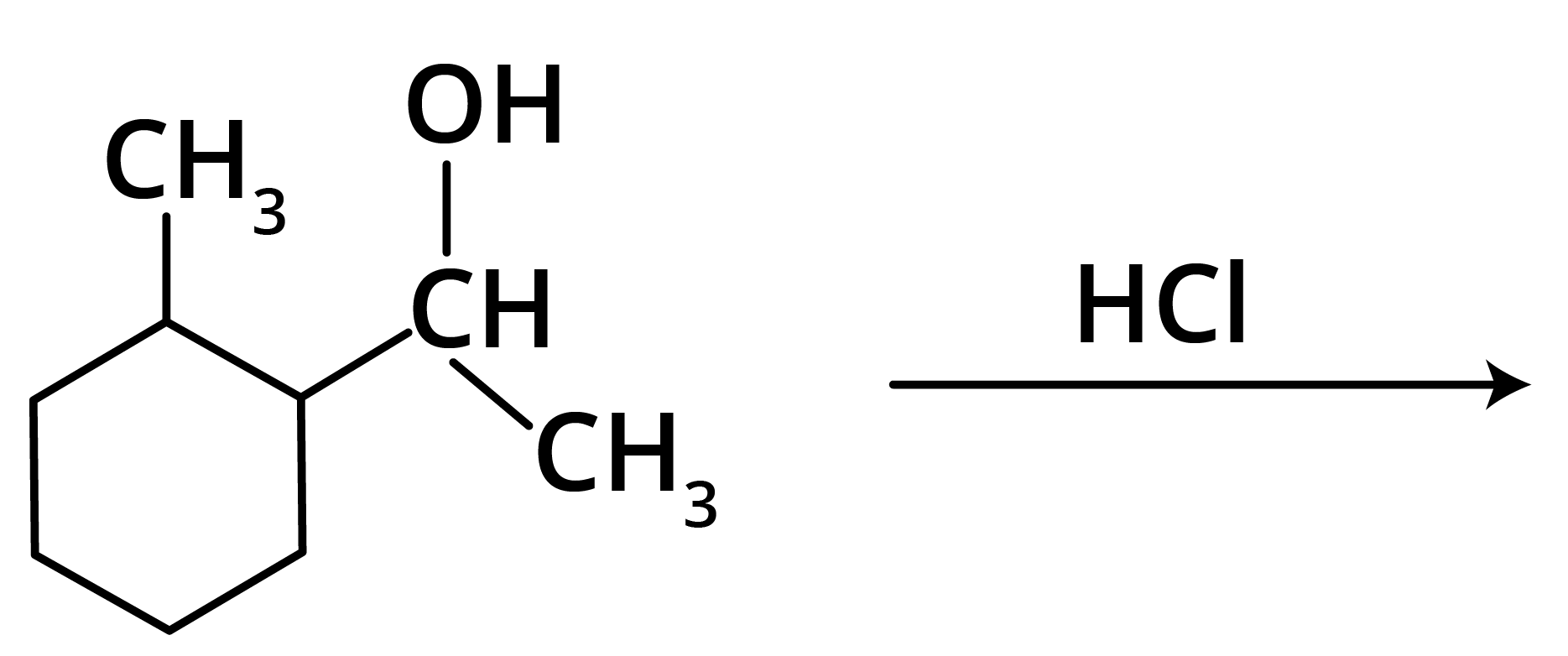
Options:
(a)
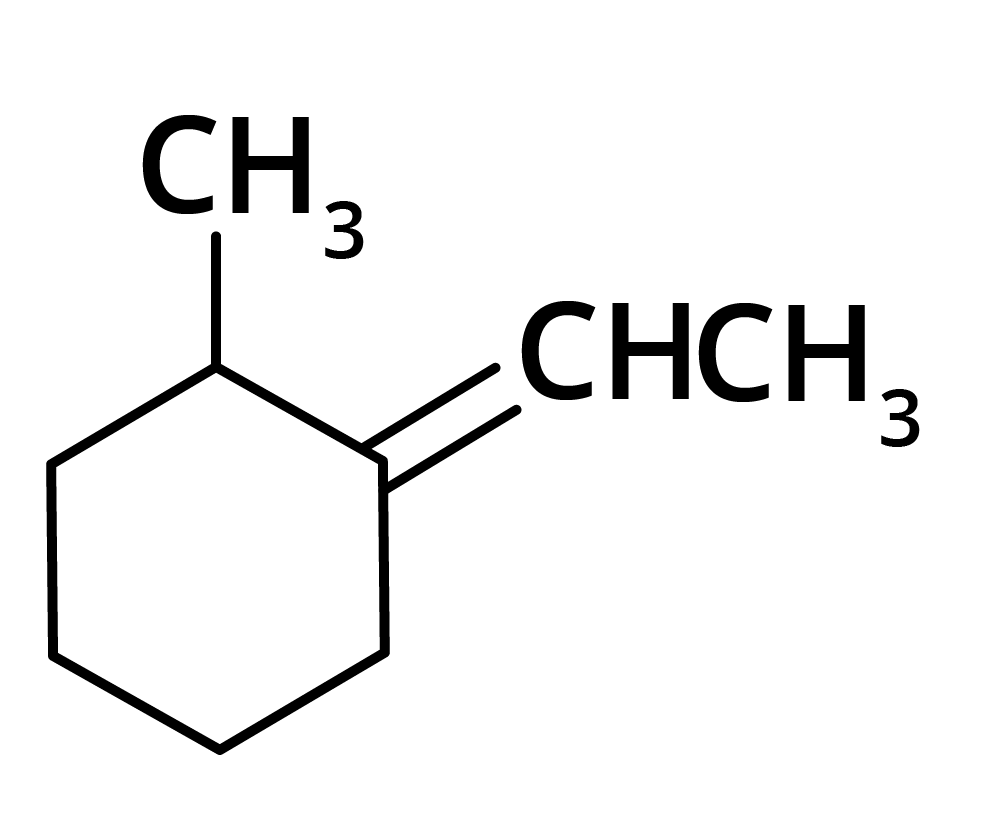
(b)
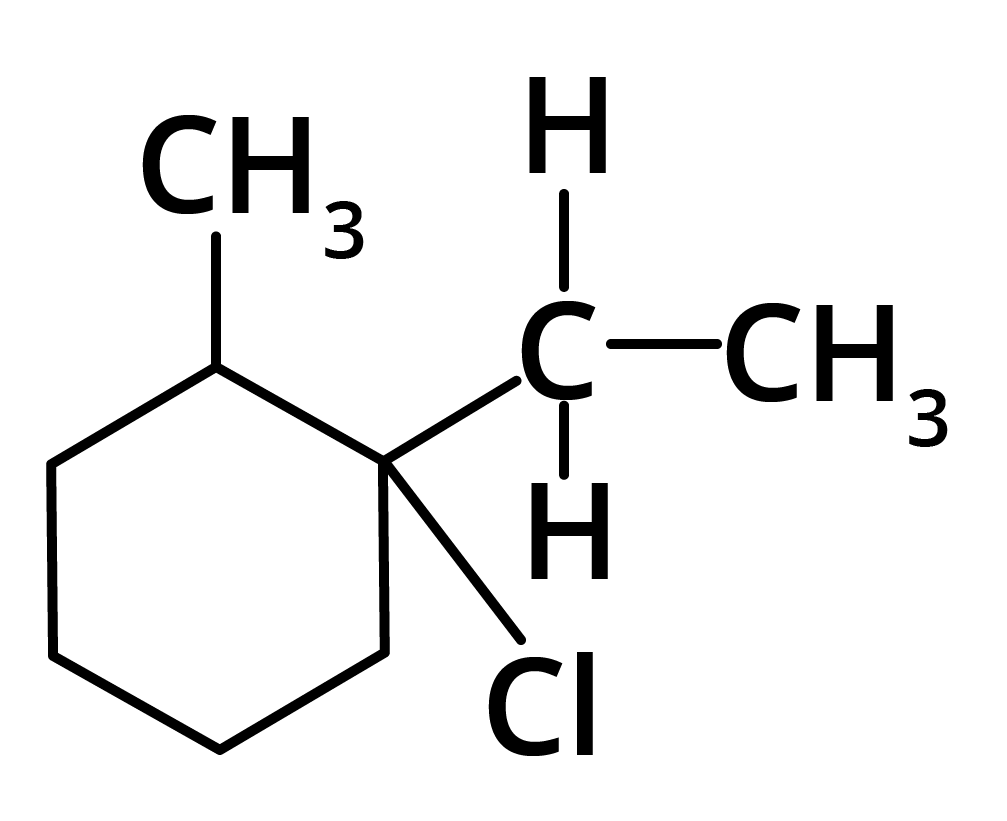
(c)
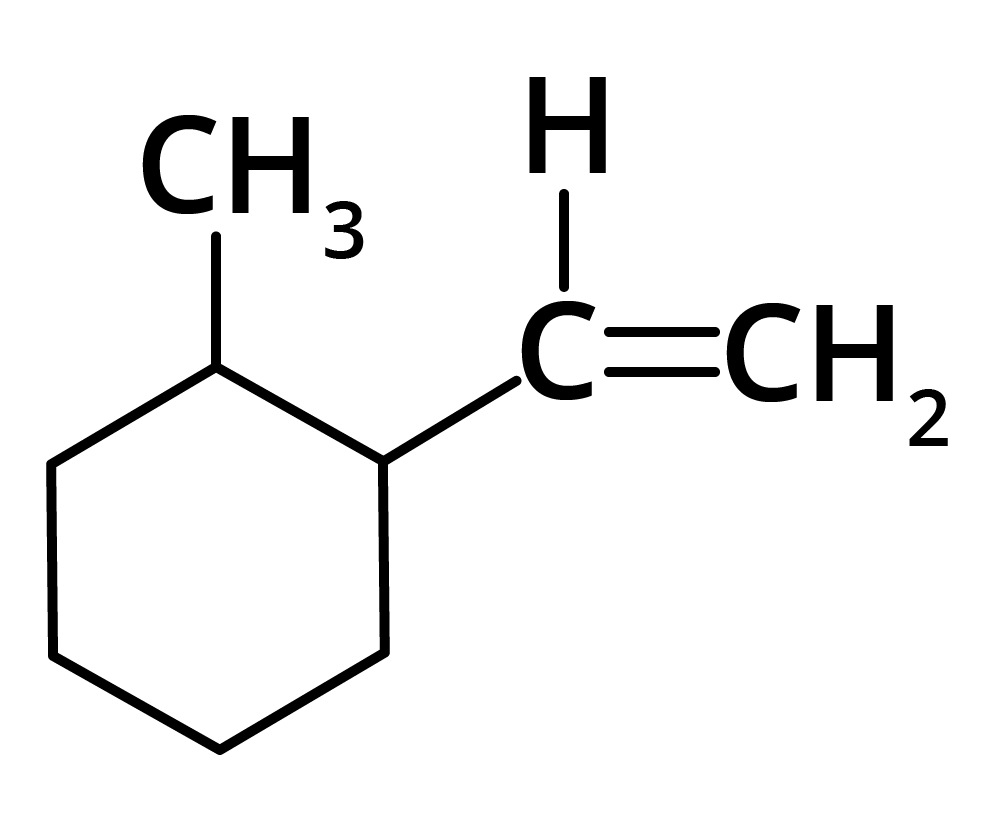
(d)
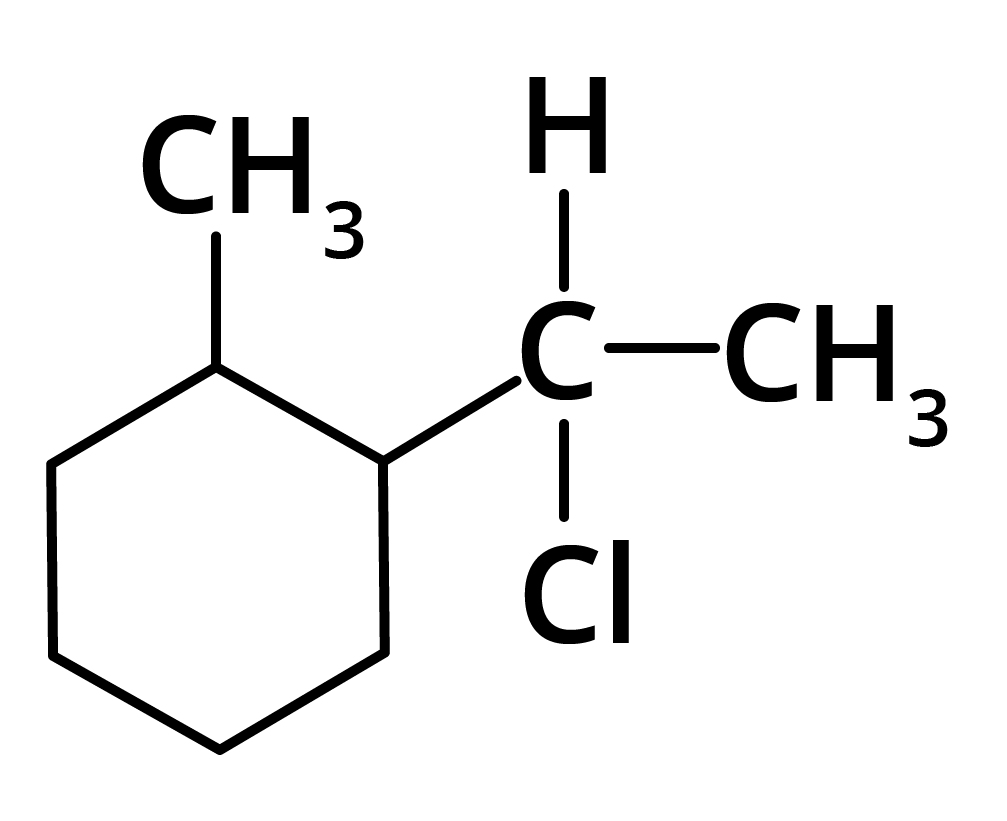
Ans: The correct answer is option b.
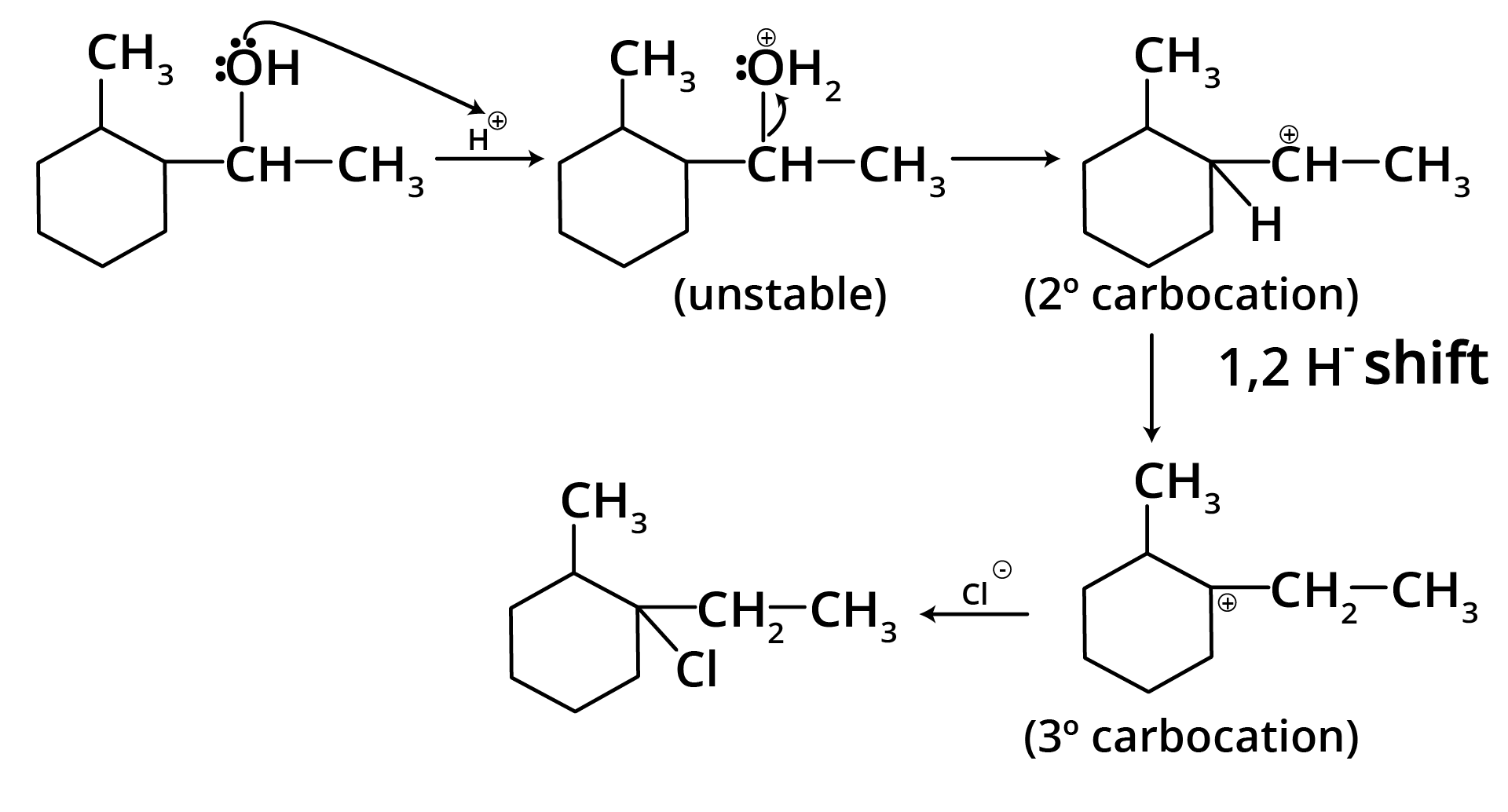
Trick: The given reaction is dependent on the formation of stable carbocation.
2. Predominant product is
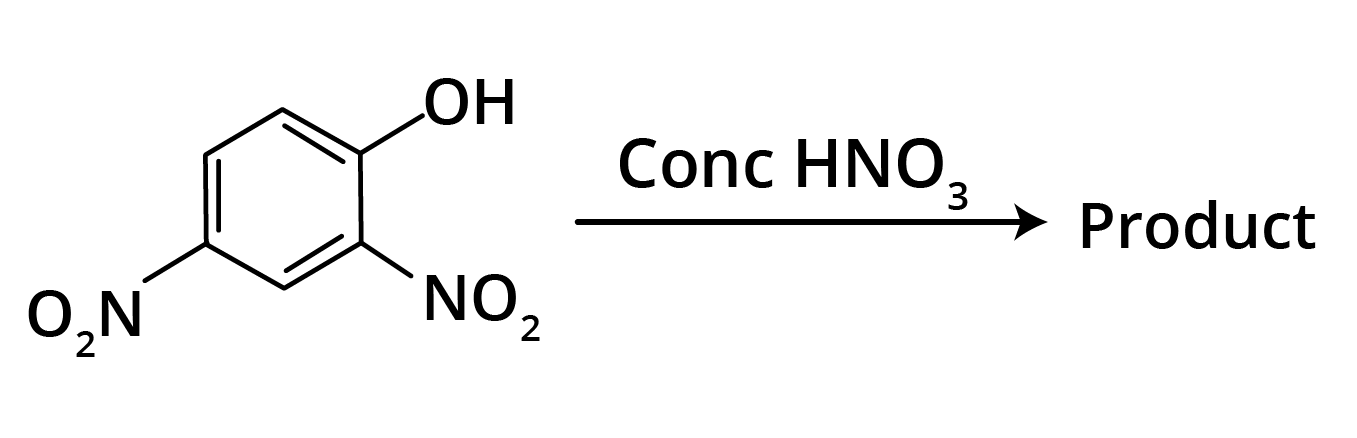
Options:
(a)
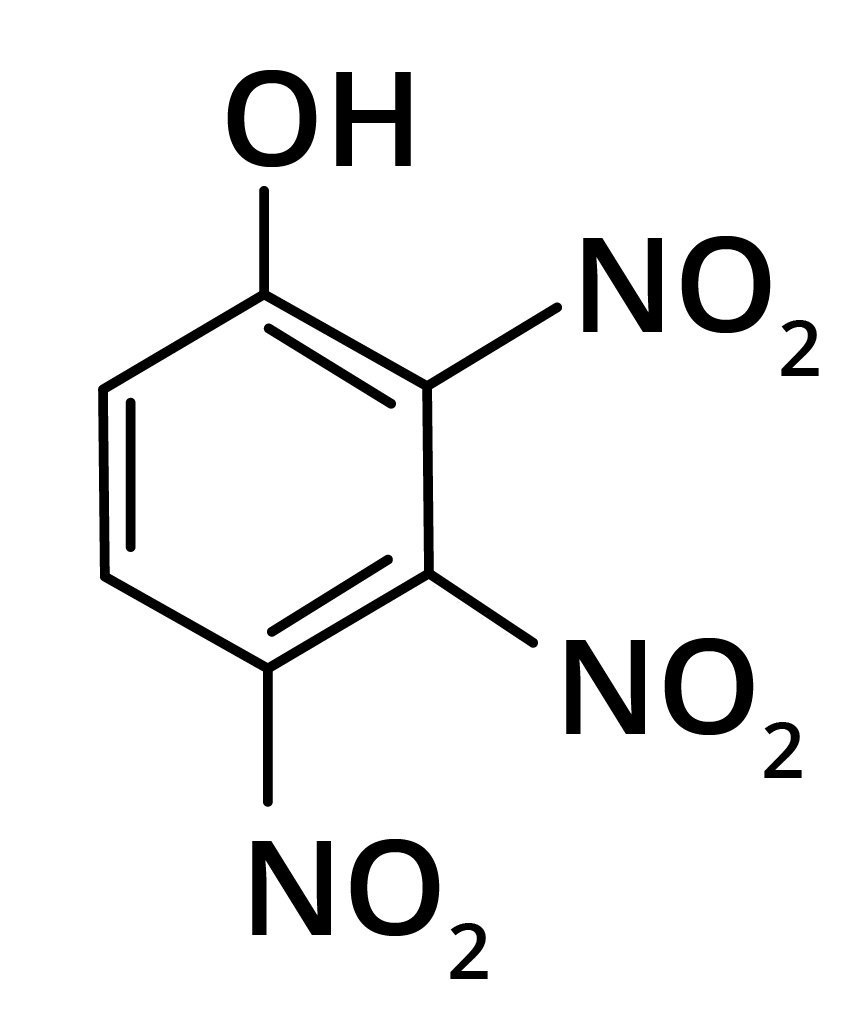
(b)
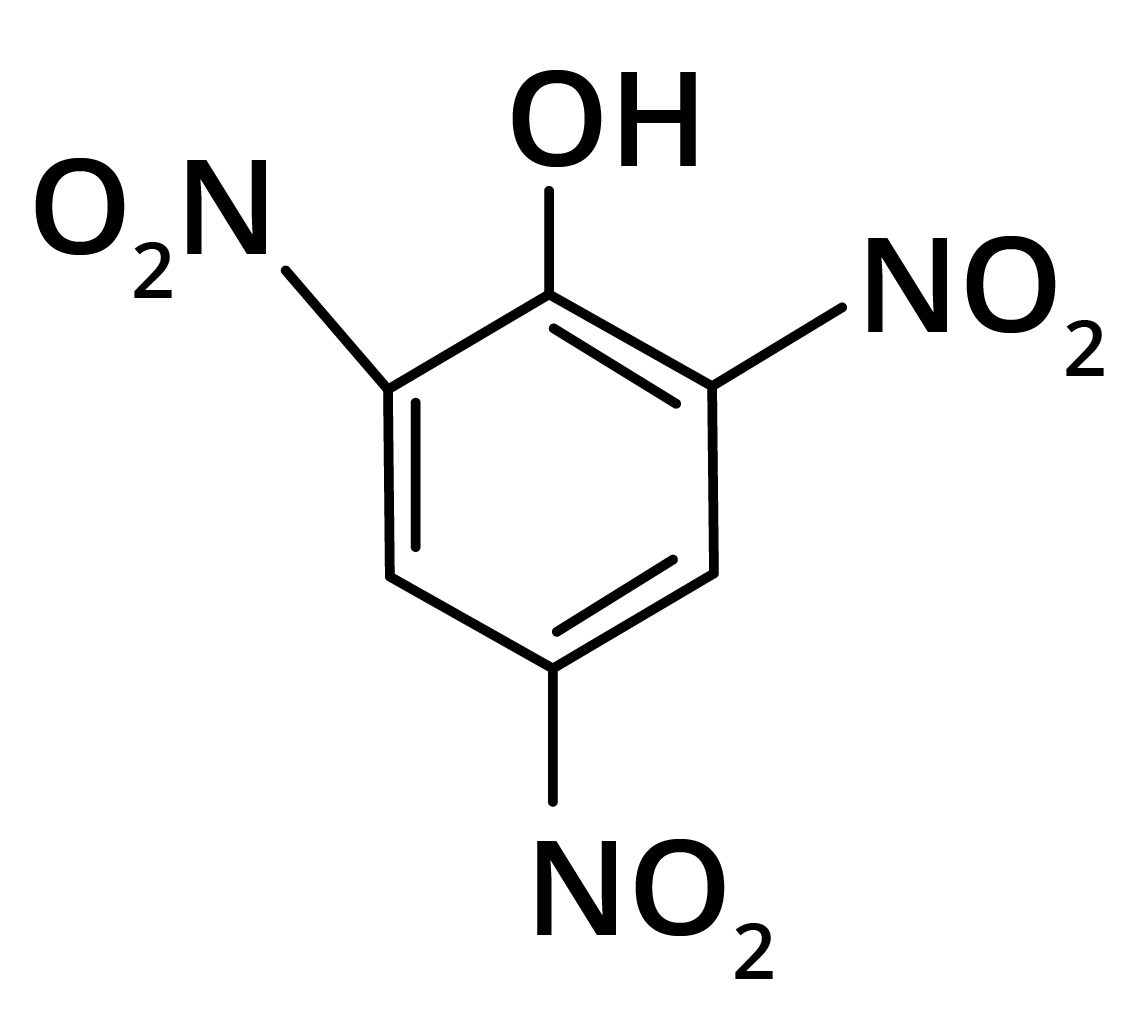
(c)
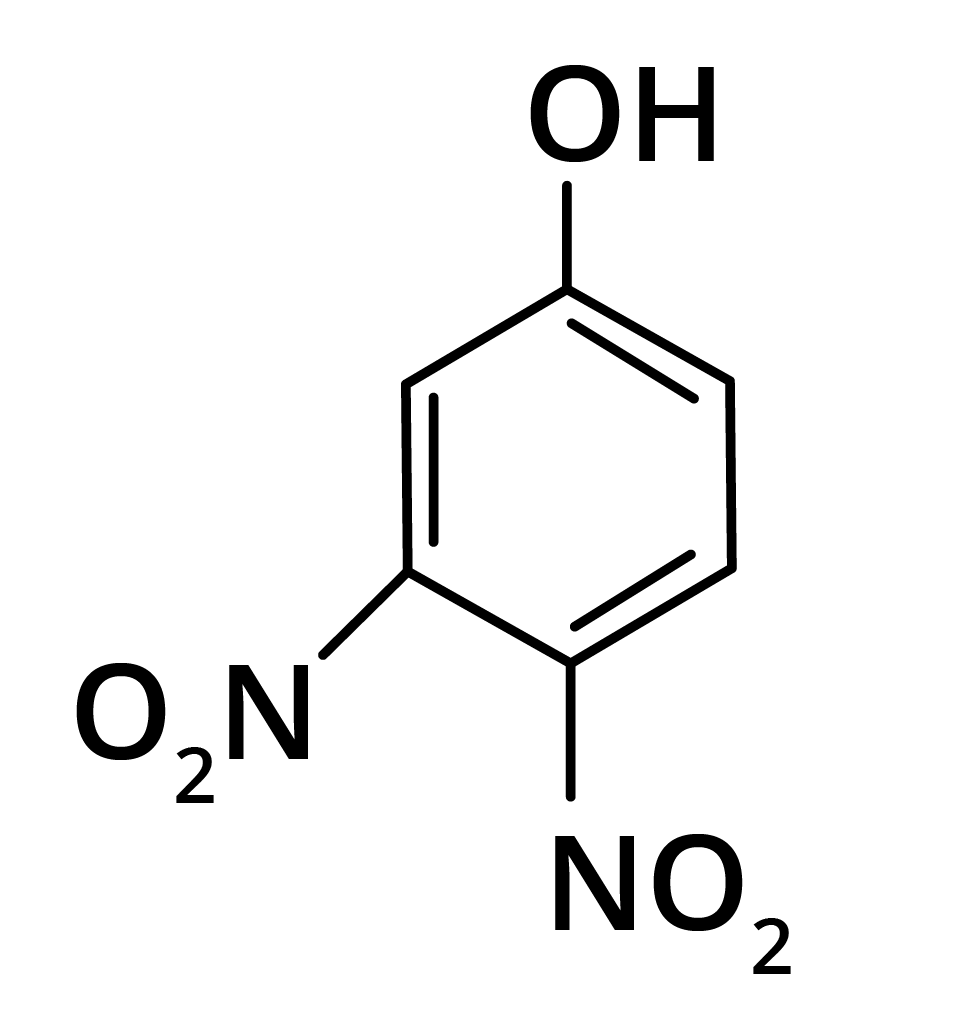
(d)
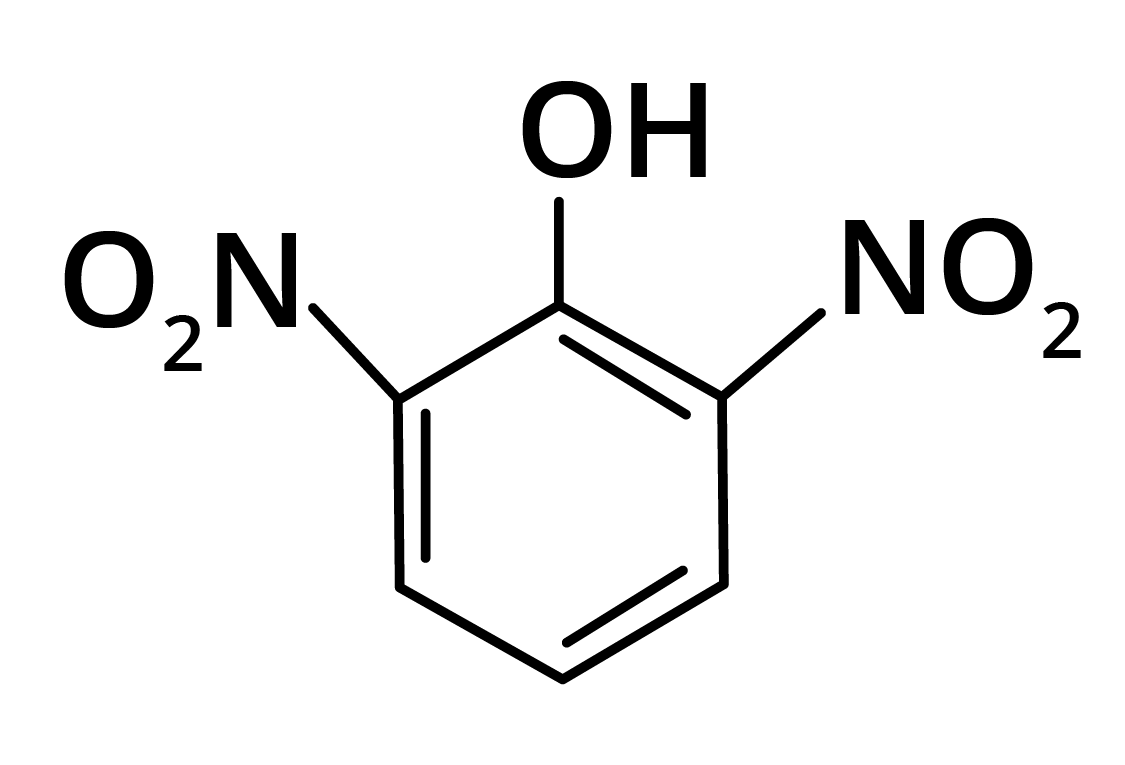
Ans: The correct answer is option b.
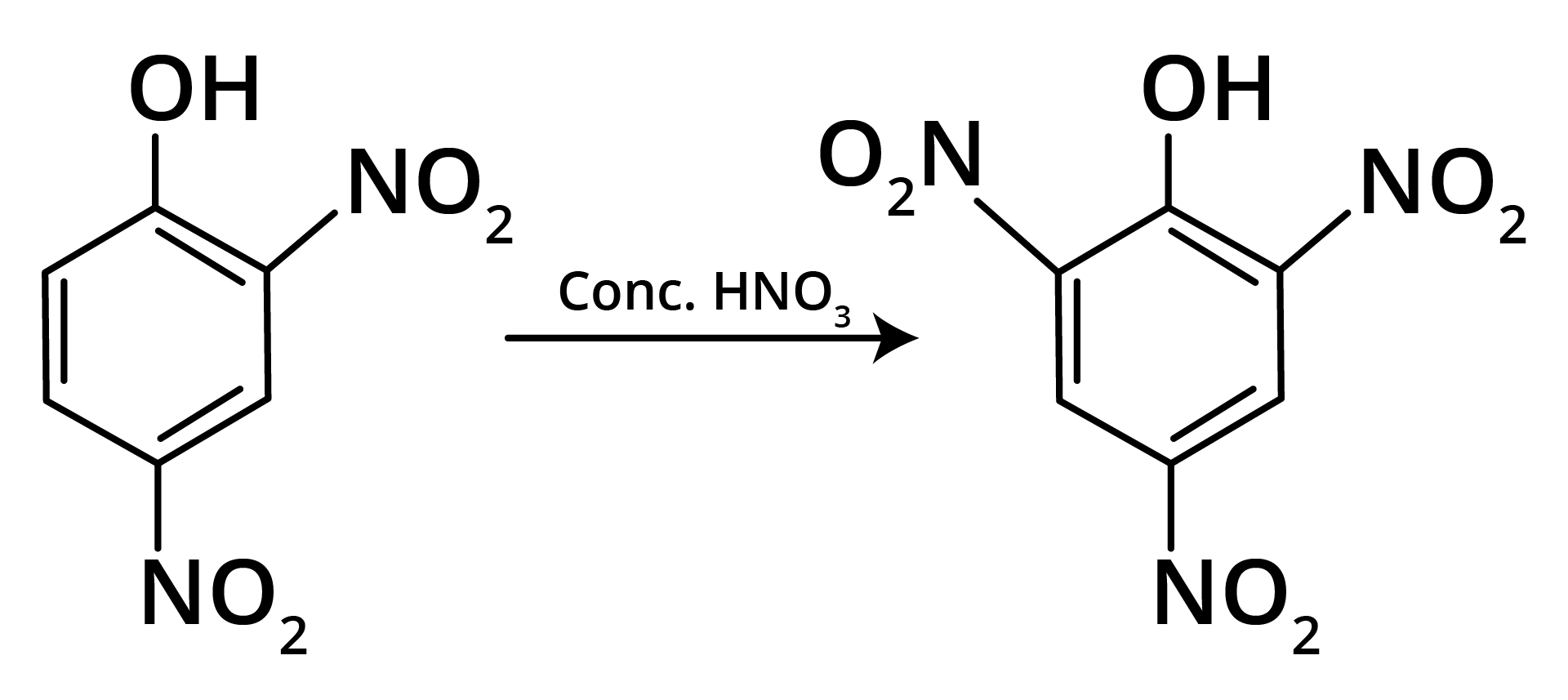
Trick: OH group is activating in nature. So, NO2 will attack at ortho and para position, but para position is already blocked; therefore, NO2 is only attached at ortho position w.r.t. OH group.
3. The structure of the starting compound P used in the reaction given below is:
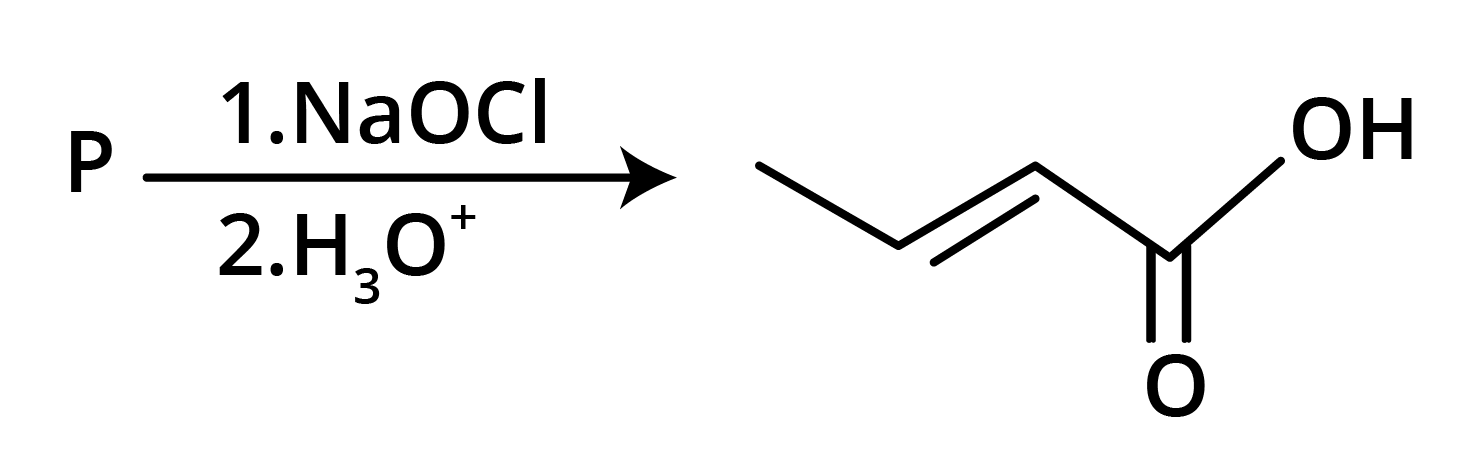
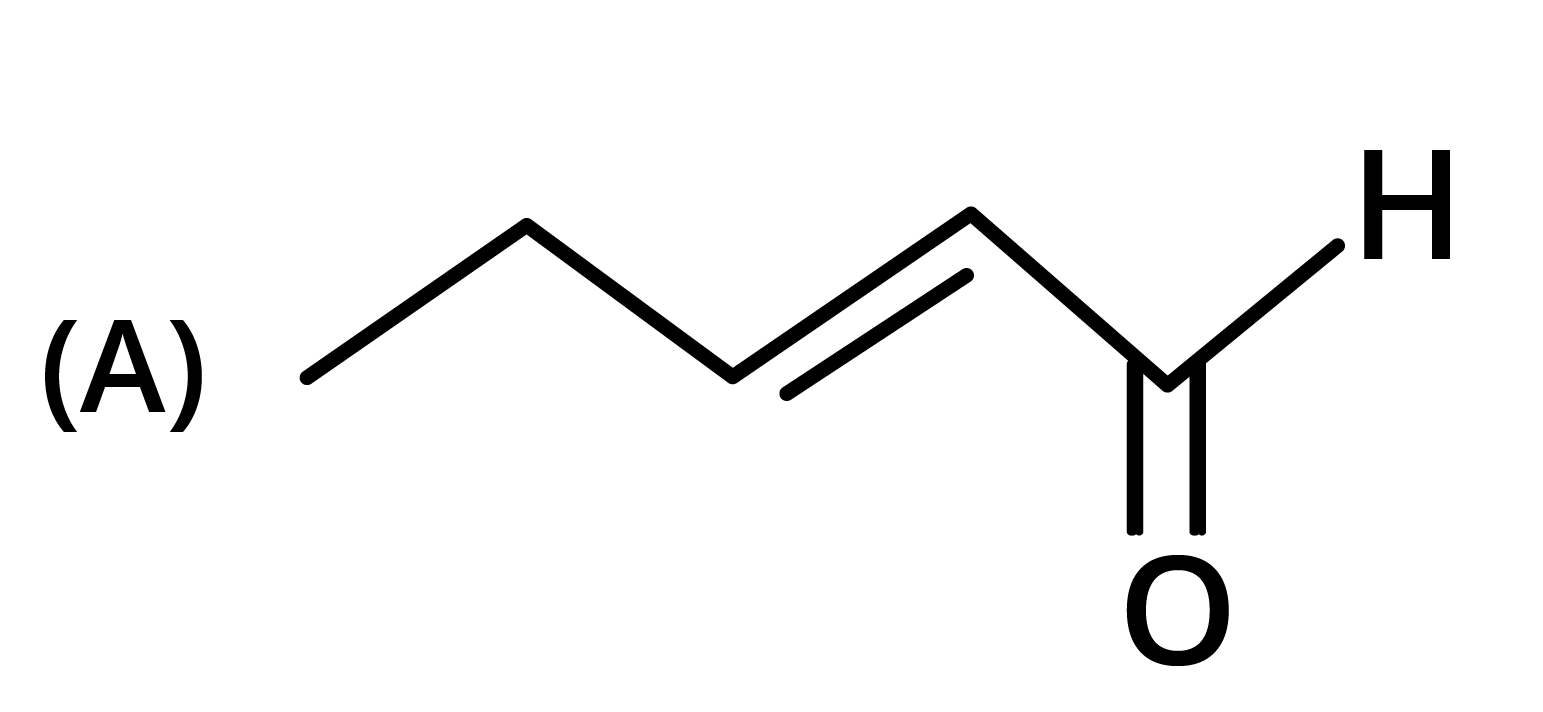
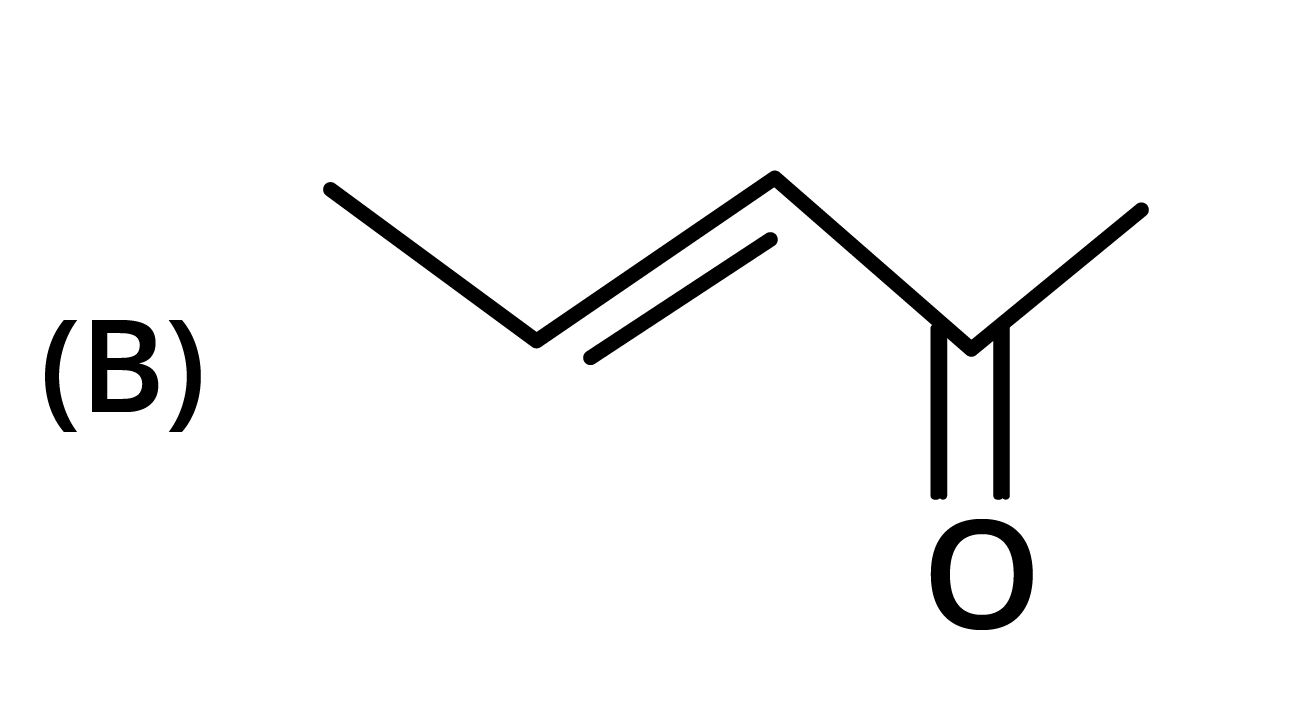
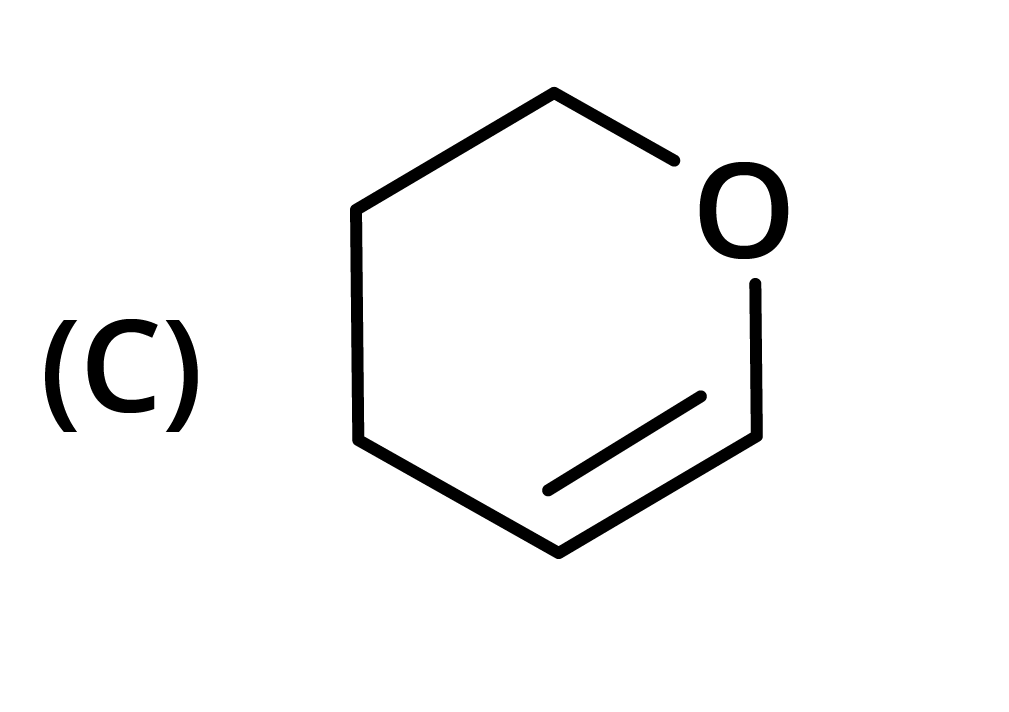
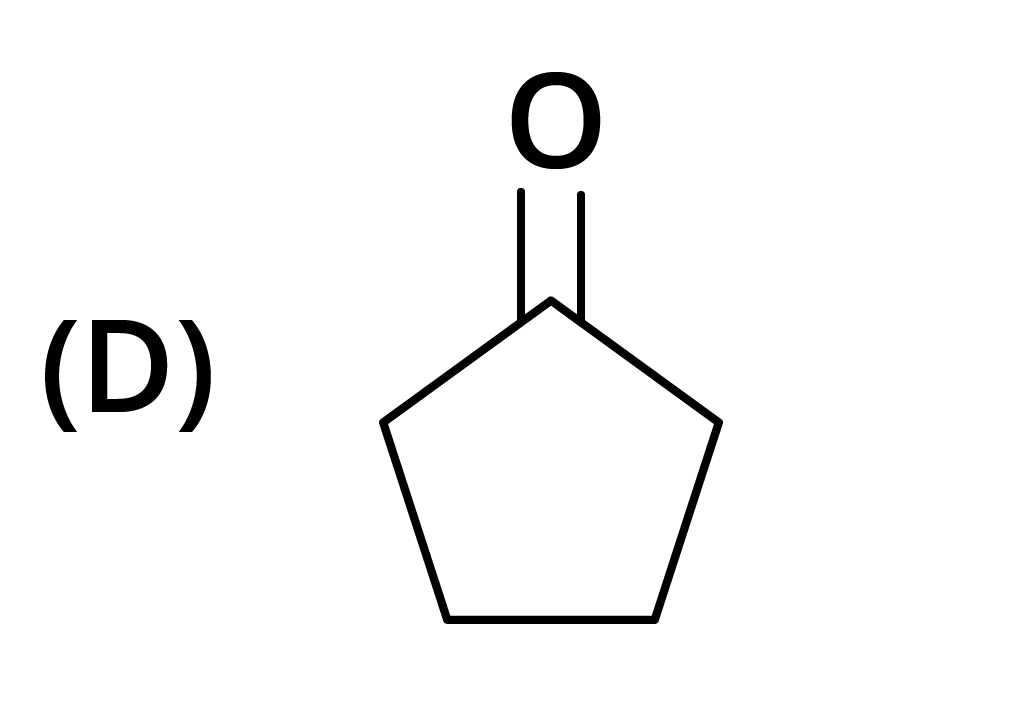
Ans: The correct answer is option B.
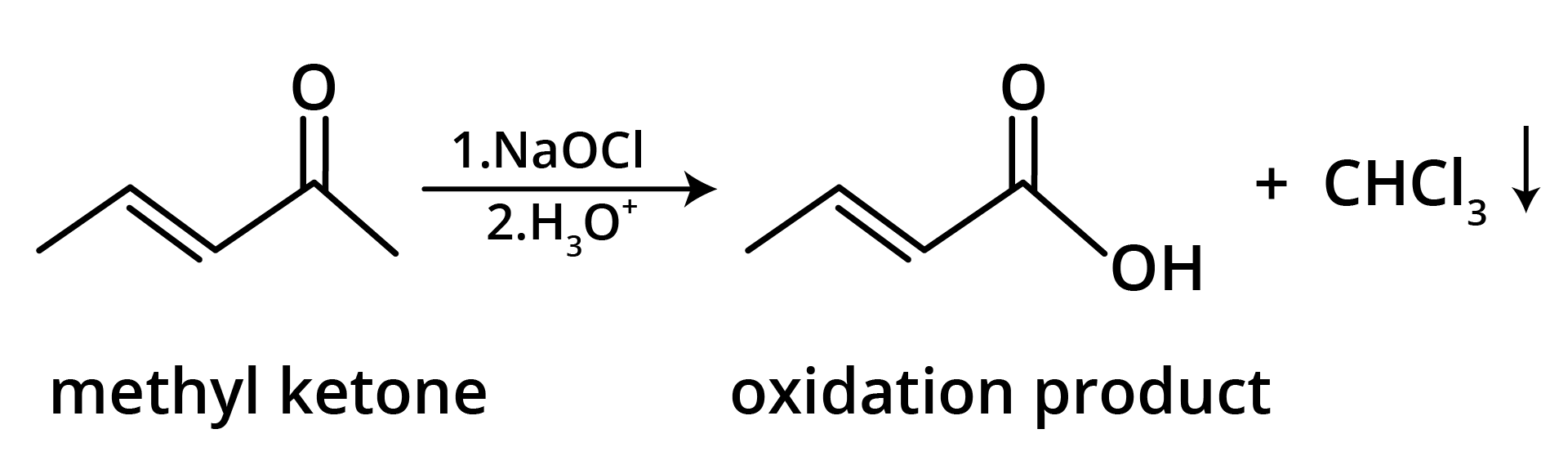
Trick: The given reaction is chloroform (CHCl3) reaction.
Practice Questions
1. Arrange the sets of compounds in order of their increasing boiling points:
Pentan-1-ol, butan-1-ol, butan-2-ol, ethanol, propan-1-ol, methanol
Ans: Methanol, ethanol, propan-1-ol, butan-2-ol, butan-1-ol, pentan-1-ol.
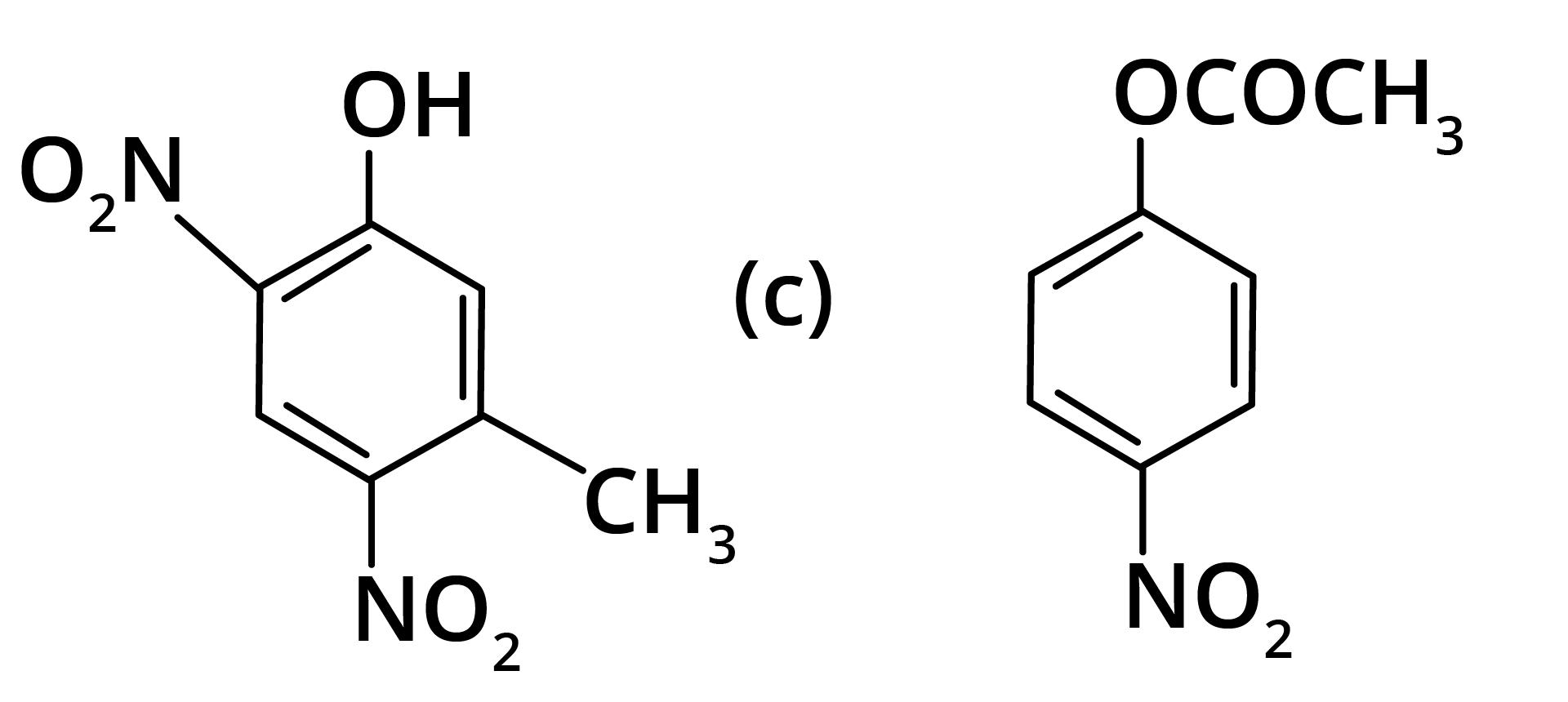
2. What will be the product formed in dinitration of 3-methylphenol?
Ans:
JEE Main Chemistry Organic Compound Contains Oxygen Study Materials
Here, you'll find a comprehensive collection of study resources for Organic Compound Contains Oxygen designed to help you excel in your JEE Main preparation. These materials cover various topics, providing you with a range of valuable content to support your studies. Simply click on the links below to access the study materials of Organic Compound Contains Oxygen and enhance your preparation for this challenging exam.
JEE Main Chemistry Study and Practice Materials
Explore an array of resources in the JEE Main Chemistry Study and Practice Materials section. Our practice materials offer a wide variety of questions, comprehensive solutions, and a realistic test experience to elevate your preparation for the JEE Main exam. These tools are indispensable for self-assessment, boosting confidence, and refining problem-solving abilities, guaranteeing your readiness for the test. Explore the links below to enrich your Chemistry preparation.
Benefits of Using Vedantu for JEE Main 2026 - Chemistry Organic Compound Contains Oxygen Chapter.
Organic Compound Contains Oxygen chapter for JEE Main 2026, Vedantu offers expert guidance, personalized learning plans, and interactive sessions with engaging examples. Regular assessments, flexible schedules, important questions, notes, and dedicated doubt resolution further enhance comprehension, making Vedantu an invaluable resource for mastering this challenging chapter:
Expert Guidance for "Organic Compound Contains Oxygen" Chapter: Vedantu provides seasoned JEE Main tutors offering expert guidance, making the intricate concepts of the "Organic Compound Contains Oxygen" chapter clear and comprehensible.
Personalised Learning Tailored to the Chapter: Tailored study plans on Vedantu cater specifically to the nuances of the "Organic Compound Contains Oxygen" chapter, adapting to individual learning styles for effective understanding.
Interactive Sessions with Fun Examples: Live interactive sessions in Vedantu's platform for the "Organic Compound Contains Oxygen" chapter include engaging examples, making learning enjoyable while students can ask questions for real-time explanations.
Regular Assessments for Reinforcement: Regular assessments and mock tests on Vedantu aid in tracking progress within the "Organic Compound Contains Oxygen" chapter, identifying areas for improvement, and reinforcing crucial concepts.
Flexible Learning Schedule Aligned with the Chapter: Vedantu's flexible learning schedule accommodates the "Organic Compound Contains Oxygen" chapter, allowing students to schedule sessions at their convenience, aligning with their daily routines.
Conclusion
The JEE Main chapter on "Organic Compounds Containing Oxygen" is a fundamental component of organic chemistry. It provides students with a comprehensive understanding of the diverse and essential compounds that contain oxygen functionalities. This knowledge is invaluable for grasping the intricate structures, nomenclature, reactions, and applications of these compounds. Additionally, it lays the foundation for advanced studies in organic chemistry and is pivotal in understanding the vast array of organic compounds encountered in various fields, from pharmaceuticals to petrochemicals. Mastery of this chapter equips JEE Main students with the necessary skills to excel in organic chemistry, enabling them to tackle complex organic reactions and syntheses effectively.
FAQs on Organic Compound Contains Oxygen Chapter - Chemistry JEE Main
1. In the JEE Main examination, how important is the alcohol chapter?
Nearly 1-2 questions arise in the exam from this chapter covering about 8 marks which makes about 2% of the total marks.
2. What are the main points to remember while tackling problems involving halogen-containing organic compounds?
Students should practise writing the mechanism of the reaction for solving the questions from the JEE Main Chemistry Organic Compounds Containing Halogens.
3. Are previous year question papers enough for scoring good marks in JEE Main?
NCERT and previous year JEE Main papers are adequate to score good marks (200+) in JEE Main. Solving previous 10-year IIT-JEE exams gives us a significant edge because 3-4 questions with the same answers are sure to be repeated every year.
4. What topics are covered in the Organic Compound Contains Oxygen chapter for JEE Main?
The chapter typically covers various organic compounds containing oxygen, including alcohols, phenols, ethers, and carbonyl compounds, exploring their structures, properties, and reactions.
5. How can Vedantu's Chemistry JEE Main course help in understanding the Organic Compound Contains Oxygen chapter?
Vedantu's course provides expert guidance, personalized learning plans, and interactive sessions, facilitating a comprehensive understanding of the Organic Compound Contains Oxygen chapter for JEE Main.























