NCERT Exemplar Class 12 Available on Vedantu
Free PDF download of NCERT Exemplar for Class 12 Chemistry Chapter 14 - Biomolecules solved by expert Chemistry teachers on Vedantu as per NCERT (CBSE) Book guidelines. All Chapter 14 - Biomolecules exercise questions with solutions to help you to revise the complete syllabus and score more marks in your examinations
Access NCERT Exemplar Solutions for Class 12 Chemistry Chapter 14 - Biomolecule
MULTIPLE CHOICE QUESTIONS TYPE 1
1: Glycogen is a branched chain polymer of $\alpha-D-$ glucose units in which chain is formed by $\mathrm{C}_{1}-\mathrm{C}_{4}$ glycosidic linkage whereas branching occurs by the formation of $\mathrm{C}_{1}-\mathrm{C}_{6}$ glycosidic linkage. Structure of glycogen is similar to
(a) Amylose
(b) Amylopectin
(c) Cellulose
(d) Glucose
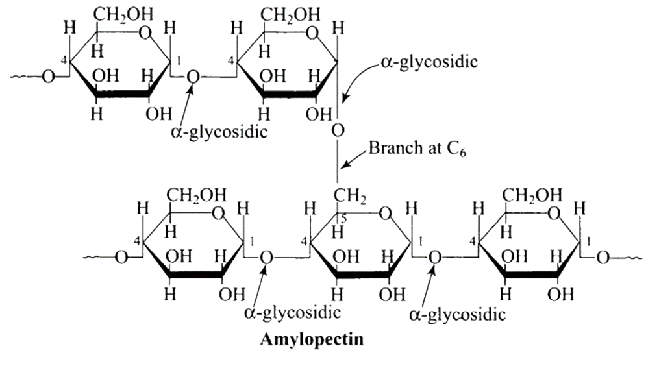
Ans: The Correct option is $b$ Glycogen resembles amylopectin in structure. It's a $\alpha-D-$ glucose unit branched chain polymer with $\mathrm{C}_{1}-\mathrm{C}_{4}$ glycosidic linkage and $\mathrm{C}_{1}-\mathrm{C}_{6}$ glycosidic linkage branching. The structure of amylopectin is depicted in the diagram below.
2: Which of the following polymers is stored in the liver of animals?
(a) Amylose
(b) Cellulose
(c) Amylopectin
(d) Glycogen
Ans: The Correct option is b. In humans and animals, glycogen is mostly synthesized and stored in the liver and skeletal muscle cells. Glycogen accounts for around 5-6% of the liver's fresh weight, and an adult liver weighing 1.5kg can store approximately 100-120 gms of glycogen.
3: Sucrose (cane sugar) is a disaccharide. One molecule of sucrose on hydrolysis gives _________.
(a) 2 molecules of glucose
(b) 2 molecules of glucose +1 molecule of fructose
(c) 1 molecule of glucose +1 molecule of fructose
(d) 2 molecules of fructose
Ans: Sucrose is a disaccharide (sometimes known as cane sugar). One molecule of glucose and one molecule of fructose are produced when sucrose is digested. The reaction is depicted in the diagram below.
$\underset{\text { Cane sugar }}{\mathrm{C}_{12} \mathrm{H}_{22} \mathrm{O}_{11}} \stackrel{\mathrm{H}_{2} \mathrm{O}}{\stackrel{\mathrm{H}^{+}}{\longrightarrow}} \underset{D(+) \text { glucose }}{\mathrm{C}_{6} \mathrm{H}_{12} \mathrm{O}_{6}}+\underset{D(-) \text { fructose }}{\mathrm{C}_{6} \mathrm{H}_{12} \mathrm{O}_{6}}$
Correct option is c.
4: Which of the following pairs represents anomers?
(a)
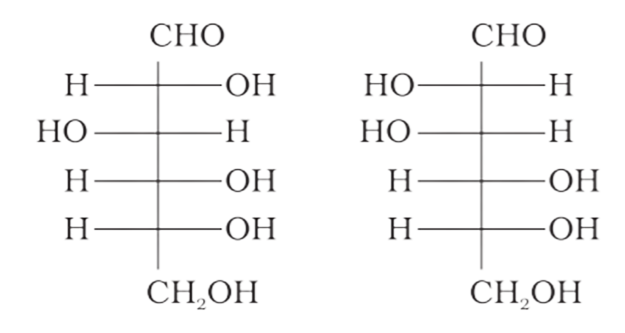
(b)
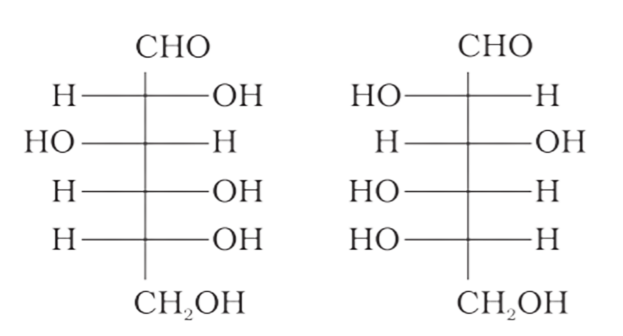
(c)
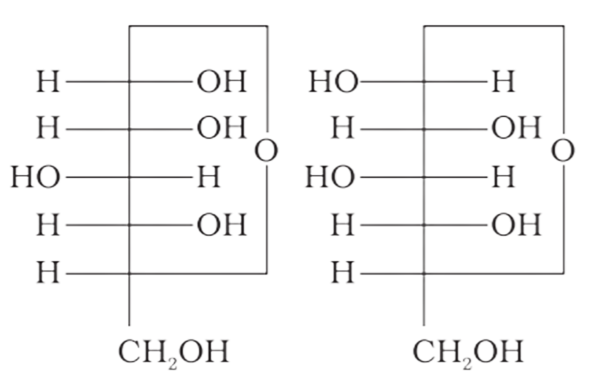
(d)
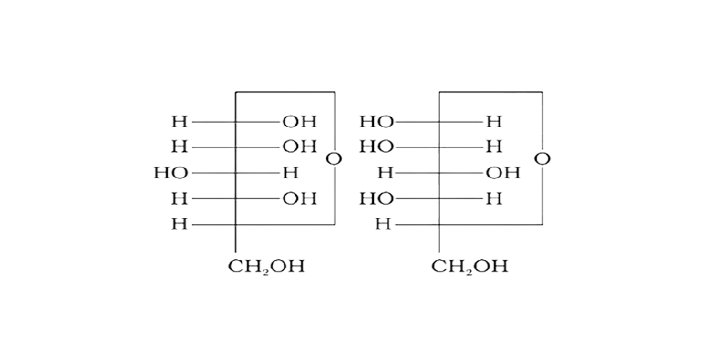
Ans: The configuration of different anomers at C-I is distinct. It is known as the form if the hydroxyl group (-OH) is present on the right side of anomeric carbon, and it is known as the $\beta-$ form if the hydroxyl group is present on the left side. As a result, option (c) represents anomers among all the supplied pairs.
Correct option is c.
5: Proteins are found to have two different types of secondary structures viz. $\alpha$-helix and $\beta$ - pleated sheet structure. $\alpha$-helix structure of protein is stabilised by
(a) Peptide bonds
(b) Van der Waals forces
(c) Hydrogen bonds
(d) Dipole-dipole interactions
Ans: Hydrogen bonds hold the $\alpha-$ helical structure of proteins together. A polypeptide chain creates all potential hydrogen bonds by twisting into a
right-handed helix with the $-\mathrm{NH}$ group of each amino acid residue hydrogen linked to $\mathrm{a}>\mathrm{C}=\mathrm{O}$ neighboring turn of helix.
Correct option is C
6: In disaccharides, if the reducing groups of monosaccharides i.e., aldehydic or ketonic groups are bonded, these are non-reducing sugars. Which of the following disaccharide is a non-reducing sugar?
(a)
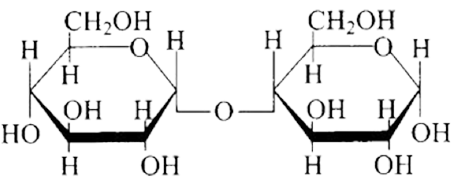
(b)
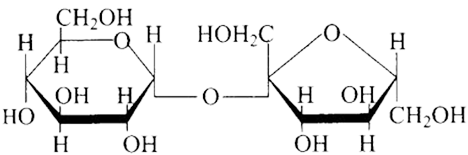
(c)
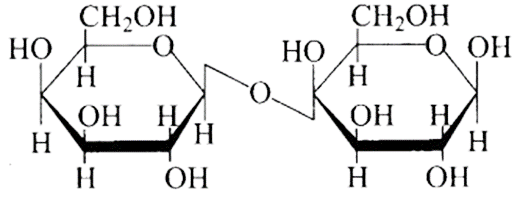
(d)
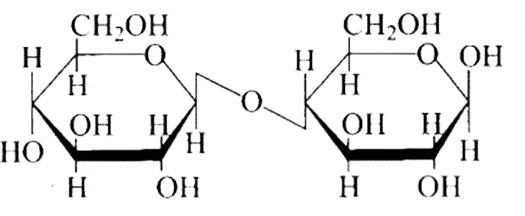
Ans: The Correct option is b.
The structure described in option (b) reflects sucrose, which has a $\mathrm{C}_{1}-\mathrm{C}_{2}$ glycosidic bond between $\alpha-D-$ glucose and $\beta-D-$ fructose.
This is a non-reducing sugar since the reducing groups of glucose and fructose are involved in the creation of glycosidic linkages.
7: Which of the following acids is a vitamin?
(a) Aspartic acid
(b) Ascorbic acid
(c) Adipic acid
(d) Saccharic acid
Ans: The Correct option is b.
Ascorbic acid is the chemical name for vitamin C. Aspartic acid is an amino acid, adipic acid is a dicarboxylic acid with an eight-carbon chain, and saccharic acid is a dicarboxylic acid made by oxidising glucose with HNO3 Vitamin C is a water-soluble vitamin that serves a variety of activities in the human body.
8: Dinucleotide is obtained by joining two nucleotides together by phosphodiester linkage. Between which carbon atoms of pentose sugars of nucleotides are these linkages present?
(a) ${ }^{5}$ and $3^{\prime}$
(b) 1 ' and 5 '
(c) $5^{\prime}$ and $5^{\prime}$
(d) $3^{\prime}$ and $3^{\prime}$
Ans: The Correct option is a.
Phosphodiester links the two nucleotides together to form a dinucleotide.
Between the pentose sugars of nucleotides, there are $5^{\prime}$ and $3^{\prime}$ connections. In the process of adding phosphate to a dinucleotide, a significant quantity of energy is consumed.
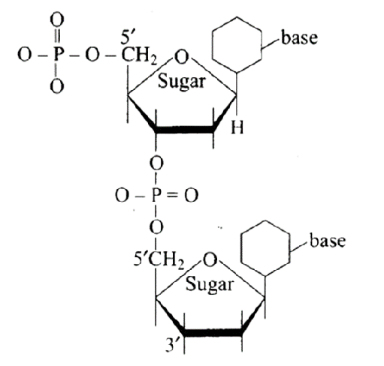
9: Nucleic acids are the polymers of ______________.
(a) Nucleosides
(b) Nucleotides
(c) Bases
(d) Sugars
Ans: The Correct option is b.
Nucleic acids are nucleotide polymers linked together by phosphodiester linkage. DNA and RNA are two examples. Nucleotides are formed by linking of nucleosides with three phosphate groups consuming a significant amount of energy making it an endothermic reaction.
10: Which of the following statements is not true about glucose?
(a) It is an aldohexose.
(b) On heating with HI it forms n-hexane.
(c) It is present in furanose form.
(d) It does not give 2-4 DNP test.
Ans: The Correct option is c.
The pyranose form of glucose, which has a five-membered ring structure, is found. As a result, option (c) is wrong.
Glucose is a six-carbon monosaccharide with an aldehyde group. As a result, it's referred to as an aldohexose. As a result, the statement in option (a) is correct.
When glucose is cooked with HI, n-hexane is produced. All six carbon atoms in glucose are linked together in a straight chain during this procedure. As a result, the statement in option (b) is correct.
The aldehyde group is not free in the cyclic structure. As a result, there is no positive 2-4 DNP test available. As a result, the statement in option (D) is correct.
11: Each polypeptide in a protein has amino acids linked with each other in a specific sequence. This sequence of amino acids is said to be ____________.
(a) primary structure of proteins.
(b) secondary structure of proteins.
(c) tertiary structure of proteins.
(d) quaternary structure of proteins.
Ans: The Correct option is a.
Proteins contain one or more polypeptide chains. When each polypeptide of a protein's amino acids is joined in a precise order, a proteins' main structure is referred to as its primary structure. This one is the linear structure with no bonds other than the ones between adjacent amino acids. The sequence of amino acids in the primary structure is pre-determined by the genetic code of an individual and thus is a specific sequence.
12: DNA and RNA contain four bases each. Which of the following bases is not present in RNA?
(A) Adenine
(B) Uracil
(C) Thymine
(D) Cytosine
Ans: The Correct option is c.
The four bases contained in DNA are adenine, guanine, thymine, and cytosine. Adenine, uracil, guanine, and cytosine are the four bases of RNA. As a result, RNA does not include thymine as in place of thymine, it has uracil which is a lesser stable nucleotide base and is somewhat responsible for RNA’s instability but also lends it some flexibility to change.
13: Which of the following B group vitamins can be stored in our body?
(a) Vitamin $\mathrm{B}_{1}$
(b) Vitamin $B_{2}$
(c) Vitamin $B_{6}$
(d) Vitamin $B_{12}$
Ans: The Correct option is $\mathrm{d}$.
Water-soluble vitamins are those in the B group. B group vitamins cannot be stored in our systems since they are quickly excreted; but, because they are $B_{12}$ insoluble in water, our bodies may store the vitamin. Because our systems are unable to store these vitamins, options (a), (b), and (c) are excluded.
14: Which of the following bases is not present in DNA?
(a) Adenine
(b) Thymine
(c) Cytosine
(d) Uracil
Ans: The Correct option is d.
Adenine, guanine, thymine, and cytosine are the four bases found in DNA. As a result, uracil is not found in DNA as we cannot identify it among the other nucleotides that are present in the DNA. Uracil is, however, seen in RNA, another nucleic acid found in most of the organisms.
15: Three cyclic structures of monosaccharides are given below which of these are anomers.
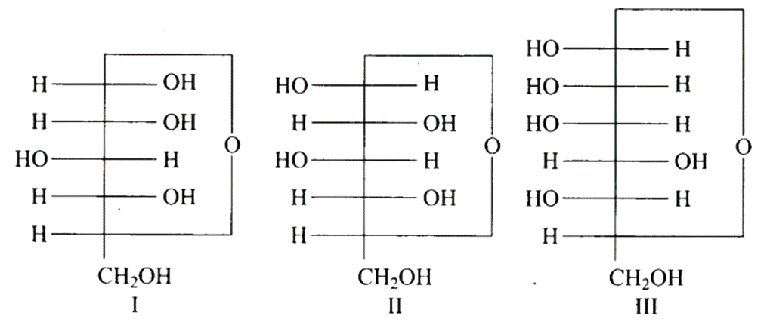
(a) I and II
(b) II and III
(c) I and III
(d) III is anomer of I and II
Ans: The Correct option is a.
Anomers are cyclic monosaccharide structures that differ structurally at carbon-1. In this scenario, I and II are anomers since they differ only at carbon -1.
16: Which of the following reactions of glucose can be explained only by its cyclic structure?
(a) Glucose forms pentaacetate.
(b) Glucose reacts with hydroxylamine to form an oxime.
(c) Pentaacetate of glucose does not react with hydroxylamine.
(d) Glucose is oxidised by nitric acid to gluconic acid.
Ans: The Correct option is c.
The absence of a free (-CHO) aldehyde group is indicated by the fact that glucose pentaacetate does not react with hydroxylamine. The open structure of glucose cannot account for this, although the open chain structure of glucose can account for all other features.
17: Optical rotations of some compounds along with their structures are given below which of them have D-configuration.
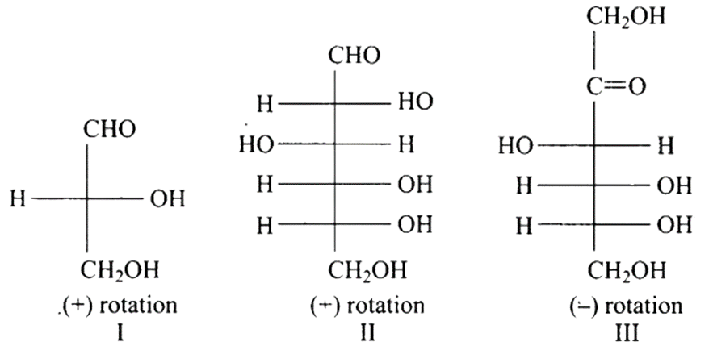
(a) I, II, III
(b) II, III
(c) I, II
(d) III
Ans: The Correct option is a.
In the same way as (+) glyceraldehyde has a group on the lowest asymmetric carbon on the right side, the I, II, and III structures have a (-OH) group on the lowest asymmetric carbon on the right side giving them a D-configuration.
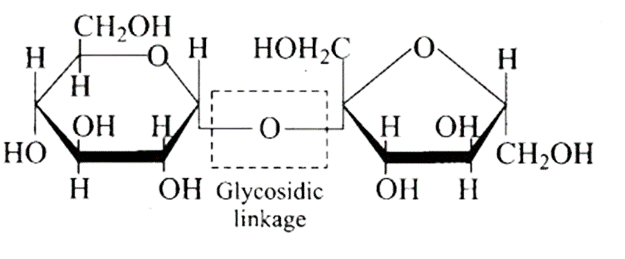
18: Structure of a disaccharide formed by glucose and fructose is given below. Identify anomeric carbon atoms in monosaccharide units.
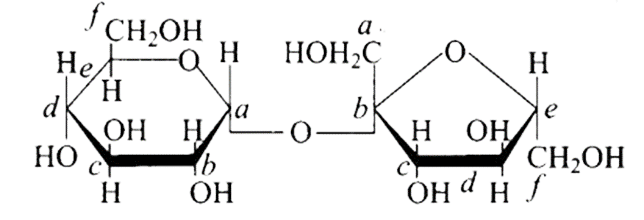
(a) ‘a’ carbon of glucose and ‘a’ carbon of fructose.
(b) ‘a’ carbon of glucose and ‘e’ carbon of fructose.
(c) ‘a’ carbon of glucose and ‘b’ carbon of fructose.
(d) ‘f’ carbon of glucose and ‘f ’ carbon of fructose.
Ans: The Correct option is c.
In the cyclic structure of glucose or fructose, anomeric carbon is carbon that is close to an oxygen atom. As shown in the structure above, atoms 'a' and 'b' are close to the oxygen atom, and the hydroxyl groups of both carbon atoms have different orientations.
19: Three structures are given below in which two glucose units are linked. Which of these linkages between glucose units are between $C_{1}$ and $C_{4}$ and which linkages are between $C_{1}$ and $C_{6}$ ?
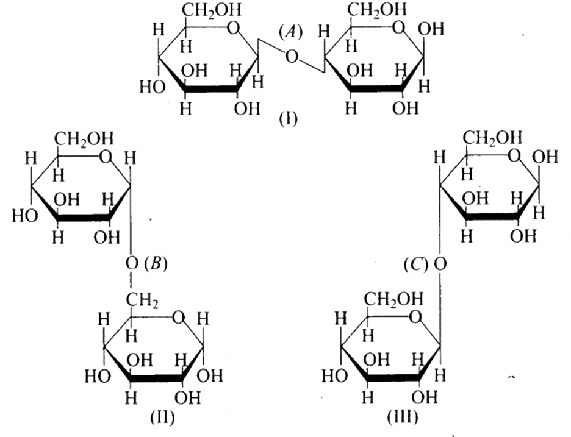
(a) (A) is between $C_{1}$ and $C_{4},(B)$ and (C) are between $C_{1}$ and $C_{6}$
(b) $(\mathrm{A})$ and $(\mathrm{B})$ are between $\mathrm{C}_{1}$ and $\mathrm{C}_{4}$, (C) is between $\mathrm{C}_{1}$ and $\mathrm{C}_{6}$
(c) $(\mathrm{A})$ and $(\mathrm{C})$ are between $\mathrm{C}_{1}$ and $\mathrm{C}_{4},(\mathrm{~B})$ is between $\mathrm{C}_{1}$ and $\mathrm{C}_{6}$
(d) $(A)$ and $(C)$ are between $C_{1}$ and $C_{6},(B)$ is between $C_{1}$ and $C_{4}$ Ans: Correct option is $\mathrm{C}$.
Ans: The links between $C_{1}$ and $C_{4}$ of glucose are shown as 'A' and ' $C$,' respectively, whilst the linkage between $C_{1}$ and $C_{6}$ of the glucose units is shown as ' $B$. ' Furthermore, option (c) is clearly correct based on the structures.
Because ' $B$ ' is the bond or link between the glucose units of $C_{1}$ and $C_{6}$ and, option (b) is wrong.
Option (a) is wrong because the relationship between $C_{1}$ and $C_{4}$ and is represented by 'C.'
Because 'A' is the hyperlink between $C_{1}$ and $C_{4}$ the glucose units of and, option $(d)$ is wrong.
Multiple Choice Questions (Type-II)
20: Carbohydrates are classified on the basis of their behaviour on hydrolysis and also as reducing or non- reducing sugar. Sucrose is a __________.
(a) monosaccharide
(b) disaccharide
(c) reducing sugar
(d) non-reducing sugar
Ans: Correct options are b and d.
Sucrose is a common disaccharide that breaks down into an equimolar mixture of D-(+)- glucose and D-(-)- fructose when hydrolyzed. These two monosaccharides are held together by a glycosidic bond between $C_{1}$ of $\alpha$ - glucose and $C_{2}$ of $\beta$ fructose. Because the reducing groups of glucose and fructose are involved in the formation of glycosidic linkages, sucrose is a non-reducing sugar.
21: Proteins can be classified into two types on the basis of their molecular shape i.e., fibrous proteins and globular proteins. Examples of globular proteins are:
(a) Insulin
(b) Keratin
(c) Albumin
(d) Myosin
Ans: Correct options are a and c.
Globular protein is a protein structure that forms when a chain of polypeptides coils around to produce a spherical shape. Insulin and albumin are two examples of water-soluble globular proteins. As a result, (a) and (c) are the correct answers.
22: Which of the following carbohydrates are branched polymers of glucose?
(a) Amylose
(b) Amylopectin
(c) Cellulose
(d) Glycogen
Ans: Correct options are b and d.
Amylopectin and Glycogen are the correct choices since they are carbohydrate storage forms found in animal tissues, whereas amylose is made up of 80-85% starch. It's also a branched glucose polymer that branches via $C_{1}$ - $C_{6}$ glycosidic linkage.
23: Amino acids are classified as acidic, basic or neutral depending upon the relative number of amino and carboxyl groups in their molecule. Which of the following are acidic?
(a)
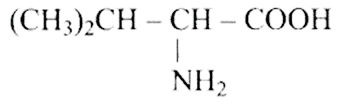
(b)
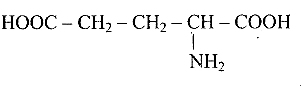
(c)
\[H_2N - CH_2 - CH_2 - CH_2 - COOH \]
(d)
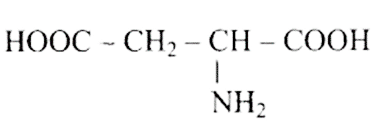
Ans: Correct options are b and d.
Acidic amino acids are divided into two groups, one -COOH group against the other - $\mathrm{NH}_{2}$ group. As a result, the acidic amino acids (b) and (d) are the only ones among the supplied structures.
24: Lysine,
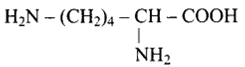
(a) $\alpha$ - Amino acid
(b) Basic amino acid
(c) Amino acid synthesized in body
(d) $\beta$ - Amino acid
Ans: Correct options are a, b and c.
Lysine is an amino acid with the structural formula of (a).
(b) Because the number of groups (two) is more than the number of -COOH groups (one), it is a basic amino acid (one).
Because it is created in human bodies, it is a non-essential amino acid.
25: Which of the following monosaccharides are present as a five membered cyclic structure (furanose structure)?
(a) Ribose
(b) Glucose
(c) Fructose
(d) Galactose
Ans: Correct options are a and c.
The cyclic structure of ribose and fructose is five-membered (furanose structures). Like compound furans, they have a five-membered ring.
26: In fibrous proteins, polypeptide chains are held together by ___________.
(a) Van der Waals forces
(b) Disulphide linkage
(c) electrostatic forces of attraction
(d) hydrogen bonds
Ans: Correct options are b and d.
Fibrous protein is generated when the polypeptide chain runs parallel and is held together by hydrogen and disulphide bonds.
27: Which of the following are purine bases?
(a) Guanine
(b) Adenine
(c) Thymine
(d) Uracil
Ans: Correct options are a and b.
Purines are made up of a six-membered nitrogen-containing ring joined with a five-membered nitrogen-containing ring.
Purine bases are guanine and adenine, while pyrimidine bases are thymine and uracil.
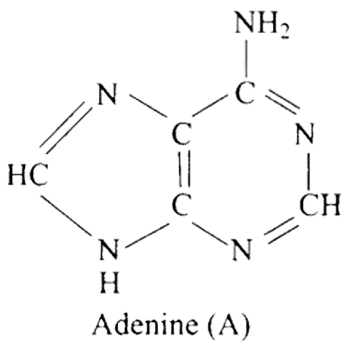
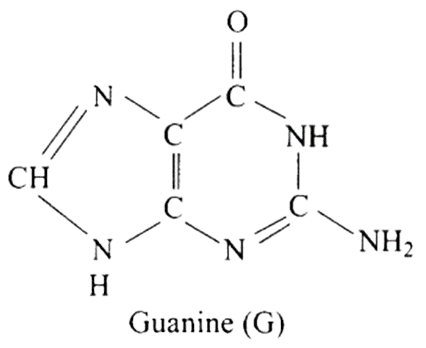
Hence (a) and (b) are correct choices for this question.
28: Which of the following terms are correct about enzyme?
(a) Proteins
(b) Dinucleotides
(c) Nucleic acids
(d) Biocatalysts
Ans: Correct options are a and d.
Enzymes are protein molecules that operate as biocatalysts in the body, facilitating chemical processes. The pace of the reaction is accelerated by these catalysts.
29: Name the sugar present in milk. How many monosaccharide units are present in it? What are such oligosaccharides called?
Ans: Lactose sugar is a naturally occurring sugar found in milk. D galactose and D glucose, two monosaccharide molecules, are linked together. As a result, disaccharides are the name given to these oligosaccharides.
30: How do you explain the presence of all the six carbon atoms in glucose in a straight chain?
Ans: n-hexane is produced when glucose is cooked with HI for a lengthy amount of time. The reaction is depicted in the diagram below.
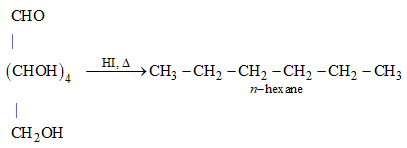
n-hexane has six carbon atoms joined in a straight chain because glucose has six carbon atoms.
31: In nucleoside a base is attached at 1′ position of sugar moiety. Nucleotide is formed by linking of phosphoric acid unit to the sugar unit of nucleoside. At which position of sugar unit is the phosphoric acid linked in a nucleoside to give a nucleotide?
Ans: The sugar moiety of a nucleoside is linked to a phosphoric acid unit, ideally at the 5′ position, to form a nucleotide.
The structure is depicted in the diagram below.
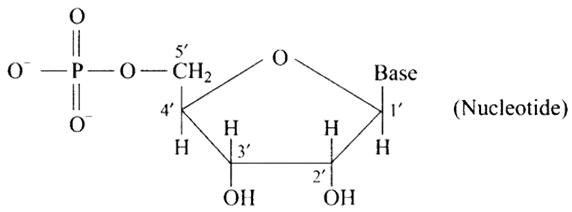
32: Name the linkage connecting monosaccharide units in polysaccharides.
Ans: A glycosidic linkage is a linkage that connects monosaccharides in polysaccharides. The covalent bond that joins two or more monosaccharides to produce a polysaccharide is known as this linkage.
33: Under what conditions glucose is converted to gluconic and saccharic acid?
Bromine water $\left(\mathrm{Br}_{2}\right)$ converts glucose to gluconic acid, which is then transformed to
saccharic acid by concentrated nitric acid $\left(\mathrm{HNO}_{3}\right)$.
The conversions are displayed in the table below.
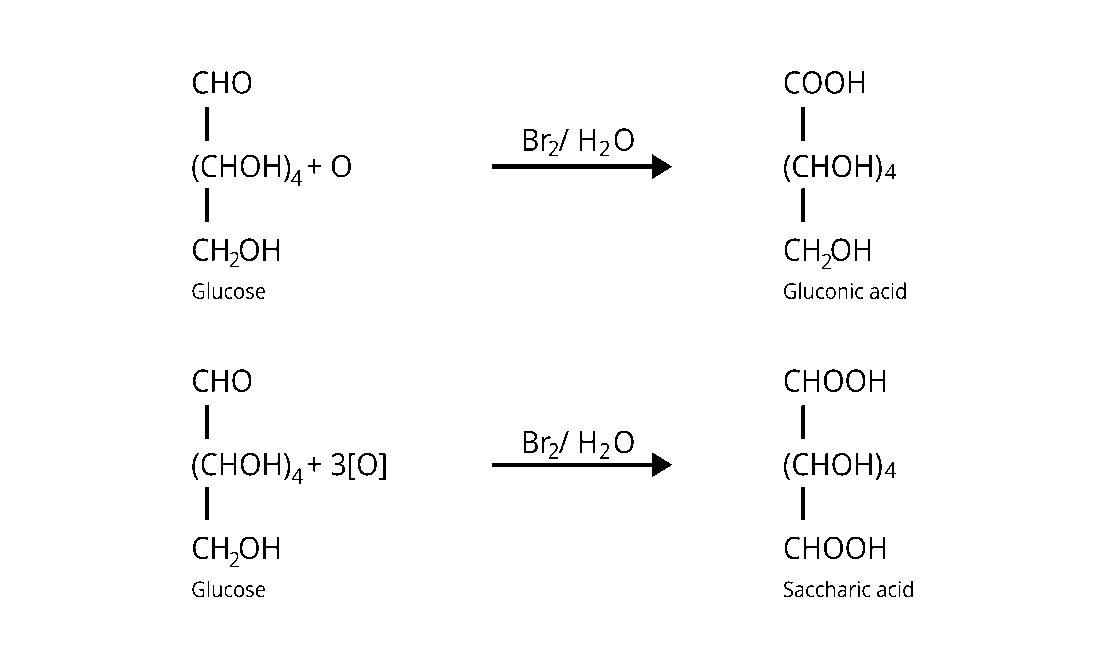
34: Monosaccharides contain carbonyl group hence are classified, as aldose or ketose. The number of carbon atoms present in the monosaccharide molecule are also considered for classification. In which class of monosaccharide will you place fructose?
Monosaccharides contain carbonyl groups. As a result, they fall under the aldose or ketose categories. When an aldehyde group is present, the monosaccharides are known as aldose, and when a ketone group is present, the monosaccharides are known as ketose. Fructose is classified as ketohexose because it has six carbons and a keto group in its chemical formula $\left(\mathrm{C}_{6} \mathrm{H}_{12} \mathrm{O}_{6}\right)$
35: The letters ‘D’ or ‘L’ before the name of a stereoisomer of a compound indicate the correlation of configuration of that particular stereoisomer. This refers to their relation with one of the isomers of glyceraldehyde. Predict whether the following compound has ‘D’ or ‘L’ configuration.
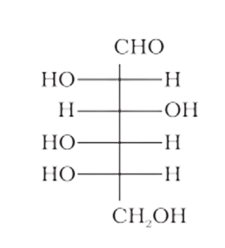
Ans: In the above given structure, the hydroxyl group $(-\mathrm{OH})$ present at the last chiral carbon atom of the molecule, that is, $\mathrm{C} 5$, is directed towards the left. As a result, the given compound has an ' $L$ '- configuration.
36: Aldopentoses named as ribose and 2-deoxyribose are found in nucleic acids. What is their relative configuration?
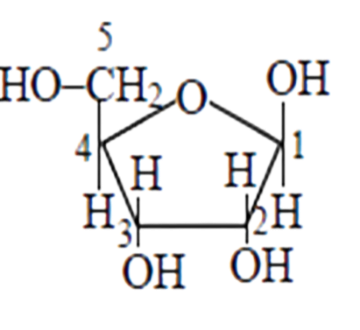
Ans: Aldo-pentoses like ribose and 2 - deoxyribose are sugar moieties found in nucleic acids like RNA and DNA. The structures of these sugars are illustrated below. Both aldopentoses' $D$ - configuration is configuration.
$\beta-D$-ribose is mentioned, and its structure is depicted below.
The structure of as $\beta-D$-deoxyribose, 2 - deoxyribose is given in the diagram below.
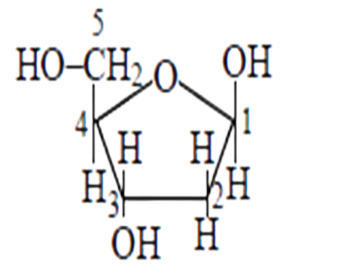
37: Which sugar is called invert sugar? Why is it called so?
Ans: Invert sugar is another name for sucrose. Sugar beet sugar is a colourless, crystalline, and sweet substance made from sugar beets. It is very water soluble and has a dextrorotatory aqueous solution $[\alpha]_{D}=+66.5^{\circ} .$ When cane sugar is hydrolyzed with dilute acids or the enzyme invertase, it creates an equimolar mixture of $D-(+)-$ glucose and $D-(-)-$ fructose. Sucrose is dextrorotatory as a result, and hydrolysis produces dextrorotatory glucose and laevorotatory fructose. Fructose's specific rotation is greater than that of glucose. As a result, the ensuing solution is laevorotatory in nature, having a rotational axis of The reaction is known as inversion, and the mixture of $D-(+)-$ glucose glucose and $D-(-)-$ fructose is known as invert sugar, because the sign of rotation changes from dextro before hydrolysis to laevo after hydrolysis by $\left(-39.9^{\circ}\right)$.
38: Amino acids can be classified as $\alpha-, \beta-, \gamma-, \delta-$ and so on depending upon the relative position of amino group with respect to carboxyl group. Which type of amino acids form polypeptide chain in proteins?
Ans: To make a polypeptide chain, the amino acid must be linked to the $\alpha-$ carbon of the molecule. The removal of water molecules from an $\alpha-$ amino acid results in the formation of a polypeptide chain.
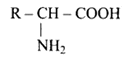
39: $\alpha-$ Helix is a secondary structure of proteins formed by twisting of polypeptide chains into right-handed screw-like structures. Which type of interactions are responsible for making the $\alpha-$ helix structure stable?
Ans: The formation of intramolecular $\mathrm{H}$-bonding between the $>\mathrm{C}=\mathrm{O}$ groups of amino acids in one turn and the $-\mathrm{NH}-$ groups of amino acids in the following turn stabilises a polypeptide chain in the a-helix shape of protein.
40: Some enzymes are named after the reaction, where they are used. What name is given to the class of enzymes which catalyse the oxidation of one substrate with simultaneous reduction of another substrate.
Ans: Oxidoreductases are enzymes that participate in redox reactions that involve oxidation reduction. They act as catalysts; one of these enzymes is Alcohol Dehydrogenase, which decreases the amount of alcohol in the human body after ingestion.
41: During curdling of milk, what happens to sugar present in it?
Ans: During the curdling process, the lactose sugar in milk is converted to lactic acid.
Milk curdling is caused by lactic acid-producing bacteria found in milk. Protein denaturation is an example. When a protein is exposed to physical or chemical alterations, it is said to be modified. The hydrogen bond has been shattered. Globules open up, helices uncoil, and proteins lose their biological function.
42: How do you explain the presence of five -OH groups in glucose molecule?
Ans: When glucose is acetylated with acetic anhydride, a penta-acetyl derivative is produced. The reaction is depicted in the diagram below.
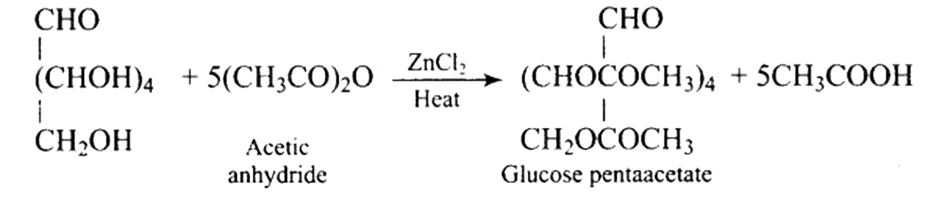
This indicates the presence of five hydroxyl or -OH groups in the glucose molecule.
43: Why does compound (A) given below not form an oxime?
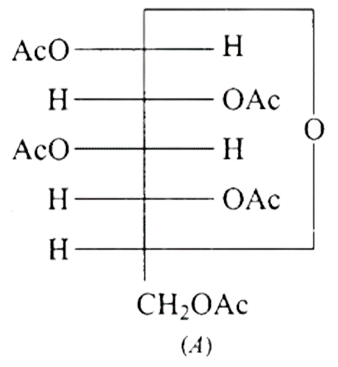
Ans: The given structure A is glucose pentaacetate, which lacks a free hydroxyl \[-OH\] or carbon -CHO group. As a result, it cannot be transferred to the open chain form to make a free group, and therefore the oxime is not produced.
44: Why must vitamin C be supplied regularly in diet?
Ans: Excess vitamin C is easily removed in the urine since it is water soluble. As a result, it can't be stored in the body and must be ingested frequently.
45: Sucrose is dextrorotatory but the mixture obtained after hydrolysis is laevorotatory. Explain.
Ans: Sucrose acts as a dextrorotatory molecule. It produces a mixture of glucose and fructose when hydrolyzed, with particular rotations of $+52.5^{\circ}$ and $-92.4^{\circ} .$ The net resultant mixture is laevorotatory because fructose laevorotation is greater than glucose dextrorotation. The sign of rotation shifts from dextro to laevo as a result of sucrose hydrolysis, and the resulting compound is known as invert sugar.
46: Amino acids behave like salts rather than simple amines or carboxylic acids. Explain.
Ans: Since both acidic $(-\mathrm{COOH})$ and basic $\left(-\mathrm{NH}_{2}\right)$ groups present in amino acids, it act like salts rather than simple amines or carboxylic acids. In Ans, a $-\mathrm{COOH}$ a group can release a proton and an amine group can take a proton, resulting in the formation of a dipolar ion known as zwitterion.
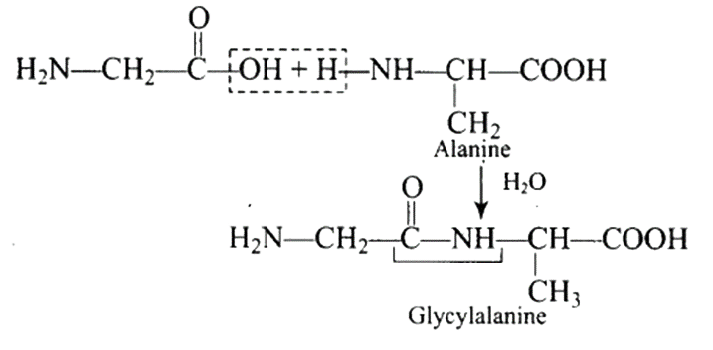
47: Structures of glycine and alanine are given below. Show the peptide linkage in glycylalanine.
$\mathrm{A}(-\mathrm{CO}-\mathrm{NH})$ a peptide bond is formed when the carboxyl group of glycine and the amino group of alanine unite to form glycylalanine. The connection is depicted below.
48: Protein found in a biological system with a unique three-dimensional structure and biological activity is called a native protein. When a protein in its native form, is subjected to a physical change like change in temperature or a chemical change like, change in pH, denaturation of protein takes place. Explain the cause.
Ans: Physical or chemical changes damage hydrogen bonding and other attractive factors. In addition, globules uncoil and the helix uncoils, resulting in a thread-like molecule. As a result, the biological activity of protein secondary and tertiary structures is lost entirely or partially. Protein denaturation is the term for this.
49: Activation energy for the acid catalysed hydrolysis of sucrose is $6.22 \mathrm{~kJ} \mathrm{~mol}^{-1}$, while the activation energy is only $2.15 \mathrm{~kJ} \mathrm{~mol}^{-1}$ when hydrolysis is catalysed by the enzyme sucrase. Explain.
Ans: Biocatalysts are enzymes that act as catalysts in biological reactions. They reduce the size of activation energy by finding a suitable path. Because the enzyme sucrase reduces the activation energy of sucrose hydrolysis from to, enzyme-catalyzed reactions occur at a much faster rate than traditionally catalysed ones.
50: How do you explain the presence of an aldehydic group in a glucose molecule?
Ans: Glucose reacts with hydroxylamine to form a monoxime, which is then combined with one molecule of hydrogen cyanide to form cyanohydrin. The reactions are shown in the table below.
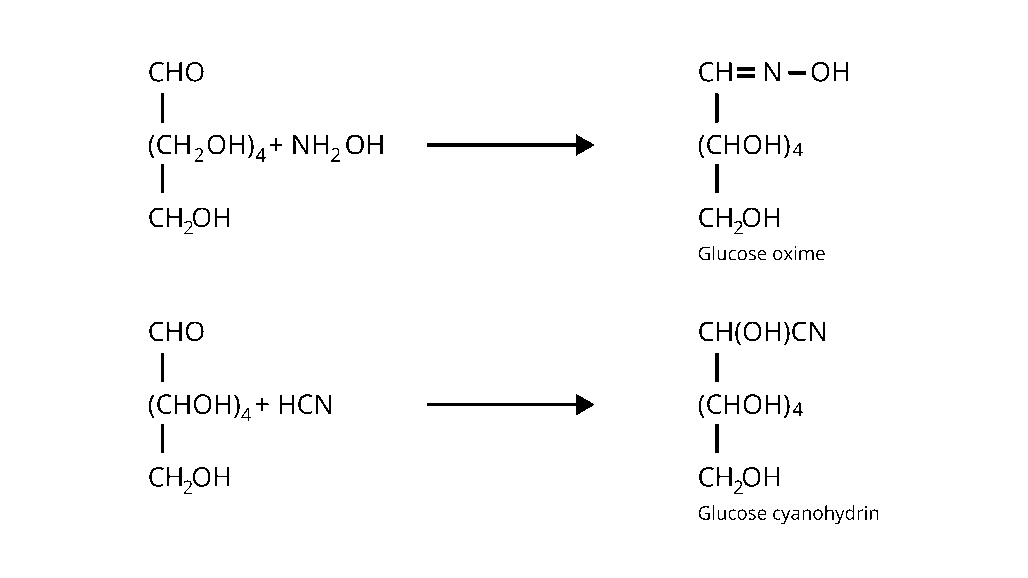
It has a carbonyl group as a consequence, which can be an aldehyde or a ketone. Gluconic acid, a carboxylic acid containing six carbon atoms, is formed when glucose is gently oxidised with bromine water.
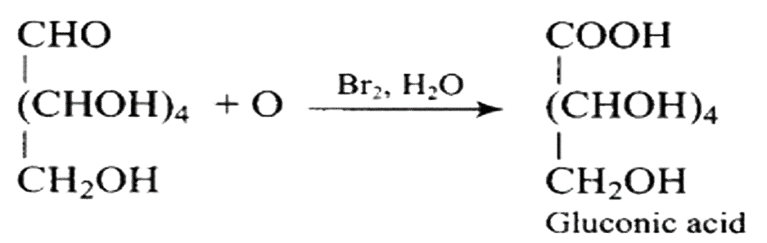
51: Which moieties of nucleosides are involved in the formation of phosphodiester linkages present in dinucleotides? What does the word diester in the name of linkage indicate? Which acid is involved in the formation of this linkage?
Ans: Nucleosides are linked to phosphoric acid at the 5′ position of the sugar moiety to produce a nucleotide. Furthermore, phosphodiester linkage between the 5′ and 3′ carbon atoms of pentose sugar bonds nucleotides (two molecules) together to generate dinucleotide. Phosphoric acid aids in the formation of this connection.
52: What are glycosidic linkages? In which type of biomolecules are they present?
Ans: Two monosaccharide molecules are linked by an oxide bond generated by the loss of a water molecule. Glycosidic linkage is the joining of two monosaccharide units by an oxygen atom.
Disaccharides, trisaccharides, and polysaccharides all have glycosidic linkage.
53: Which monosaccharide units are present in starch, cellulose and glucose and which linkages link these units?
Ans: Starch has $\alpha$ - glucose units, and cellulose has $\beta-D-$ glucose units. In starch and glycogen, there is $\alpha-I$ glycosidic linkage, and in cellulose, there is glycosidic connection $\beta$ - between glucose units.
54: How do enzymes help a substrate to be attacked by the reagent effectively?
Ans: Enzyme active sites bind the substrate molecule in a favourable location so that the reagent may attack it effectively. At each site in an enzyme, there is a substrate binding site to which the substrate binds and the reaction then proceeds.
55: Describe the term $D$ - and $L$ - configuration used for amino acids with examples.
Ans: Sugars are mainly classified into two configurations: $D-$ configuration and $L-$ configuration, each of which have individual distinct structures. These configurations are expressed in terms of the standard glyceraldehyde which are basically of two types:
$D-(+)-$ Glyceraldehyde and $L-(-)-$ Glyceraldehyde. The $D-$ configuration has $-\mathrm{OH}$ attached to the carbon near to $-\mathrm{CH}_{2} \mathrm{OH}$ on the right, whereas the $L-$ configuration has $-\mathrm{OH}$ attached to the carbon adjacent to $-\mathrm{CH}_{2} \mathrm{OH}$ on the left. The sugars are designated $D-$ and $L-$ depending on whether the molecule's conformation is related to $D-$ glyceraldehyde or L-glyceraldehyde. It has been discovered that all naturally occurring sugars, such as $D-$ glucose, $D-$ ribose, and $D-$ fructose, belong to the $D-$ series.
56: How will you distinguish $1^{\circ}$ and $2^{\circ}$ hydroxyl groups present in glucose? Explain with reactions.
Ans: Glucose with acetic anhydride in the presence of pyridine or a few drops of concentrated $\mathrm{H}_{2} \mathrm{SO}_{4}$, leads to productions of penta-acetyl derivatives which shows that there are five hydroxyl groups. One hydroxyl group is a primary $\left(1^{\circ}\right)$ alcoholic, whereas the other four $(\mathrm{C} 2, \mathrm{C} 3, \mathrm{C} 4$, and $\mathrm{C} 5)$ hydroxyl or $-\mathrm{OH}$ groups are all labelled as secondary $\left(2^{\circ}\right)$ alcoholics.
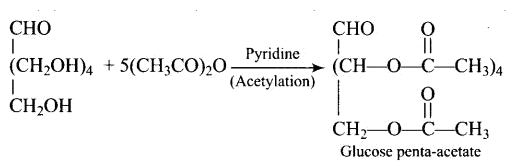
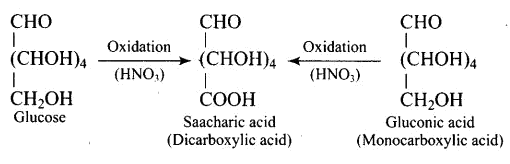
When glucose (or gluconic acid) is oxidized in the presence of $\mathrm{HNO}_{3}$, it produces saccharic acid (a dicarboxylic acid), showing that the primary $\left(1^{\circ}\right)$ alcoholic group is oxidized to $-\mathrm{COOH}$, but only under extreme of the conditions, the secondary $\left(2^{\circ}\right)$
hydroxyl groups undergo oxidation and participate in the reaction.
57: Coagulation of egg white on boiling is an example of denaturation of protein. Explain it in terms of structural changes.
Ans: When an egg is cooked, the soluble globular protein albumin transforms into an insoluble fibrous protein. Bioactivity is lost throughout the denaturation process.
(ii) Albumin protein's secondary and tertiary structures are destroyed, but the primary structure (which contains the -amino acid sequence) is unaffected.
Although egg white is a fluid Ans, when it is cooked, the proteins get denatured and solidify as a result of coagulation.
MATCHING TYPE QUESTIONS
58: Match the vitamins given in Column I with the deficiency disease they cause given in Column II.
Column A | Column B | ||
1 | Vitamin A | a | Pernicious anaemia |
2 | Vitamin B1 | b | Increased blood clotting time |
3 | Vitamin B12 | c | Xerophthalmia |
4 | Vitamin C | d | Rickets |
5 | Vitamin D | e | Muscular weakness |
6 | Vitamin E | f | NIght blindness |
7 | Vitamin K | g | Beri Beri |
h | Bleeding gums | ||
i | Osteomalacia |
Ans:
Column A | Answer | Column B | ||
1 | Vitamin A | c)Xerophthalmia f)NIght blindness | a | Pernicious anaemia |
2 | Vitamin B1 | f)NIght blindness g)Beri Beri | b | Increased blood clotting time |
3 | Vitamin B12 | a)Pernicious anaemia | c | Xerophthalmia |
4 | Vitamin C | h)Bleeding gums | d | Rickets |
5 | Vitamin D | d)Rickets i)Osteomalacia | e | Muscular weakness |
6 | Vitamin E | e)Muscular weakness | f | NIght blindness |
7 | Vitamin K | b)Increased blood clotting time | g | Beri Beri |
h | Bleeding gums | |||
i | Osteomalacia |
59: Match the following enzymes given in Column I with the reactions they catalyse given in Column II.
Column I (Enzymes) | Column II ( Reactions) | ||
1 | Invertase | a | Decomposition in urea into NH3 andCO2 |
2 | Maltase | b | Conversion of glucose into ethyl alcohol |
3 | Pepsin | c | Hydrolysis of maltose into glucose |
4 | Urease | d | Hydrolysis of cane sugar |
5 | Zymase | e | Hydrolysis of proteins into peptides |
Ans:
Column I (Enzymes) | Answer | Column II ( Reactions) | ||
1 | Invertase | Hydrolysis of cane sugar | a | Decomposition in urea into NH3 andCO2 |
2 | Maltase | Hydrolysis of maltose into glucose | b | Conversion of glucose into ethyl alcohol |
3 | Pepsin | Hydrolysis of proteins into peptides | c | Hydrolysis of maltose into glucose |
4 | Urease | Decomposition in urea into NH3 andCO2 | d | Hydrolysis of cane sugar |
5 | Zymase | Conversion of glucose into ethyl alcohol | e | Hydrolysis of proteins into peptides |
ASSERTION AND REASON TYPE
60: Assertion: $D(+)-$ Glucose is dextrorotatory in nature.
Reason: ' $D$ ' represents its dextrorotatory nature.
(i) Assertion and reason both are correct statements and reason explains the assertion.
(ii) Both assertion and reason are wrong statements.
(iii) Assertion is correct statement and reason is wrong statement.
(iv) Assertion is wrong statement and reason is correct statement.
(v) Assertion and reason both are correct statements but reason does not explain
assertion.
Ans: (iii)
Because $D$ (+) - glucose causes plane polarised light to rotate to the right, it is
dextrorotatory. D denotes the relative configuration of glucose in regard to
glyceraldehyde in this situation. As a result, assertion is the proper statement, while
reason is the incorrect statement.
61: Assertion: Vitamin D can be stored in our body.
Reason: Vitamin D is fat soluble vitamin.
(i) Assertion and reason both are correct statements and reason explains the assertion.
(ii) Both assertion and reason are wrong statements.
(iii) Assertion is correct statement and reason is wrong statement.
(iv) Assertion is wrong statement and reason is correct statement.
(v) Assertion and reason both are correct statements but reason does not explain assertion.
Ans: (i)
Vitamins that are fat soluble are soluble in fats and oils but insoluble in water. They can be stored in the liver as well as adipose (fat-storing) tissues and are not excreted in urine . As a result, both assertion and reason are true assertions, and reason clarifies the assertion.
62: Assertion: $\beta$ - glycosidic linkage is present in maltose.
Reason: Maltose is composed of two glucose units in which $\mathrm{C}-1$ of one glucose unit is linked to $C-4$ of another glucose unit.
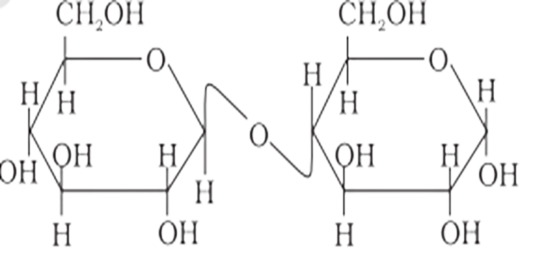
(i) Assertion and reason both are correct statements and reason explains the assertion.
(ii) Both assertion and reason are wrong statements.
(iii) Assertion is correct statement and reason is wrong statement.
(iv) Assertion is wrong statement and reason is correct statement.
(v) Assertion and reason both are correct statements but reason does not explain assertion.
Ans: (iv)
Maltose is made up of two $\alpha-D-$ glucose units, one of which, $C-1$, is coupled to the $\mathrm{C}-4$ of the other one. As a result, maltose contains a $\alpha-$ glycosidic linkage. As a result, the assertion is a false statement, but the reason is true.
63: Assertion: All naturally occurring $\alpha-$ amino acids except glycine are optically active.
Reason: Most naturally occurring amino acids have L- configuration.
(i) Assertion and reason both are correct statements and reason explains the assertion.
(ii) Both assertion and reason are wrong statements.
(iii) Assertion is correct statement and reason is wrong statement.
(iv) Assertion is wrong statement and reason is correct statement.
(v) Assertion and reason both are correct statements but reason does not explain assertion.
Ans: (v)
All other naturally occurring alpha-amino acids, with the exception of glycine, are optically active due to the asymmetry of the $\alpha$ - carbon atom. Both of these ' $D$ ' and ' $L$ ' configurations are available. The majority of amino acids found in nature have a $L-$ configuration. The group on the left side is used to designate $L-$ amino acids.
naturally occurring amino acids have a configuration. The $-\mathrm{NH}_{2}$ group on the left side is used to indicate amino acids.
64: Assertion: Deoxyribose, $\mathrm{C}_{5} \mathrm{H}_{10} \mathrm{O}_{4}$ is not a carbohydrate.
Reason: Carbohydrates are hydrates of carbon so compounds which follow $C_{x}\left(\mathrm{H}_{2} \mathrm{O}\right)_{y}$ formula are carbohydrates.
(i) Assertion and reason both are correct statements and reason explains the assertion.
(ii) Both assertion and reason are wrong statements.
(iii) Assertion is correct statement and reason is wrong statement.
(iv) Assertion is wrong statement and reason is correct statement.
(v) Assertion and reason both are correct statements but reason does not explain assertion.
Ans: (ii)
When DNA (or RNA) is completely hydrolyzed, it produces a 5 carbon based sugar called pentose sugar, phosphoric acid, and nitrogen-containing heterocyclic molecules (called bases). The sugar moiety of the nucleic acid, DNA is $\beta-D-2-$ deoxyribose. Therefore, both assertion and reason are wrong statements.
65: Assertion: Glycine must be taken through diet.
Reason: It is an essential amino acid.
(i) Assertion and reason both are correct statements and reason explains the assertion.
(ii) Both assertion and reason are wrong statements.
(iii) Assertion is correct statement and reason is wrong statement.
(iv) Assertion is wrong statement and reason is correct statement.
(v) Assertion and reason both are correct statements but reason does not explain assertion.
Ans: (ii)
Non-essential amino acids are amino acids that can be synthesized in the body and need not be taken from outside through diet. Glycine is an example of non-essential amino acids and is available in plenty of foods.
66: Assertion: In presence of enzyme, substrate molecule can be attacked by the reagent effectively. Reason: Active sites of enzymes hold the substrate molecule in a suitable position.
(i) Assertion and reason both are correct statements and reason explains the assertion.
(ii) Both assertion and reason are wrong statements.
(iii) Assertion is correct statement and reason is wrong statement.
(iv) Assertion is wrong statement and reason is correct statement.
(v) Assertion and reason both are correct statements but reason does not explain assertion.
Ans: (i)
Because enzyme active sites hold the substrate molecule in a favourable location, a reagent can successfully attack the substrate molecule in the presence of enzyme. As a result, reactions catalysed by enzymes are stereospecific.
LONG ANSWER TYPE QUESTIONS
67: Write the reactions of $D-$ glucose which can’t be explained by its open-chain structure. How can cyclic structure of glucose explain these reactions?
Ans: The open chain structure of glucose explained the majority of its properties. However, it fails to explain the following facts:
I Although glucose has an aldehydic group, it does not undergo all aldehydic reactions. (a) Glucose does not react with ammonia, for example.
(b) Glucose does not create an addition product when it combines with sodium bisulphite.
(c) Glucose does not pass the Schiff's test or the 2, 4-DNP test, unlike other aldehydes.
(ii) Glucose forms an oxime when it reacts with hydroxylamine, but glucose pentaacetate does not. This proves that glucose pentaacetate contains no aldehyde groups.
(iii) There are two stereoisomeric forms of glucose: glucose and glucose. The melting temperatures and optical rotations of these two crystalline forms are different.
(iv) Mutarotation occurs in an aqueous Ans of glucose, which means that its specific rotation decreases when compared to glucose and increases when compared to glucose.
(v) Glucose produces isomeric methyl glucosides. Two isomeric monomethyl compounds are generated when glucose is burnt with methanol in the presence of dry hydrogen chloride gas.
68: On the basis of which evidences $D-$ glucose was assigned the following structure?
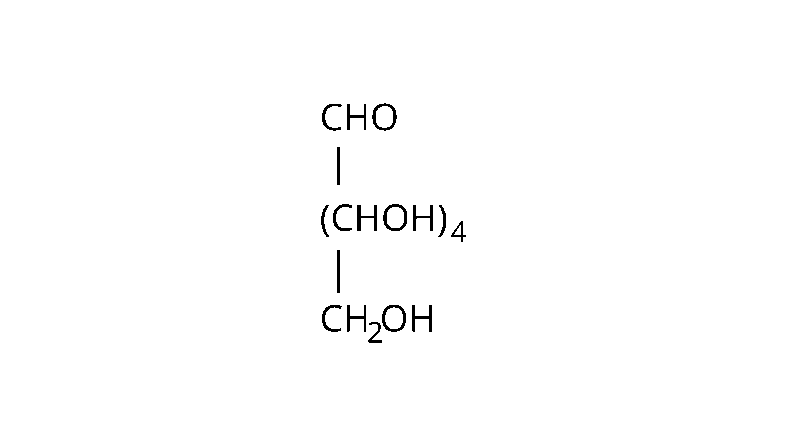
Ans: The following evidences are used to assign the given structure:
Molecular formula; of the molecule glucose is found to be $\mathrm{C}_{6} \mathrm{H}_{12} \mathrm{O}_{6}$.
Straight chain structure: When an aqueous solution of glucose reacts with sodium amalgam, sorbitol (a hexahydric alcohol) is produced by the reaction.
Glucose after a prolonged time of heating with hydroiodic acid and red phosphorus at 100oC gives a mixture of n-hexane and 2-iodohexane as two products. The reaction is depicted below:
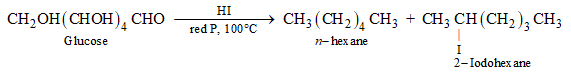
Five hydroxyl groups: When glucose is acetylated in the presence of acetic anhydride, it produces pentaacetate after the reaction. This confirms the presence of five -OH hydroxyl groups in the glucose molecule. The presence of two or more -OH groups on the same carbon atom is known to make the molecules unstable.
Presence of a single primary alcoholic group: When oxidized with concentrated nitric acid, glucose and gluconic acid, these all molecules yield the same product, dicarboxylic acid, saccharic acid, or glucaric acid. The main alcoholic group $\left(\mathrm{CH}_{2} \mathrm{OH}\right)$ is always present at the end of the carbon chain in the molecule.
Presence of an aldehyde group: Glucose molecule combines with the hydroxylamine $\left(\mathrm{NH}_{2} \mathrm{OH}\right)$ to produce glucose $\mathrm{CHO}$ oxime as the product. This implies that glucose has carbonyl $(\mathrm{CHOH})_{4}(>\mathrm{C}=\mathrm{O})$ groups as it is needed to react in order to produce an oxime product.
69: Carbohydrates are essential for life in both plants and animals. Name the carbohydrates that are used as storage molecules in plants and animals, also name the carbohydrate which is present in wood or in the fibre of cotton cloth.
Ans: Plants and animals employ the following carbohydrates as storage molecules:
i) Starch, cellulose, sucrose, and other sugars make up the majority of plant material.
(ii) Animals' bodies contain glycogen. Glycogen is commonly referred to as animal starch as a result of this. Glycogen is found in the liver, muscles, and brain, and an enzyme converts glycogen to glucose when the body wants it.
(iii) Cellulose is a type of cellulose that can be found in wood and fabric fibre.
70: Explain the terms primary and secondary structure of proteins. What is the difference between $\alpha$-helix and $\beta$ - pleated sheet structure of proteins?
Ans: Proteins can have a single polypeptide chain or many polypeptide chains as their basic structure. A considerable number of $\alpha$ - amino acids are joined in a particular sequence in each polypeptide chain. The specific order in which the various $\alpha-$ amino acids found in a protein are linked to one another is the protein's primary structure. Any change to the main structure, i.e. the amino acid sequence, results in a different protein.
Protein secondary structure: Secondary structure defines how polypeptide chains are folded or structured. As a result, the shape or conformation of the protein molecule is determined. This is explained by the planar geometry of the peptide bond and the hydrogen link between the and groups of separate peptide bonds.
An $\alpha$ - helix is a polypeptide chain that twists into a right-handed screw (helix), with the $-\mathrm{NH}$ group of each amino acid residue hydrogen bonded to the the $-\mathrm{C}=\mathrm{O}$ group of the next turn of the helix. The a-helix structure is also known as the $3.613$ helix structure.
$\beta$ - pleated sheet structure of proteins: The polypeptide chains are zigzagged side by side in this shape, with alternative R groups on the same side spaced at fixed intervals. The two nearby polypeptide chains are held together by intermolecular H-bonds. This type of interbounded chain can be used to make a sheet. These sheets are then stacked one on top of the other to create a three-dimensional structure, similar to the pages of this book.
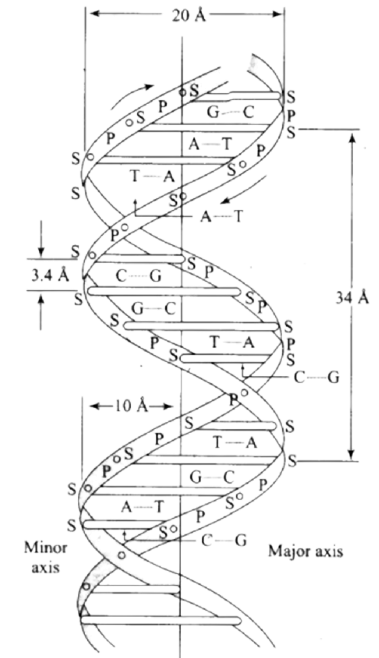
71: Write the structures of fragments produced on complete hydrolysis of DNA. How are they linked in DNA molecule? Draw a diagram to show pairing of nucleotide bases in double helix of DNA.
Ans: When DNA is fully degraded, a pentose sugar, phosphoric acid, and nitrogen-containing heterocyclic compounds known as bases are produced. Below is a diagram illustrating the structure of sugar and phosphoric acid.
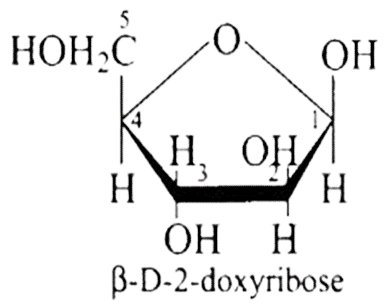
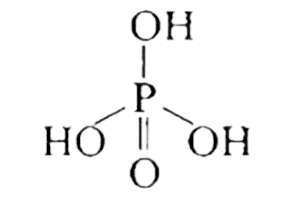
DNA contains four bases adenine (A), guanine (G), cytosine (C) and thymine (T). The structures of all bases are shown below.
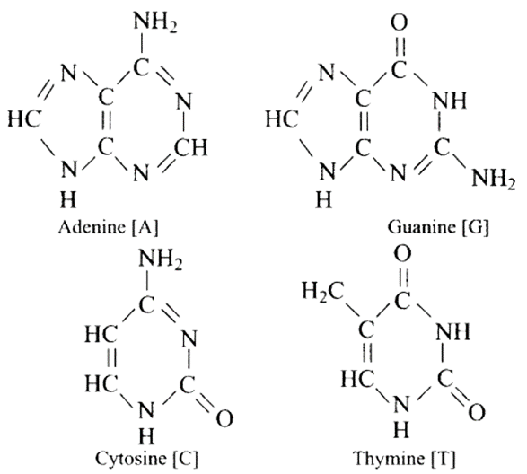
The attachment of a base to the 1'-position of sugar produces a nucleoside. When a nucleoside attaches to phosphoric acid at the sugar moiety's 5′ position, a nucleotide is formed. Nucleotides are linked through the phosphodiester linkage between the 5' and 3' carbon atoms of the pentose sugar.
In DNA, two nucleic acid chains wrap around each other and are held together by hydrogen bonds between their bases.
Summary of the Chapter
Chapter 14 on Biomolecules starts by defining what a molecule is. Complex organic compounds that govern the common activities of all living organisms are named biomolecules. Students then learn about Carbohydrates in detail. The classification of the carbohydrates into monosaccharides, oligosaccharides, polysaccharides and reducing/non-reducing sugars has been explained through the help of examples.
Glucose is an important sub-topic that has been dealt with next. A vast description on it has been provided along with a description of Fructose. The distinction between them has then been made clear. Students then learn about disaccharides and polysaccharides. Nucleic Acids, enzymes, hormones come next. The chapter finishes with a brief note on Biomolecules.
The NCERT Exemplar for Class 12 Chemistry - Biomolecules along with solutions is available for free download in PDF format from Vedantu. The solutions are written by experts keeping in mind the student’s intellect, the guidelines by CBSE and the competitive requirement of exams. These thoughtfully created solutions are the perfect companion to have along with the NCERT Exemplar during your preparatory stage. Together these will help you strengthen your understanding of concepts and also boost your confidence in approaching the exam.
FAQs on NCERT Exemplar for Class 12 Chemistry - Biomolecules - Free PDF Download
1. What are Nucleic acids in Class 12’s Chemistry -Chapter 14?
Nucleic acids are a vital class of macromolecules that are found in all the cells as well as viruses. In-depth information about Nucleic acids has been given in NCERT Exemplar for Class 12 Chemistry Chapter 14 - Biomolecules. The chapter has been entirely encapsulated in this book which is available for students on Vedantu. Related questions along with their proposed solutions are also there which makes it easier for all students to understand the properties of the acid in their own terms.
2. How do I find NCERT Exemplar for Class 12 Chemistry Chapter 14 - Biomolecules online?
You can check out Vedantu for the relevant material. This PDF of solved exercises has been created by Chemistry professionals and teachers and is a great guide. By solving all the exercises, one is bound to do well in the examinations. This book will also help decrease your time of solving papers as time management will be a skill that’ll come in handy during tests and otherwise. One can scan this book before one takes assessments or oral exams and feel confident in the subject.
3. How can I increase my speed of solving papers for my Class 12 Chemistry exam?
You can increase the speed of solving all papers by referring to NCERT Exemplar for Class 12 Chemistry available on Vedantu. The book has been curated by Chemistry teachers and is a real boon for those who are looking for practice questions. You can download these papers in PDF format and then study and solve those with ease. Timing yourself while you solve these papers will prove beneficial before the exams that take place. Make sure that you are regular at it and do not give up when the exercises seem too challenging. Instead, observe how they have been solved by the experts and learn from those.
4. Who has created the NCERT Exemplar for Class 12 Chemistry Chapter 14 - Biomolecules?
The PDF has been created in collaboration with Chemistry teachers from leading institutions as well as experts. The book has been made keeping the NCERT guidelines in mind and so is an ideal guide for exams and competitive tests. Solving exercises from this book will clarify all those concepts that needed revision as well as those which weren’t quite clear. The book is available on Vedantu and will not cost you anything. One can rest assured that only the most useful material has been included in the book and that nothing is out of the curriculum. Free resources are available for easy access on the Vedantu app and also the website.
5. Where can I find the test for biomolecules for Class 12 Chemistry Chapter 14?
You will find relevant material on the above at Vedantu if you check out NCERT Exemplar for Class 12 Chemistry Chapter 14 - Biomolecules. The test for biomolecules as well as other related matters has been explained in the notes and the solved book of exercise questions will further bolster your knowledge in the subject.
These concepts need to be absolutely crystal clear for the students to comprehend the later concepts in the successive grades and the book takes care of that aspect.

























