NCERT Exemplar for Class 12 Chemistry - Haloalkanes and Haloarenes - Free PDF Download
Free PDF download of NCERT Exemplar for Class 12 Chemistry Chapter 10 - Haloalkanes and Haloarenes solved by expert Chemistry teachers on Vedantu as per NCERT (CBSE) Book guidelines. All Chapter 10 - Haloalkanes and Haloarenes exercise questions with solutions to help you to revise the complete syllabus and score more marks in your examinations.
Organic Chemistry is the most challenging part of chemistry (According to most students! Not us)
What if we tell you that Organic Chemistry is not all that tough? We understand that if your initial reaction to this was a lot of confusion, it is only because of how you have been looking at the subject. Organic Chemistry once studied properly can potentially be one of your favourite parts of Chemistry as a whole. That is something we can promise.
Access NCERT Exemplar Solutions for Class–12 Chemistry Chapter 10- Haloalkanes and Haloarenes
Exercise
I. Multiple Choice Questions (Type-I)
1. The order of reactivity of following alcohols with halogen acids is
(A) $C{H_3}C{H_2} - C{H_2} - OH$
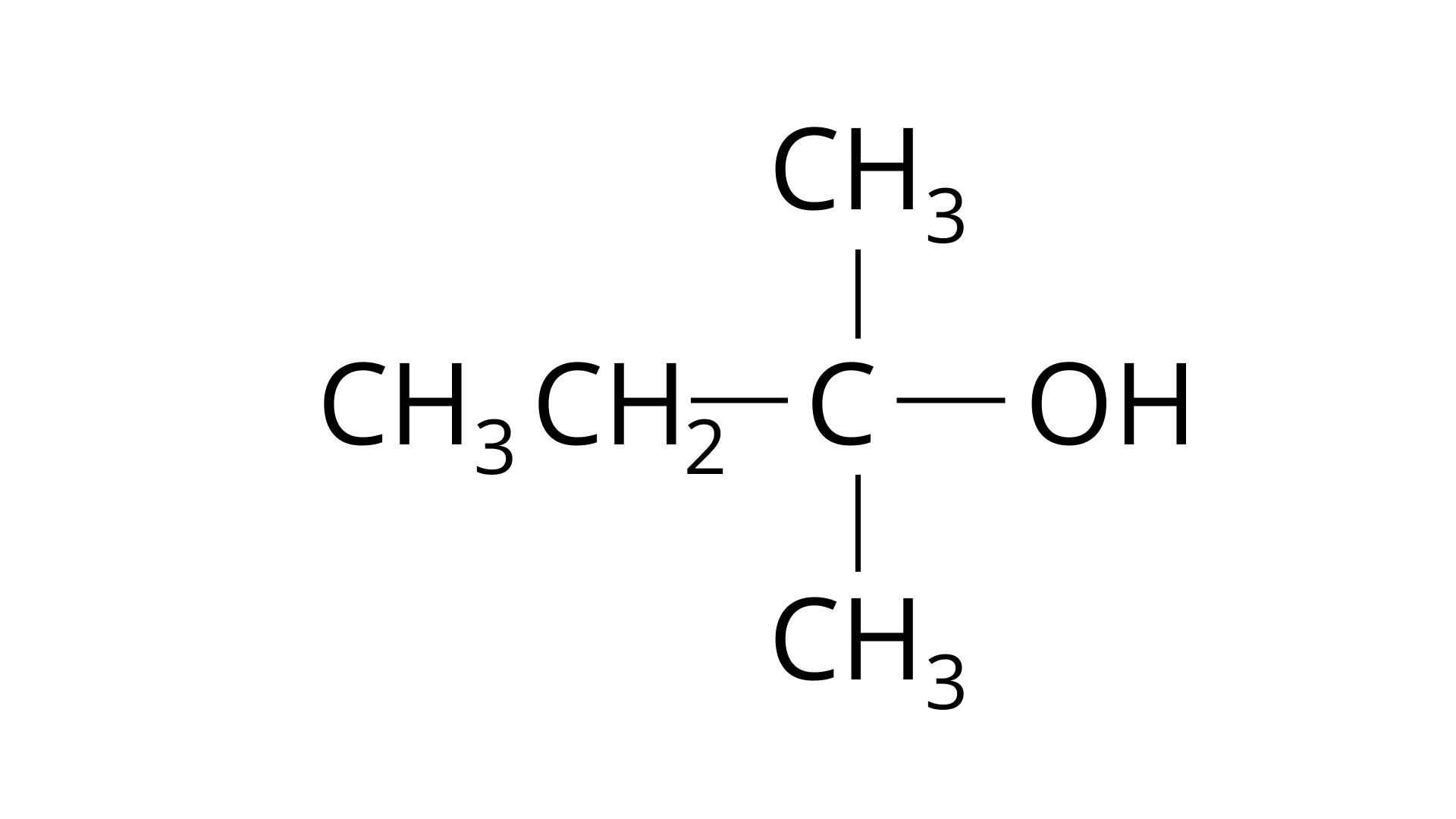
(B)
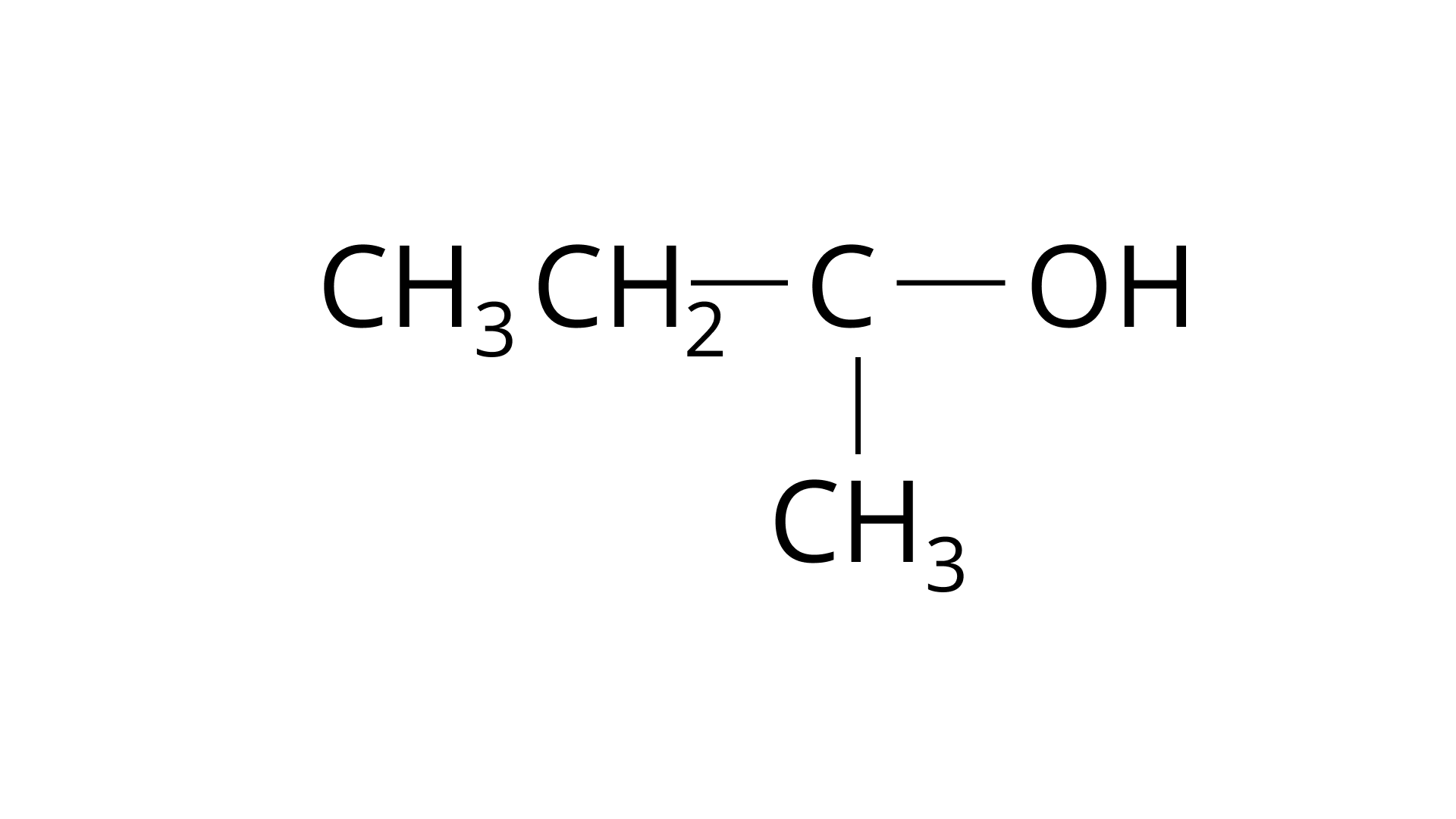
(C)
(i) \[(A) > (B) > (C)\]
(ii) \[(C) > (B) > (A)\]
(iii) \[(B) > (A) > (C)\]
(iv) \[(A) > (C) > (B)\]
Ans: The Correct Answer is Option (ii). Haloalkanes are made by combining alcohols with halogen acids, in which the hydroxyl group of the alcohol is replaced by the halogen. Primary, secondary, and tertiary alcohols are represented by options (A), (B), and (C). Tertiary alcohols are more reactive than secondary and primary alcohols, and they generate haloalkanes from haloacids without the need of catalysts at ambient temperature. Alcohols have a reactivity order of \[3^\circ > 2^\circ > 1^\circ \]. As a result, the proper option is (ii).
2. Which of the following alcohols will yield the corresponding alkyl chloride on reaction with concentrated \[HCl\] at room temperature?
(i) \[C{H_3}C{H_2} - C{H_2} - OH\]
(ii) \[C{H_3}C{H_2} - \mathop {CH}\limits_{\mathop |\limits_{C{H_3}} } - OH\]
(iii) \[C{H_3}C{H_2} - \mathop {CH}\limits_{\mathop |\limits_{C{H_3}} } - C{H_2}OH\]
(iv)
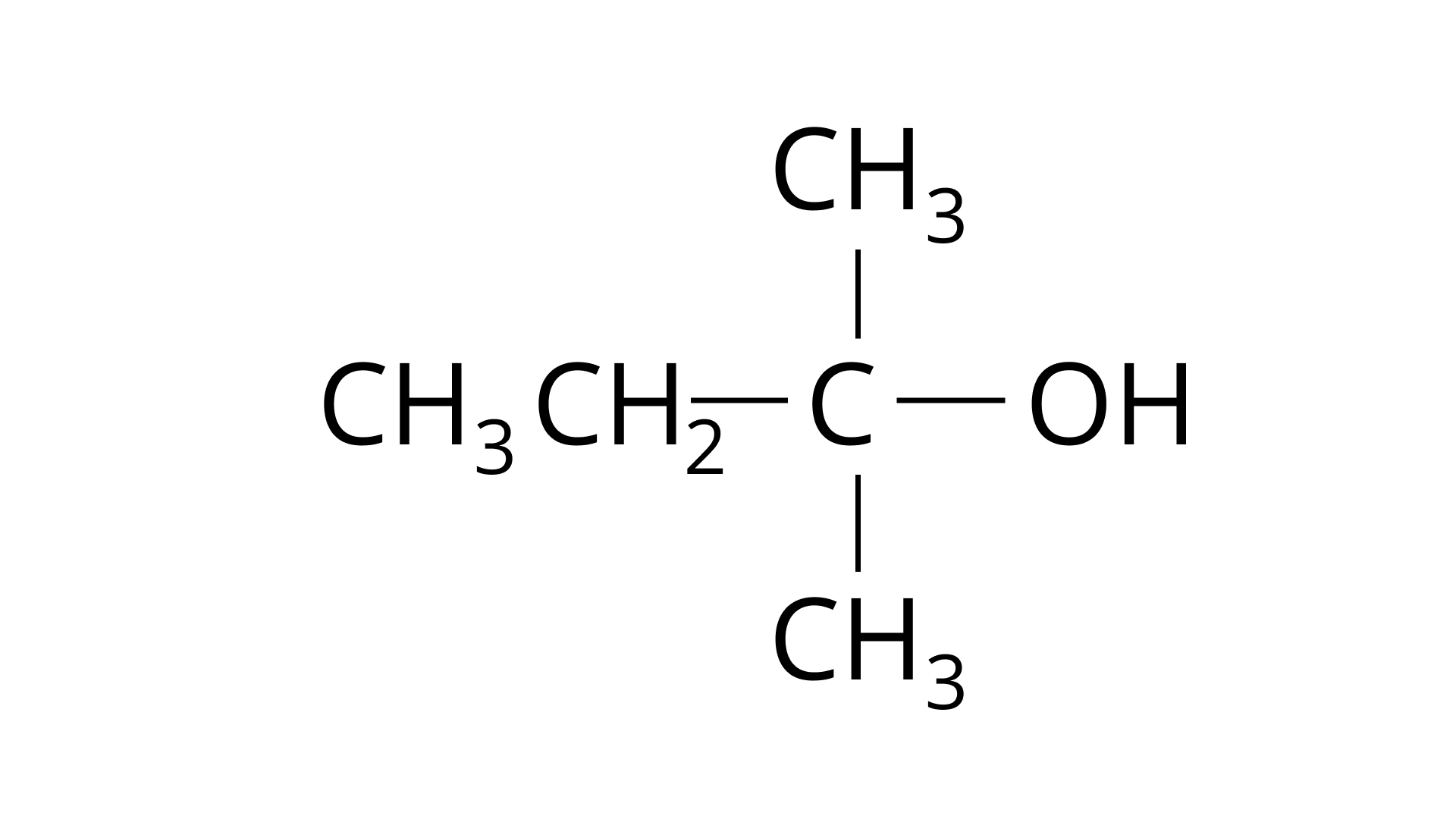
Ans: The Correct Answer is Option (iv)Because the tertiary carbocation is the most stable, it interacts the most with concentrated \[{\text{HCl}}\]. As a result, for tertiary alcohol, room temperature is sufficient for the reaction. However, for primary and secondary alcohols, the presence of a catalyst (\[{\text{ZnC}}{{\text{l}}_{\text{2}}}\]) is required.
As a result, option (iv) is accurate.
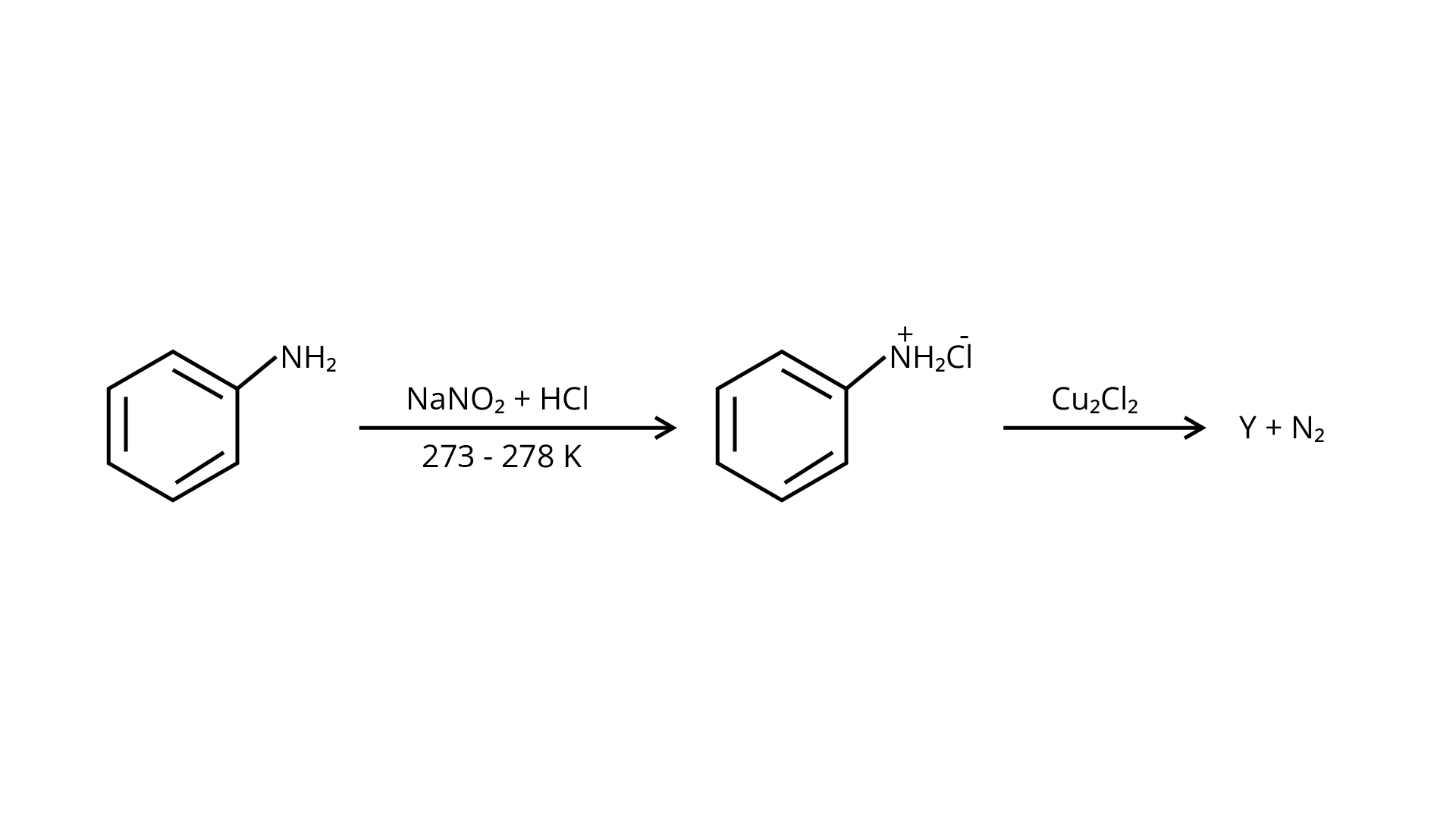
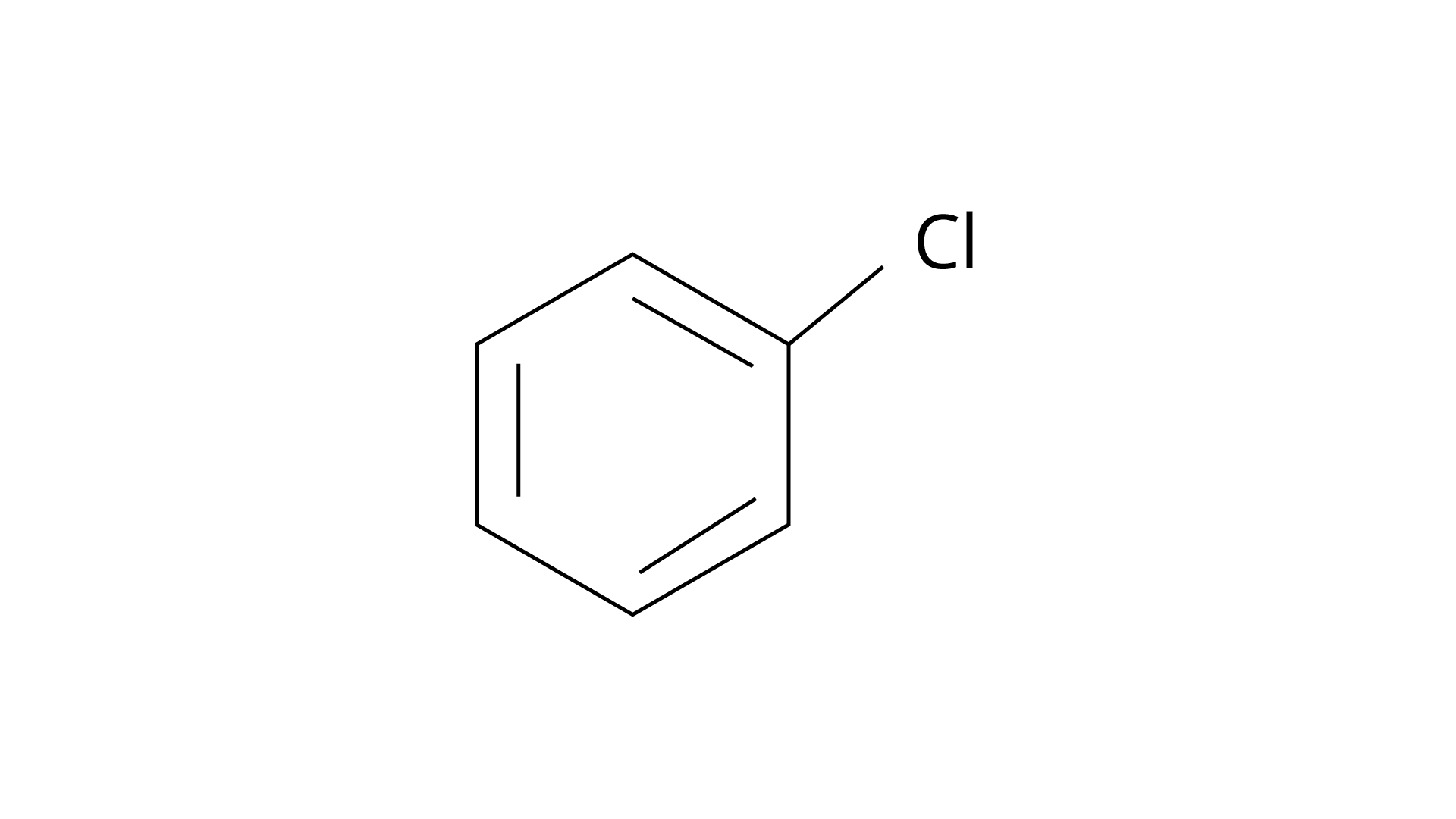
3. Identify the compound \[Y\] in the following reaction.
(i).
(ii).
(iii).
(iv).
Ans: The correct answer is (i). Sandmeyer's reaction can be used to synthesise haloarenes from amines. A primary aromatic amine that has been dissolved or suspended in cold aqueous mineral acid is treated with sodium nitrite to create a diazonium salt in this procedure. When this freshly produced salt is combined with cuprous chloride, the diazonium group is replaced with \[{\text{ - Cl}}\], resulting in aryl chloride. Option I is the chemical Y, which is an aryl chloride.
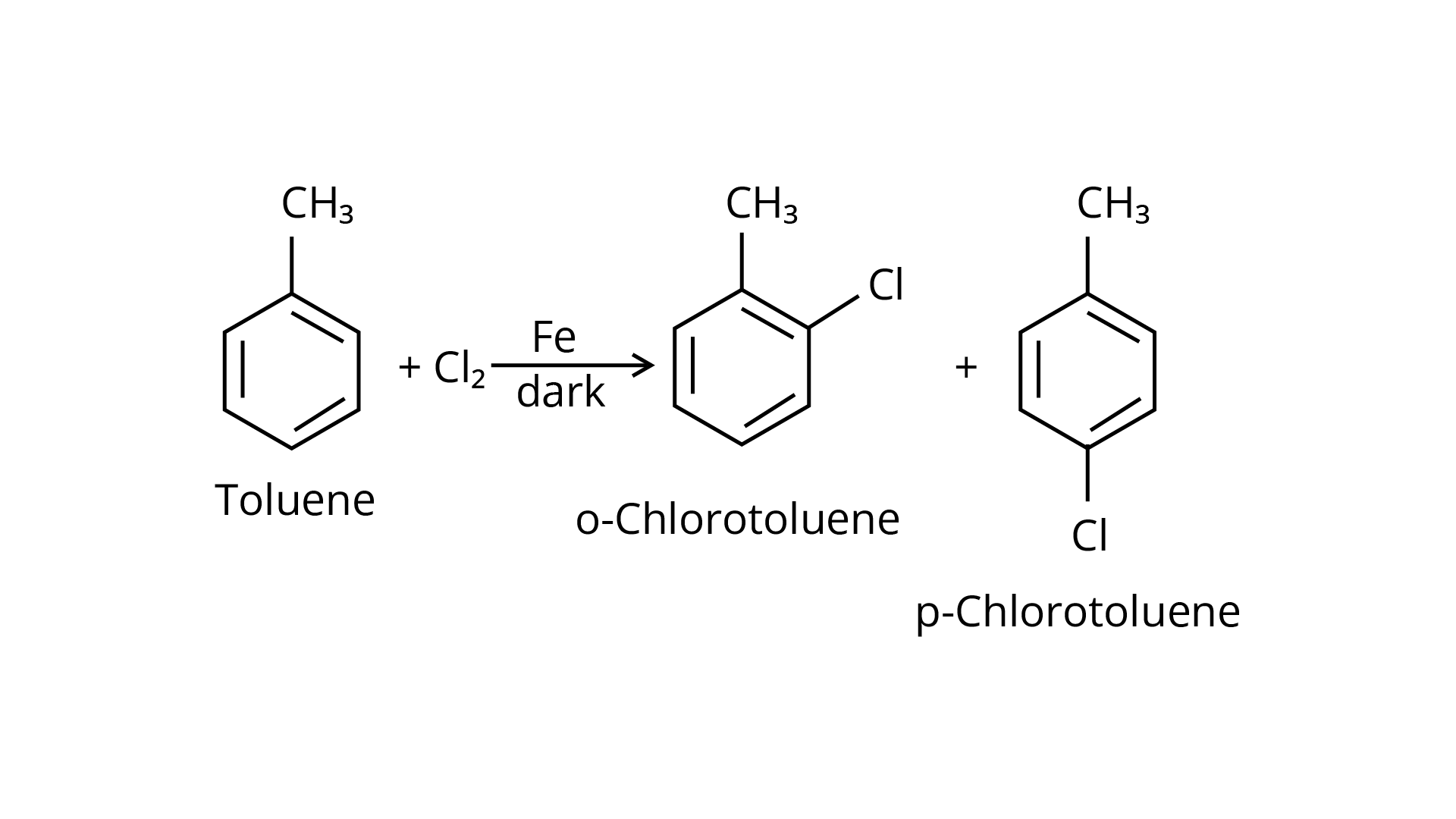
4. Toluene reacts with a halogen in the presence of iron (III) chloride giving ortho and para halo compounds. The reaction is
(i) Electrophilic elimination reaction
(ii) Electrophilic substitution reaction
(iii) Free radical addition reaction
(iv) Nucleophilic substitution reaction
Ans: The Correct Answer is Option (ii). Toluene is an aromatic hydrocarbon (\[{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}{\text{---C}}{{\text{H}}_{\text{3}}}\]) that may be treated with halogens and utilised in an electrophilic substitution process to create aryl halides in the presence of the Lewis acid catalyst iron (III) chloride. In the absence of light, the reaction is carried out using chlorine or bromine, and the products are o- and p- haloarenes.
5. Which of the following is halogen exchange reaction?
(i) $\mathrm{RX}+\mathrm{NaI} \longrightarrow \mathrm{RI}+\mathrm{NaX}$
(ii)
(iii) \[R - OH + HX\mathop {\xrightarrow{{}}}\limits^{ZnC{l_2}} R - X + {H_2}O\]
(iv)
Correct Answer: option (i)
Ans: The correct answer is option (i). Option (i) is an example of a Finklestein reaction, which is a halogen exchange reaction that produces alkyl iodide by treating alkyl halides with \[{\text{NaI}}\] in dry acetone. Option (ii) is an addition reaction in which an alkene is transformed to the equivalent alkyl halide. Option (iii) is a substitution reaction, whereas option (iv) is an electrophilic substitution reaction.
6. Which reagent will you use for the following reaction?
(i) light
(ii) \[NaCl + {H_2}S{O_4}\]
(iii) \[C{l_2}\]gas in dark
(iv) \[C{l_2}\] gas in the presence of iron in dark
Ans: The correct Answer is Option (i).
Chlorination in the presence of UV light can be used to produce alkyl chlorides from alkanes. Under the action of UV radiation, the chlorine molecule generates free radicals, which react with alkanes to create a mixture of isomeric mono- and poly haloalkanes.
Step 1: $\mathrm{Cl}-\mathrm{Cl} \underset{\text { light }}{\stackrel{\mathrm{UV}}{\longrightarrow}} 2 \stackrel{\bullet}{\mathrm{C}} \mathrm{l}$
$\dot{\mathrm{Cl}}+\mathrm{CH}_{3}-\mathrm{CH}_{2}-\mathrm{CH}_{2}-\mathrm{CH}_{3} \longrightarrow \mathrm{CH}_{3} \mathrm{CH}_{2} \mathrm{CH}_{2}-\dot{\mathrm{C}} \mathrm{H}_{2}+\mathrm{HCl}$
Step 2: $\mathrm{CH}_{3}-\mathrm{CH}_{2}-\mathrm{CH}_{2}-\dot{\mathrm{C}} \mathrm{H}_{2}+\mathrm{Cl}_{2} \longrightarrow \mathrm{CH}_{3}-\mathrm{CH}_{2}-\mathrm{CH}_{2}-\mathrm{CH}_{2} \mathrm{Cl}+\dot{\mathrm{Cl}}$
Step 3: $\mathrm{CH}_{3}-\mathrm{CH}_{2}-\mathrm{CH}_{2}-\dot{\mathrm{C}} \mathrm{H}_{2}+\dot{\mathrm{Cl}} \longrightarrow \mathrm{CH}_{3} \mathrm{CH}_{2} \mathrm{CH}_{2} \mathrm{CH}_{2} \mathrm{Cl}$
7. Arrange the following compounds in the increasing order of their densities.
(a)
(b)
(c)
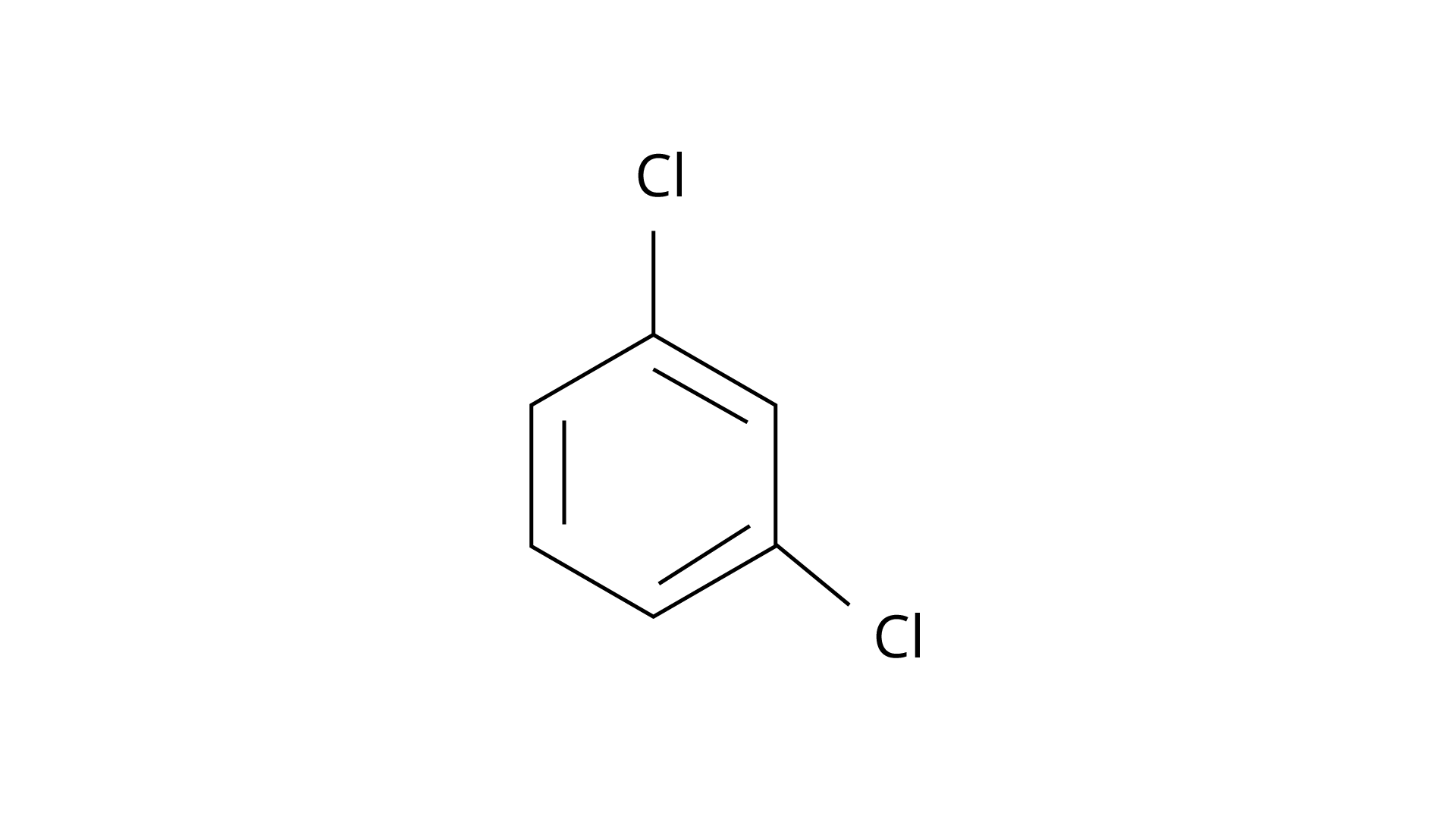
(d)
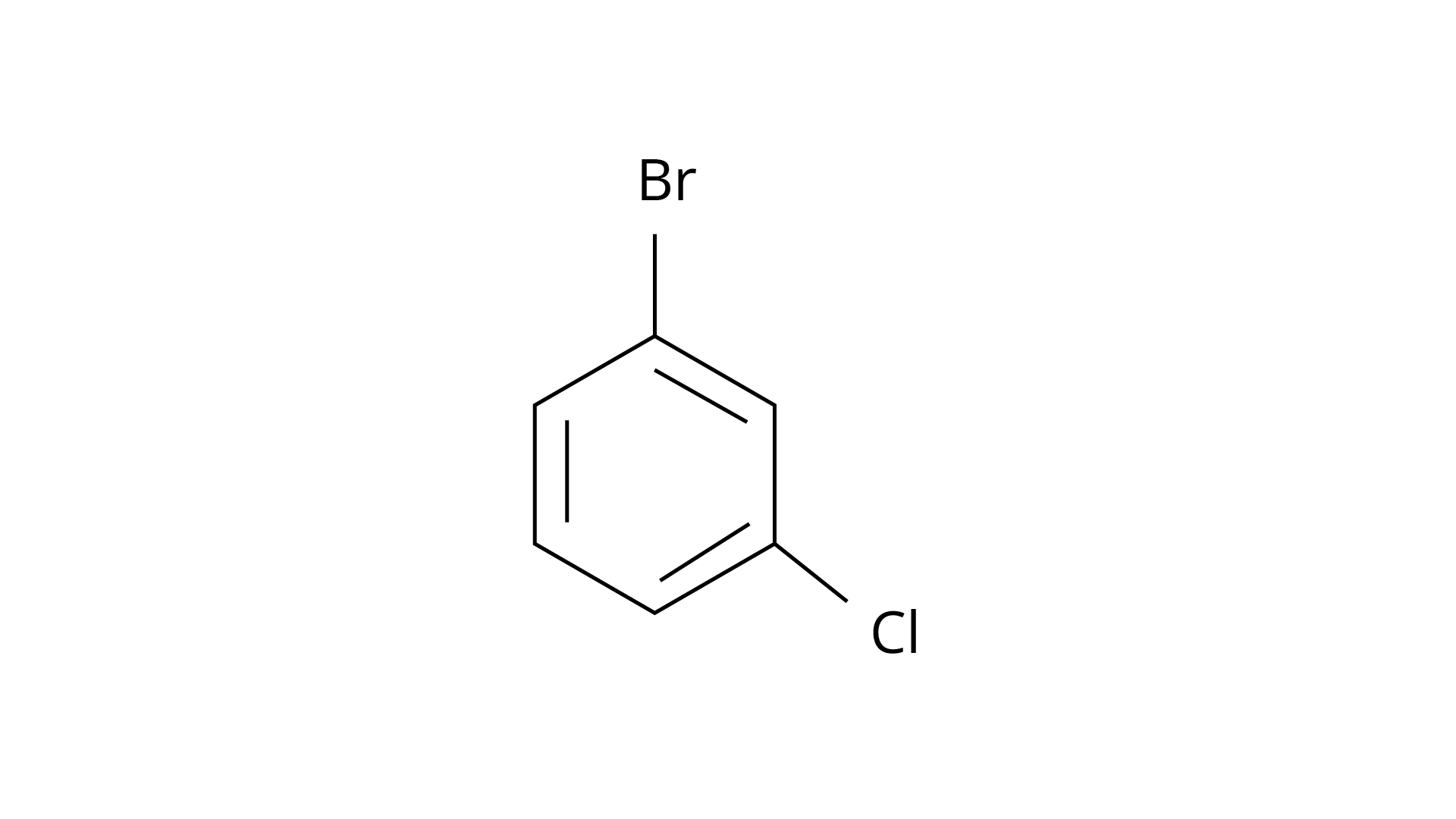
(i) \[(a) < (b) < (c) < (d)\]
(ii) \[(a) < (c) < (d) < (b)\]
(iii) \[(d) < (c) < (b) < (a)\]
(iv) \[(b) < (d) < (c) < (a)\]
Ans: The Correct Answer is Option (i).
Alkyl halides have a higher density than water. Their densities are determined by the masses of the halogen atoms, the number of halogen atoms, and the number of carbon atoms. Simply expressed, \[{\text{Br}}\] has an atomic mass of 79 while \[{\text{Cl}}\] has an atomic mass of 35. (d) will be the heaviest of the molecules, followed by (c), (b), and (a). Because density is exactly proportional to mass, the order of decreasing densities will be the same. The right answer is (i).
8. Arrange the following compounds in increasing order of their boiling points.
(a) \[{}_{{}_{C{H_3}}/}^{{}^{C{H_3}}\backslash }CH - C{H_2}Br\]
(b) \[C{H_3}C{H_2}C{H_2}C{H_2}Br\]
(c) \[{H_3}C - \mathop C\limits_{\mathop |\limits_{Br} }^{\mathop |\limits^{C{H_3}} } - C{H_3}\]
(i) \[(b) < (a) < (c)\]
(ii) \[(a) < (b) < (c)\]
(iii) \[(c) < (a) < (b)\]
(iv) \[(c) < (b) < (a)\]
Ans: The Correct Answer is Option(iii).
Isomeric alkyl halides' boiling points are related to their branching, with a reduction in B.P. as branching increases. As a result, the boiling point of the tertiary isomer is the lowest, while that of the primary isomer is the highest. Thus, the order of lowering boiling points is (b) > (a) > (c).
9. In which of the following molecules carbon atom marked with asterisk (*) is asymmetric?
(a)
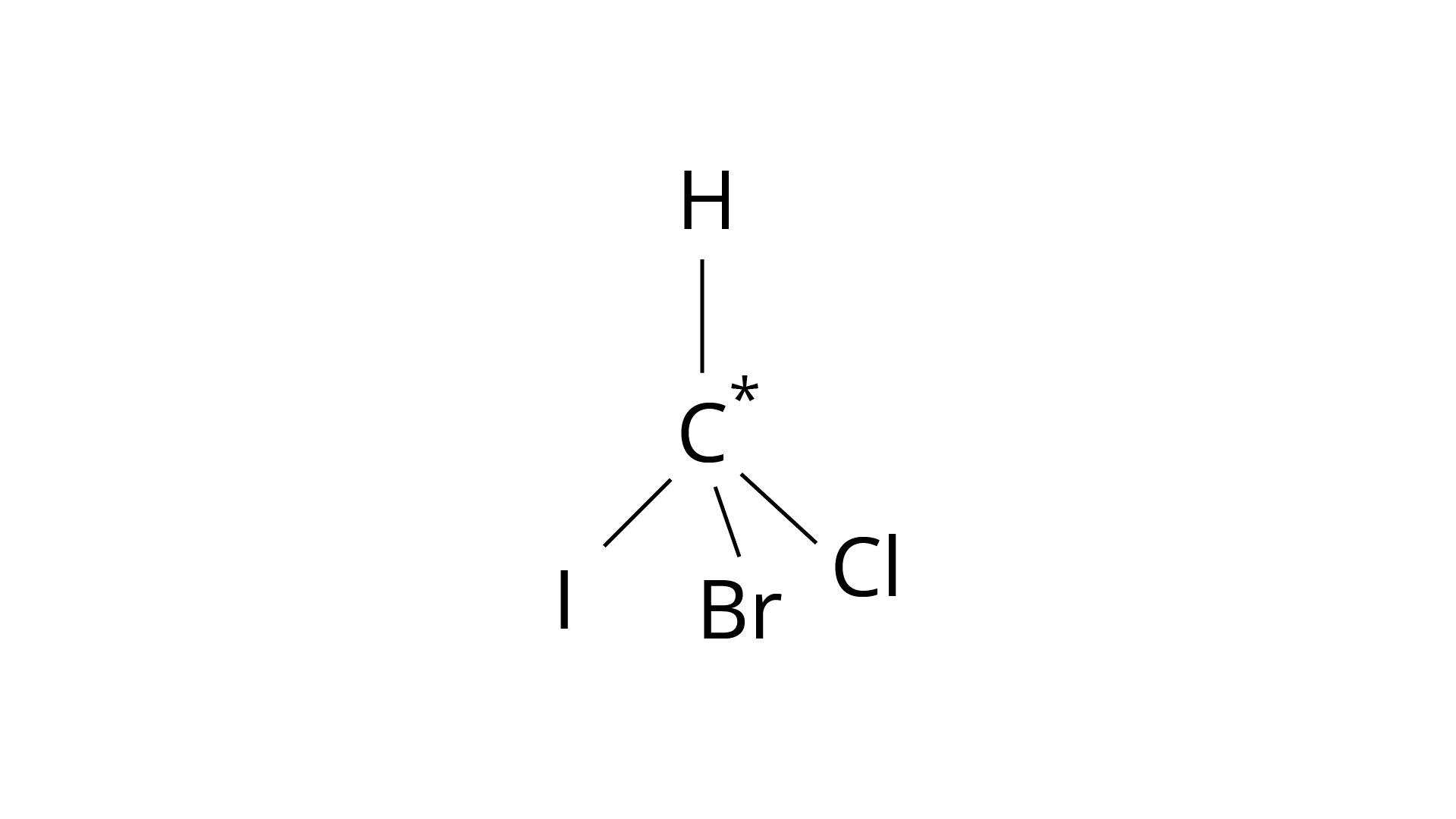
(b)
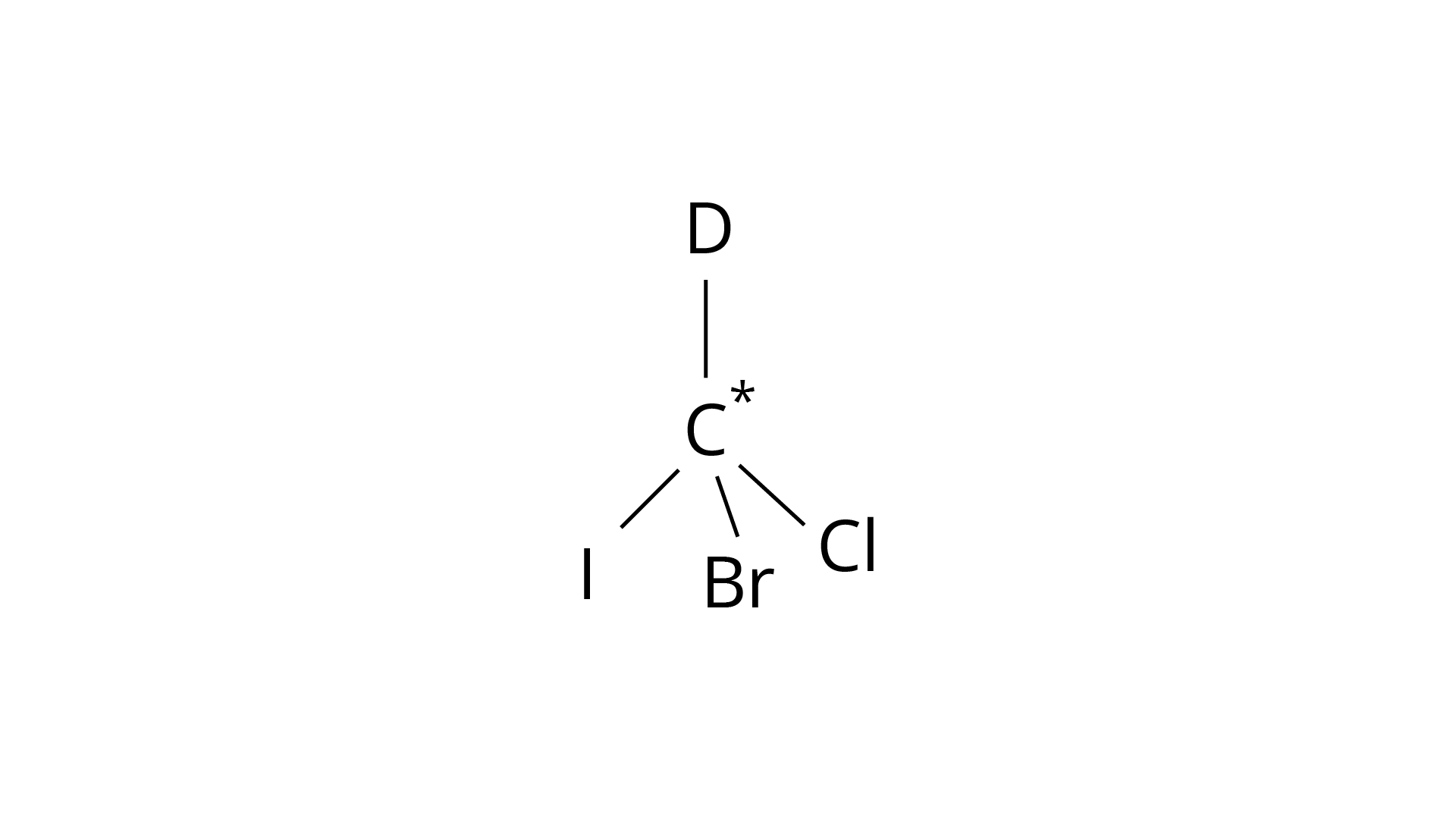
(c)
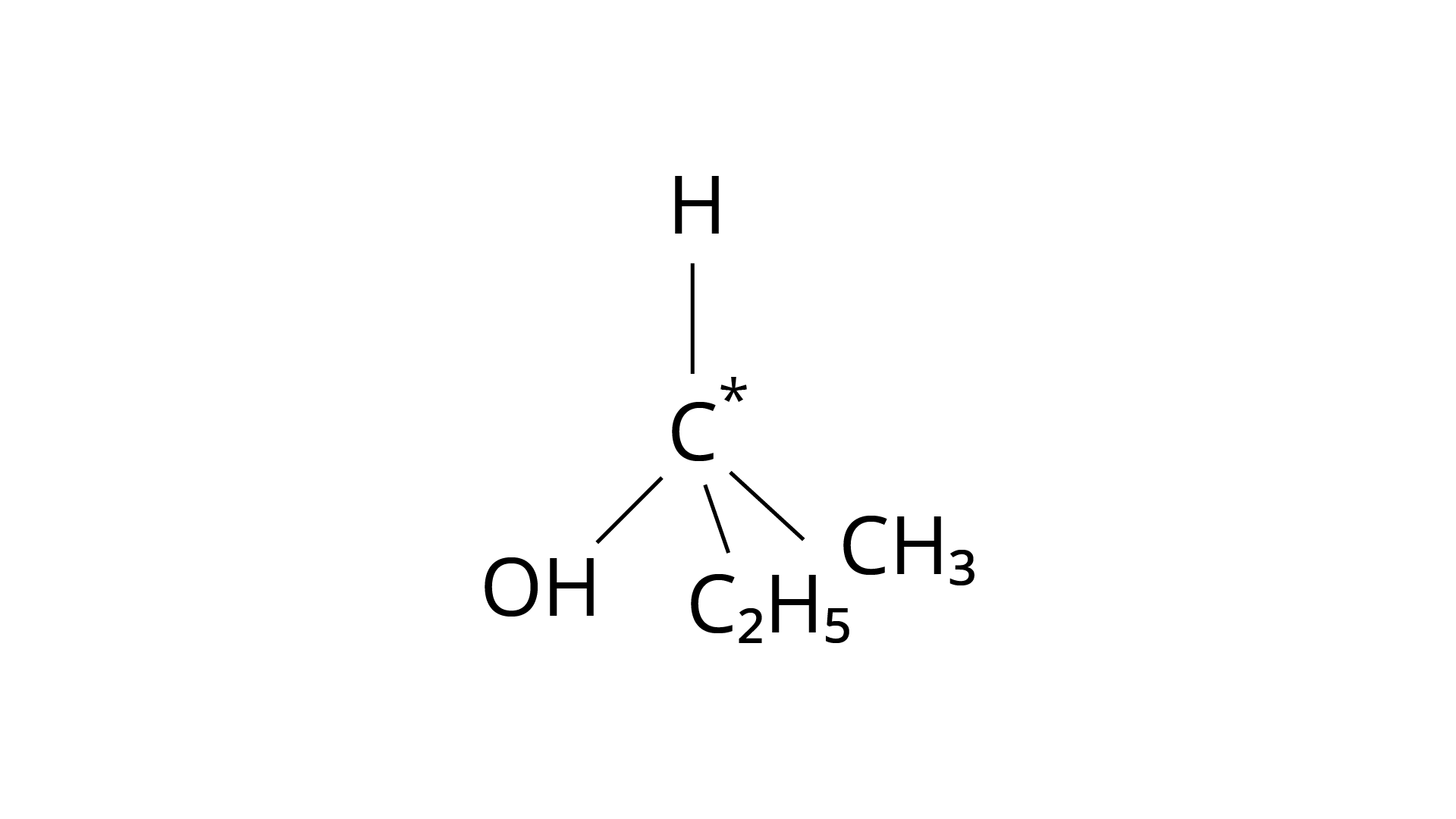
(d)
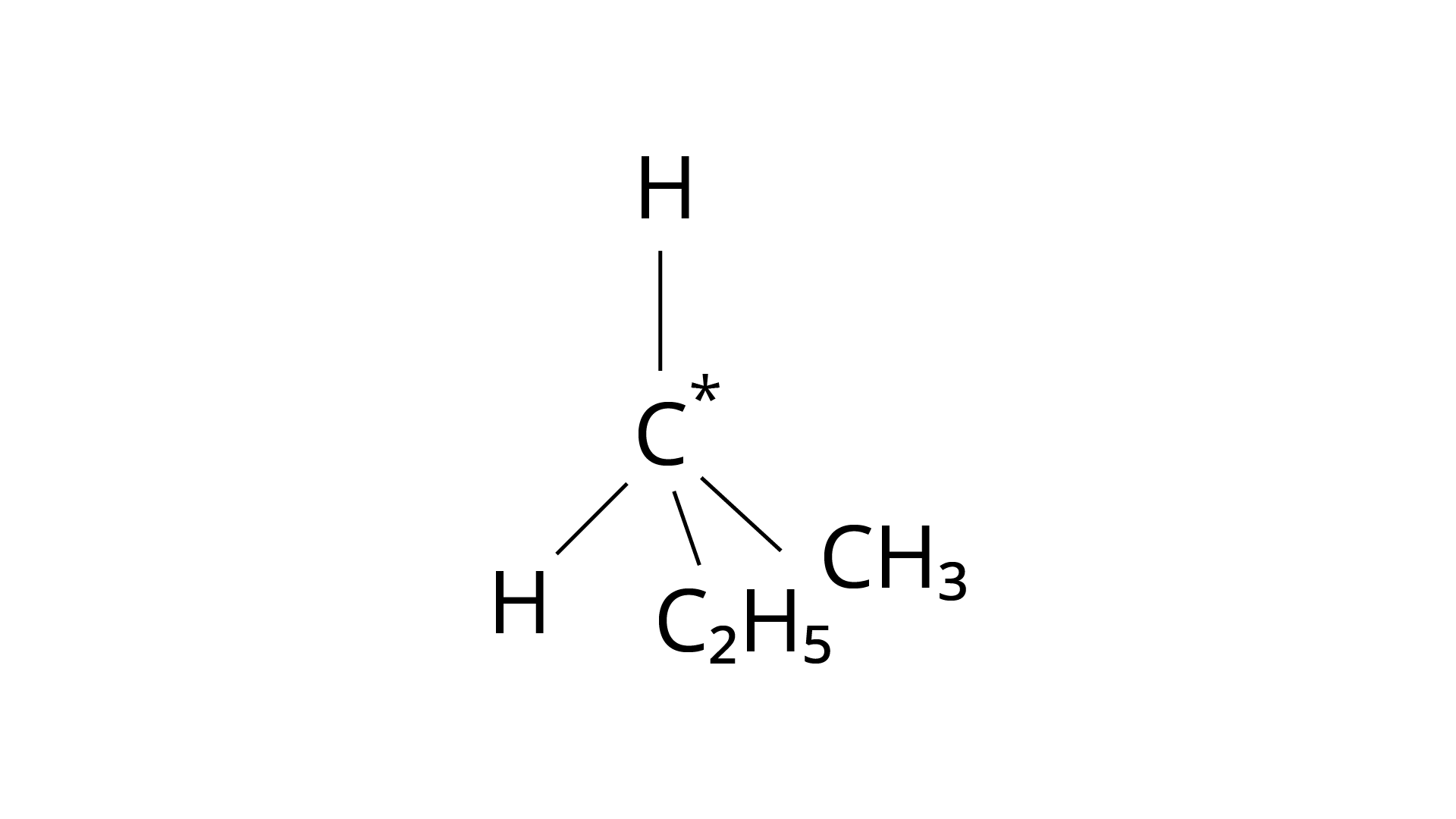
Correct Answer: Option (ii)
(I) \[(a), (b), (c), (d)\]
(ii) (a), (b), (c)
(iii) (b), (c), (d)
(iv) (a), (c), (d)
Ans: The correct answer is (ii).The mirror copies of the molecules (a), (b), and (c) are non-superimposable. Because all four species linked to the carbon atom are identical, molecule (d) cannot have an asymmetric carbon atom, thus the mirror image of (d) when rotated \[180^\circ \] is superimposable on the original picture.
10. Which of the following structures is enantiomeric with the molecule (A) given below :
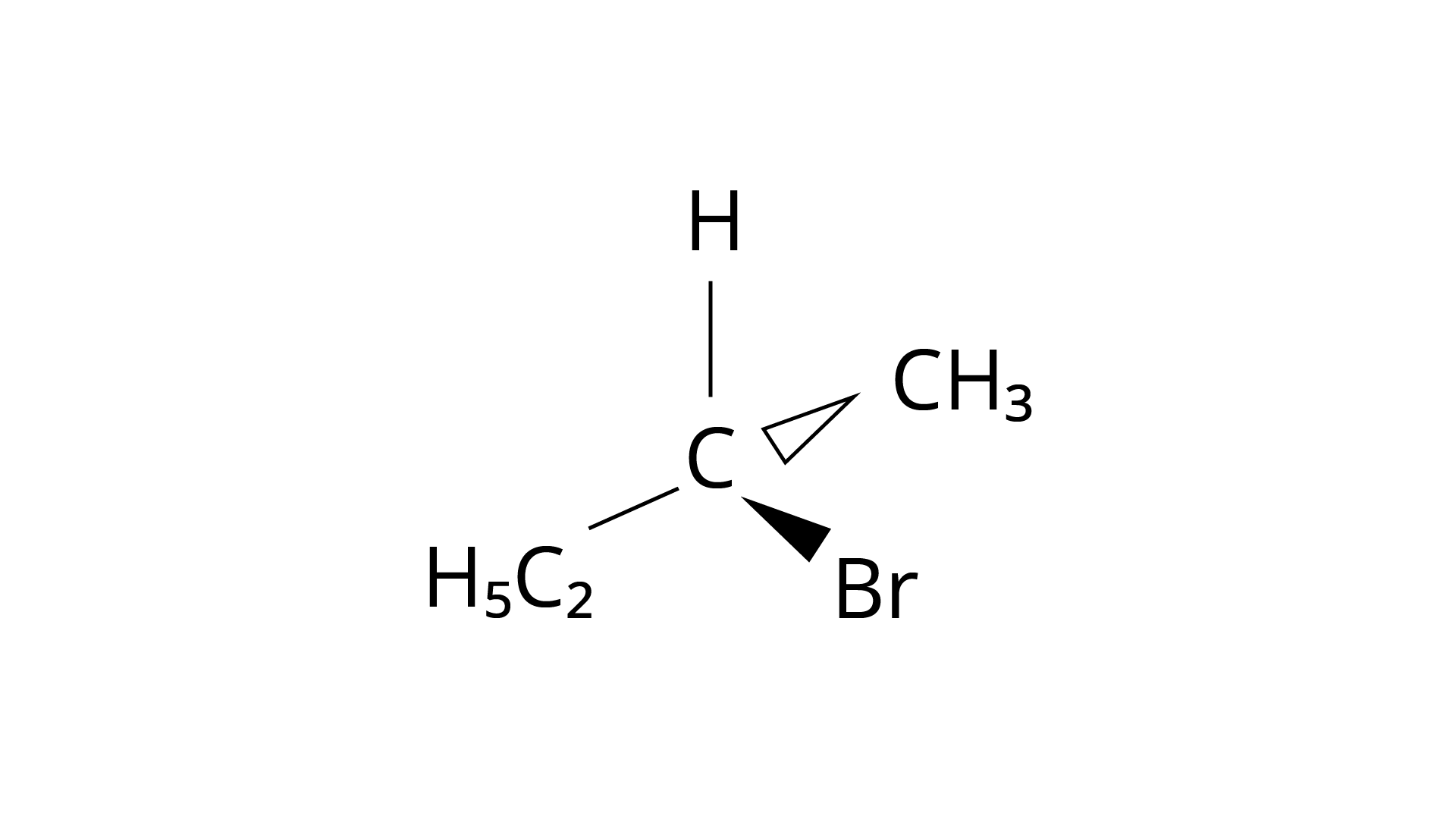
(i)
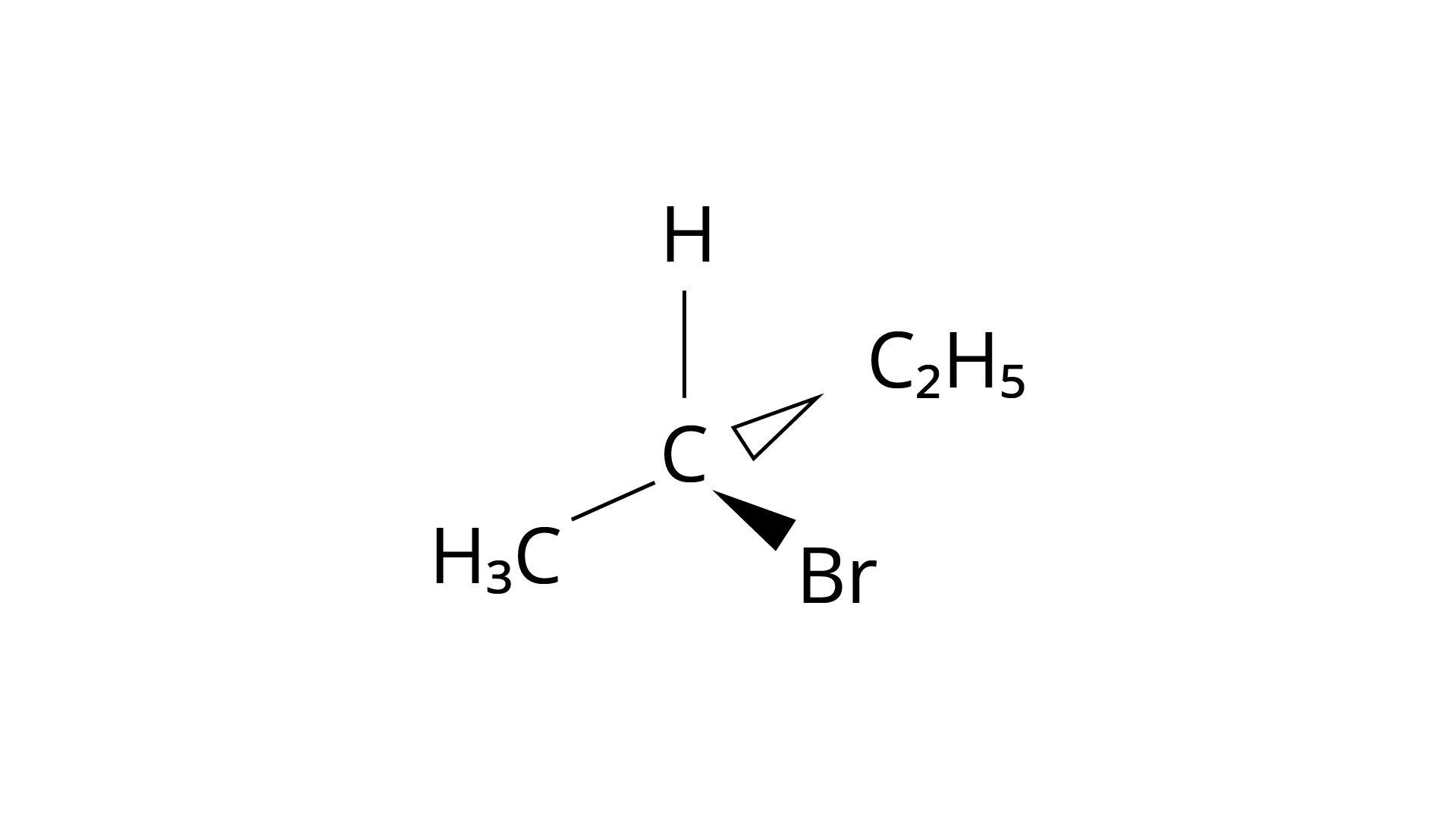
(ii)
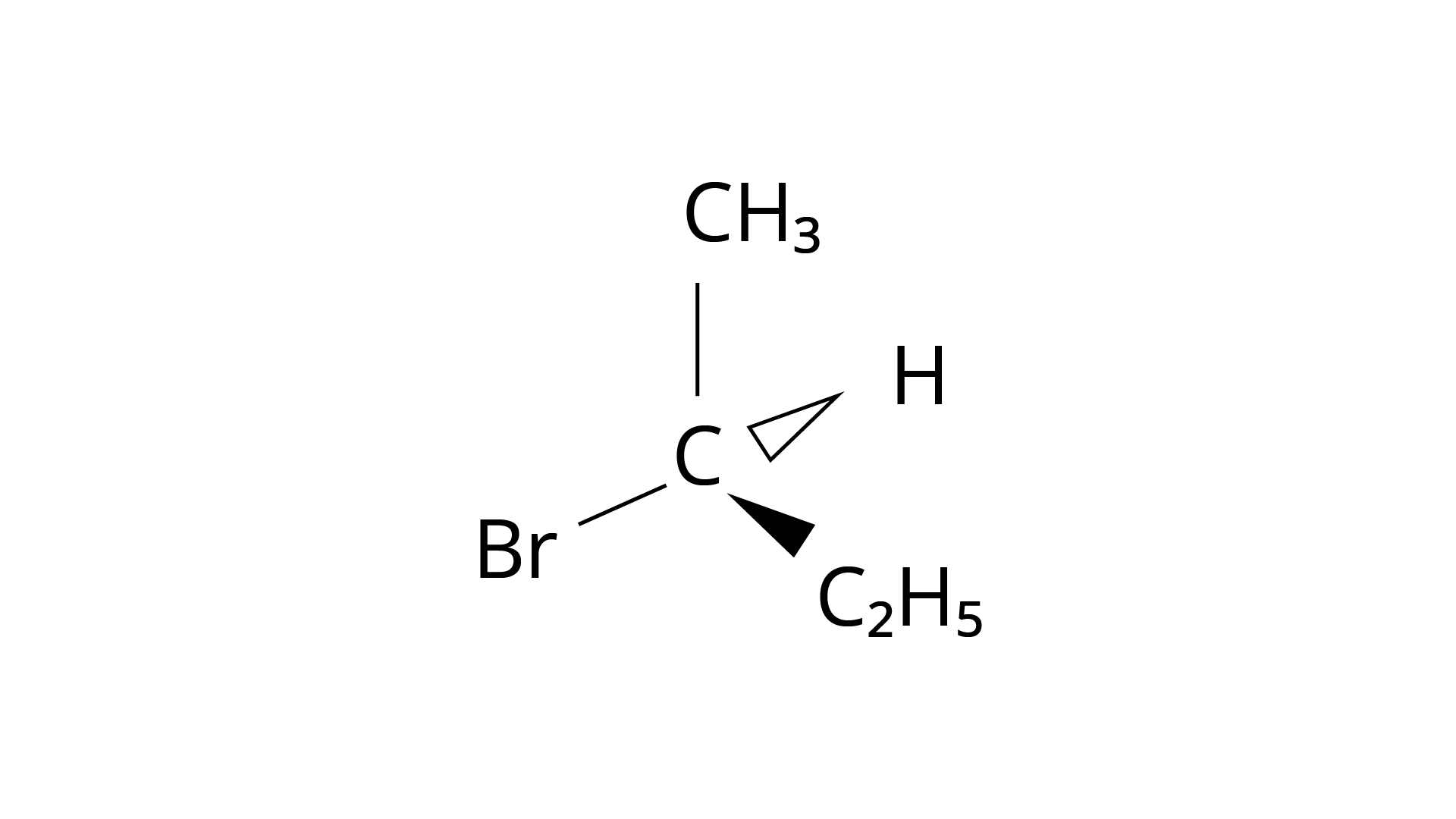
(iii)
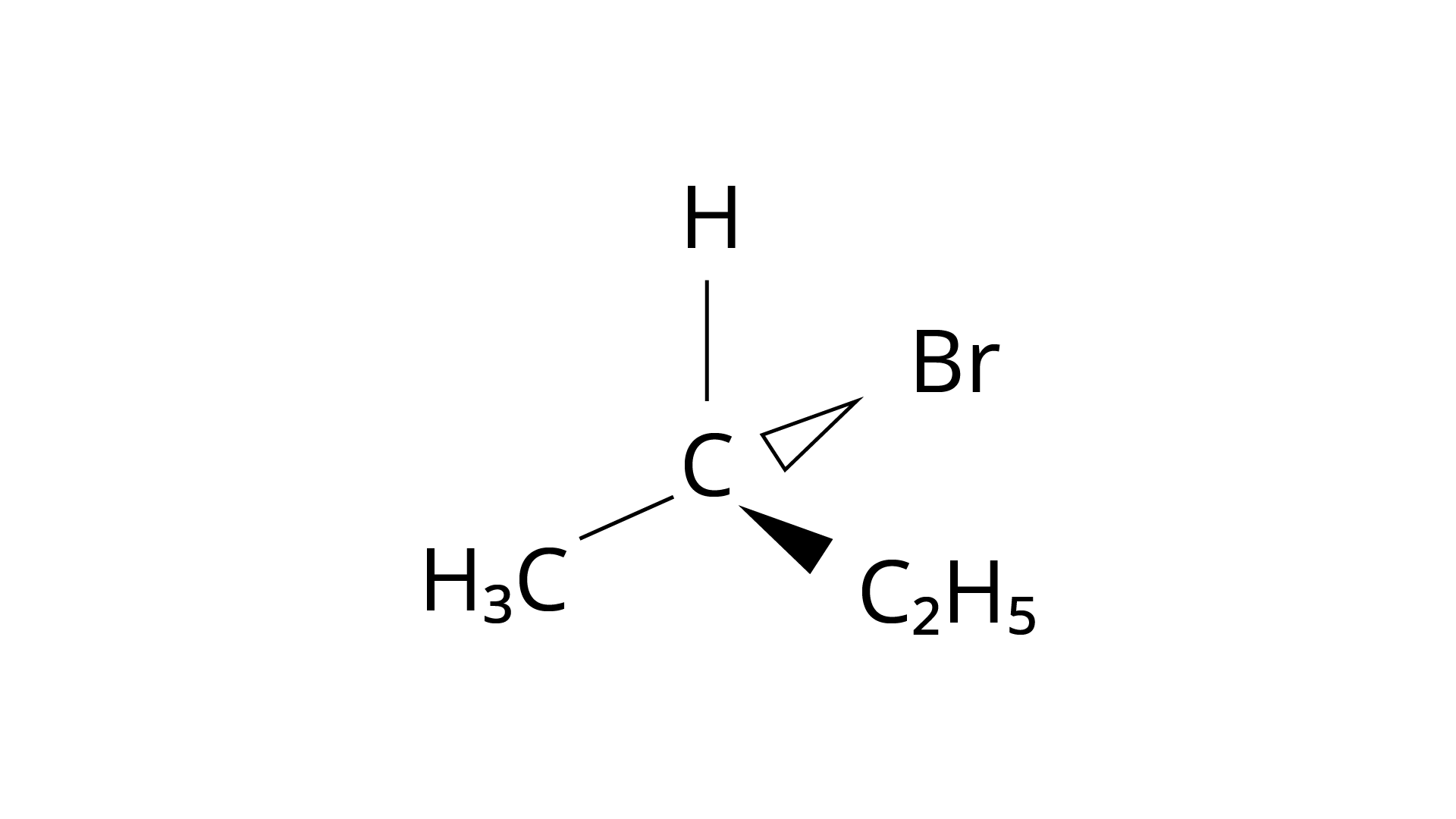
(iv)
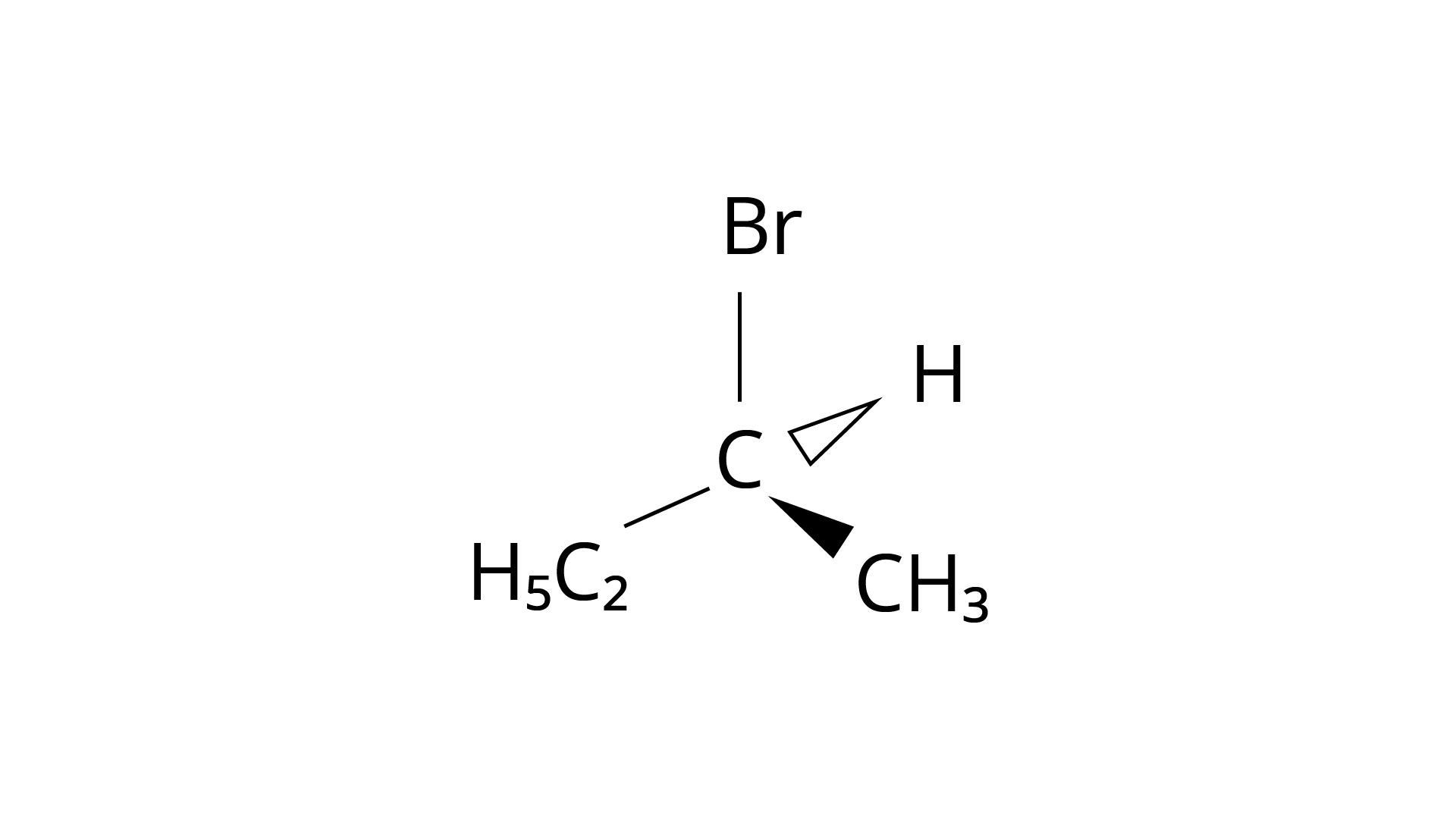
Ans: The Correct Answer is Option (i).
Enantiomers are stereoisomers of a substance that are not superimposable on each other. The carbon atom in the molecule (A) is asymmetric. The molecule's mirror image (i) and \[180^\circ \] rotation cannot be superimposed on each other (A). The molecules (ii), (iii), and (iv) can all be superimposed on one other (A). The molecules (iii) and (iv) have \[180^\circ \] rotations that are superimposable on one another (A).
11. Which of the following is an example of vic-dihalide?
(i) Dichloromethane
(ii) 1,2 -dichloroethane
(iii) Ethylidene chloride
(iv) Allyl chloride
Ans: The Correct Answer is Option (iv).
Geminal halides or gem-dihalides and vicinal halides or vic-dihalides are dihaloalkanes with the same halogen. Vic-dihalides are dihaloalkanes with halogen atoms on two adjacent carbon atoms, whereas Gem-dihalides are molecules with halogen atoms on two adjacent carbon atoms. Gem-dihalides are called alkylidene halides in the conventional naming system, whereas vic-dihalides are called alkylene dihalides. Because dichloromethane has just one carbon, it cannot contain neighbouring halogen atoms. Two carbon atoms are sandwiched between two halogen atoms in 1,2-dichloroethane. Ethylidene chloride is a gem-dihalide, as its name implies. There is only one chlorine atom in allyl chloride.
12. The position of \[ - Br\] in the compound in \[C{H_3}CH = CHC(Br){\left( {C{H_3}} \right)_2}\] can be classified as
(i) Allyl
(ii) Aryl
(iii) Vinyl
(iv) Secondary
Ans: The correct answer isOption (i).
When the halogen atom is linked to an \[{\text{s}}{{\text{p}}^{\text{3}}}\]-hybridised carbon atom next to an allylic carbon, a carbon-carbon double bond is formed.
Aryl halides are produced when a halogen atom is directly linked to an aromatic ring's \[{\text{s}}{{\text{p}}^{\text{2}}}\]-hybridised carbon atom.
When a halogen atom is linked to a \[{\text{s}}{{\text{p}}^{\text{2}}}\]-hybridised carbon atom in a carbon-carbon double bond, vinylic bonds are produced. Simply defined, it's an ethylene molecule with one hydrogen atom substituted by another \[{\text{--R}}\] group.
When the halogen is attached to a secondary carbon atom in the molecule, secondary bonds form. The \[ - {\text{Br}}\] is linked to a tertiary carbon atom in the supplied molecule, which is then bonded to an \[{\text{s}}{{\text{p}}^{\text{3}}}\]-hybridised carbon-carbon double bond. As a result, this \[ - {\text{Br}}\] has an allylic position.
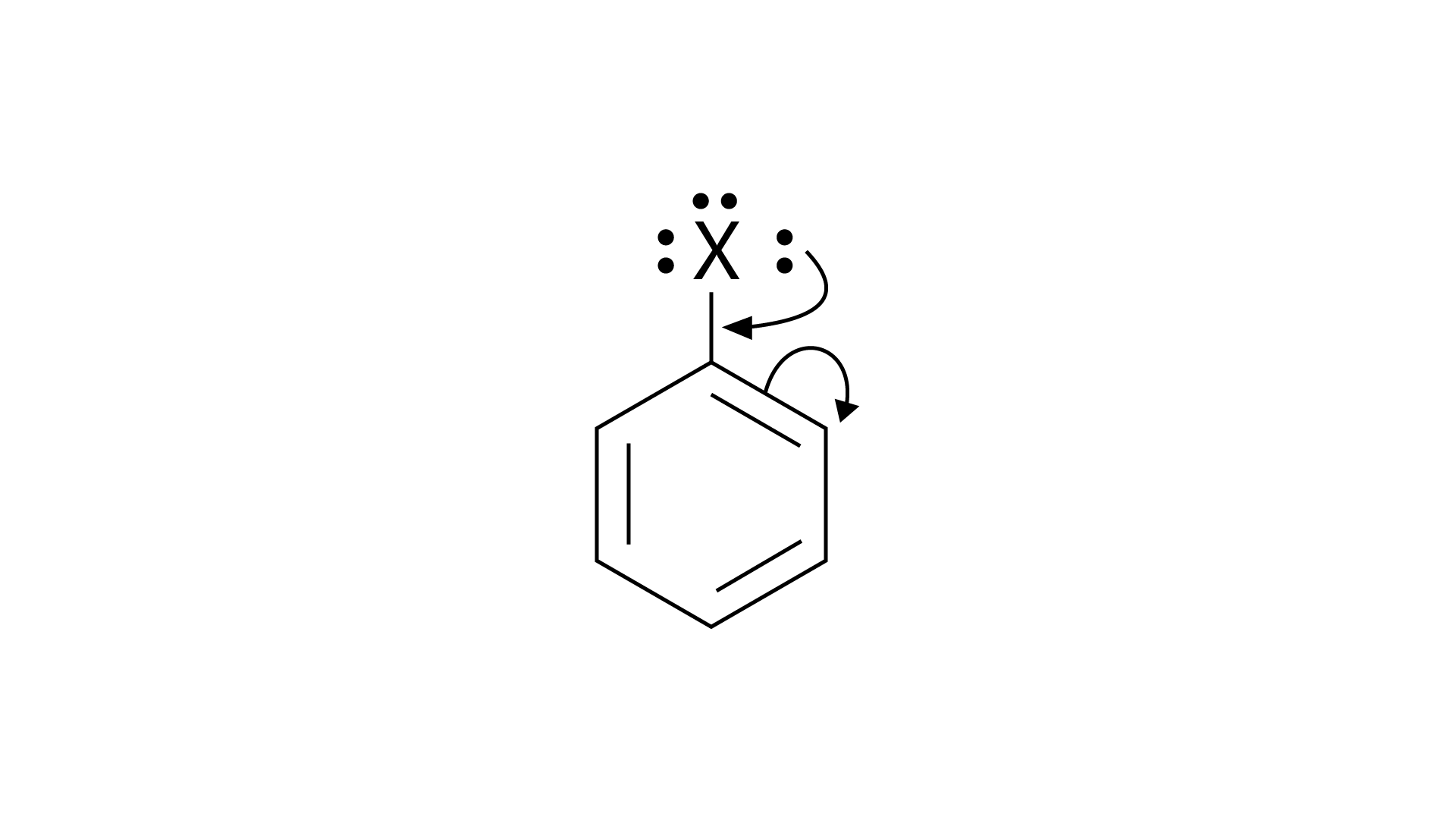
13. Chlorobenzene is formed by reaction of chlorine with benzene in the presence of \[AlC{l_3}\].Which of the following species attacks the benzene ring in this reaction?
(i) \[C{l^ - }\]
(ii) \[C{l^ + }\]
(iii) \[AlC{l_3}\]
(iv) \[{\left[ {AlC{l_4}} \right]^ - }\]
Ans: The correct answer is option ii.
Aluminum chloride is a Lewis acid catalyst that functions similarly to \[{\text{FeC}}{{\text{l}}_{\text{3}}}\]. By chlorinating benzene in the presence of \[{\text{AlC}}{{\text{l}}_3}\], benzene is transformed to chlorobenzene. Electrophilic substitution is used to carry out the reaction. \[{\text{C}}{{\text{l}}_{\text{2}}}\] forms a coordination complex with \[{\text{AlC}}{{\text{l}}_3}\] called \[{\text{C}}{{\text{l}}^{\text{ + }}}{\text{AlC}}{{\text{l}}_{\text{4}}}^{\text{ - }}\], which has a small positive charge on \[{\text{Cl}}\] and is negatively charged on \[{\text{AlC}}{{\text{l}}_{\text{4}}}^{\text{ - }}\]. This \[{\text{C}}{{\text{l}}^{\text{ + }}}\] then interacts with the benzene ring's aromatic double bond to generate an addition product, followed by deprotonation to produce chlorobenzene, \[{\text{AlC}}{{\text{l}}_3}\], and \[{\text{HCl}}\] as side products. The best choice is (ii).
14. Ethylidene chloride is a/an
(i) vic-dihalide
(ii) gem-dihalide
(iii) allylic halide
(iv) vinylic halide
Ans: The Correct Answer is Option (ii).
Dihaloalkanes with two halogen atoms of the same kind bonded to the same carbon atom are known as gem-dihalides. Alkylidene dihalides is the most frequent naming scheme for gem-dihalides. As a result, ethylidene dichloride is a gem-dihalide. The best choice is (ii).
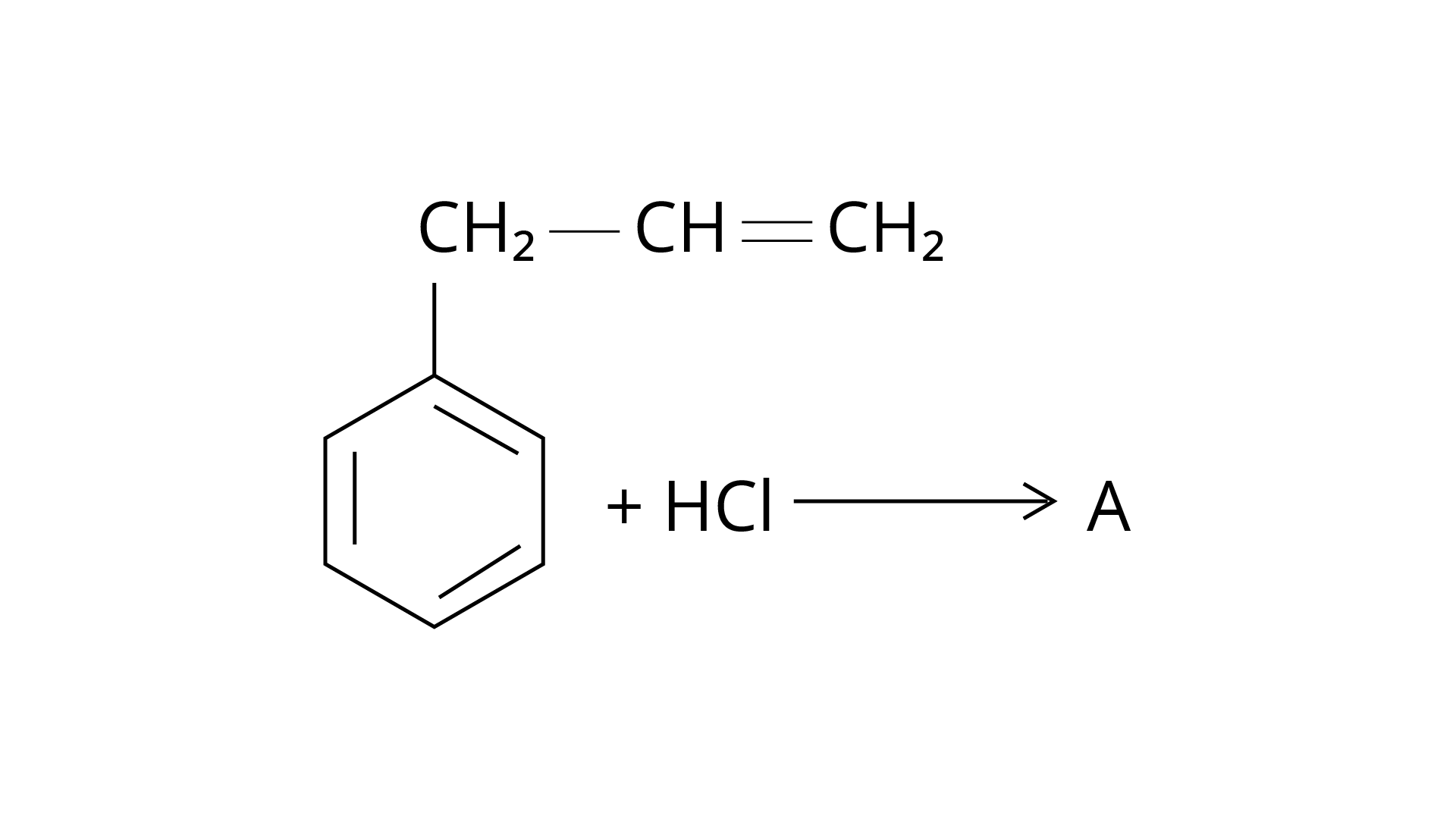
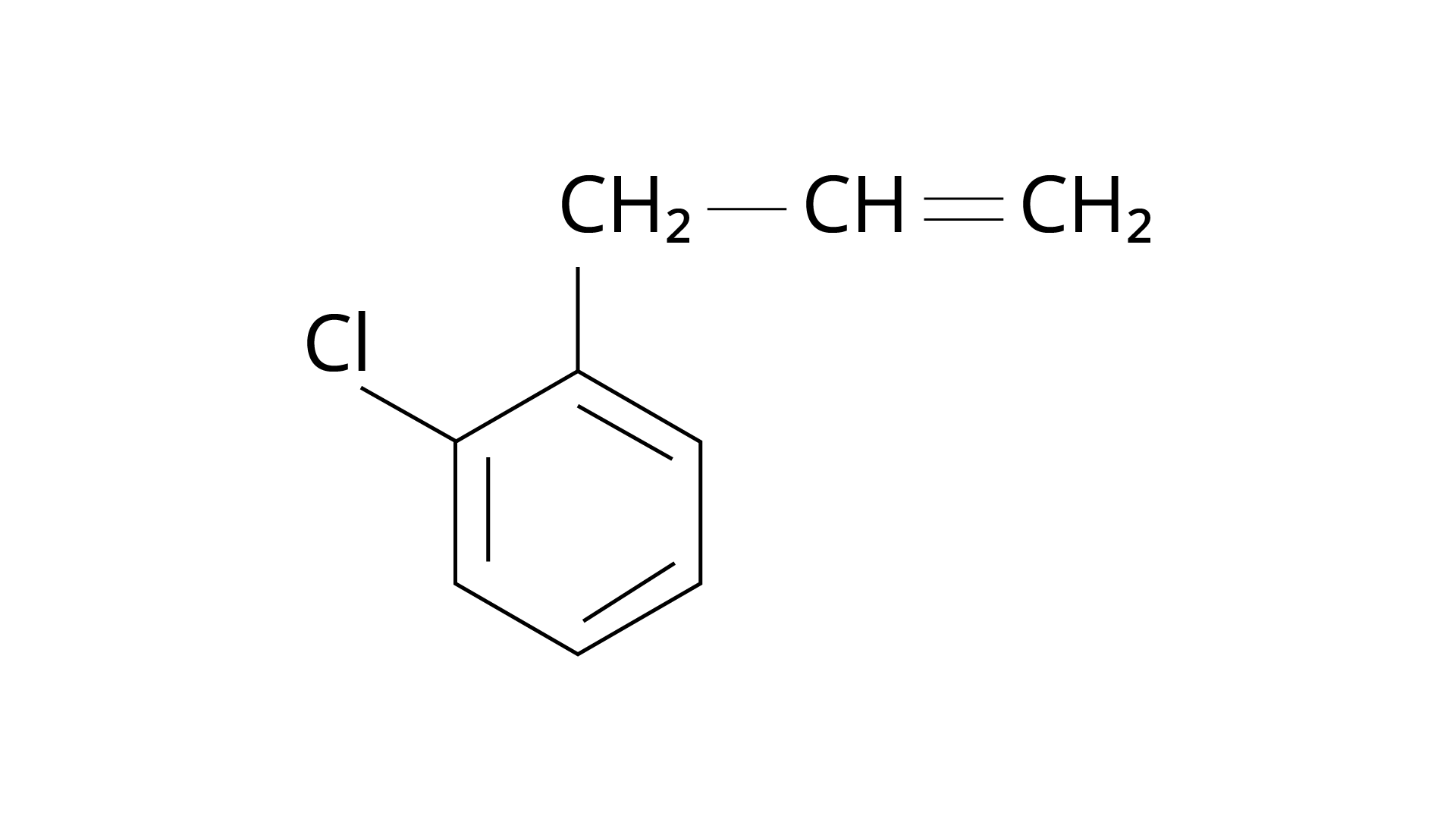
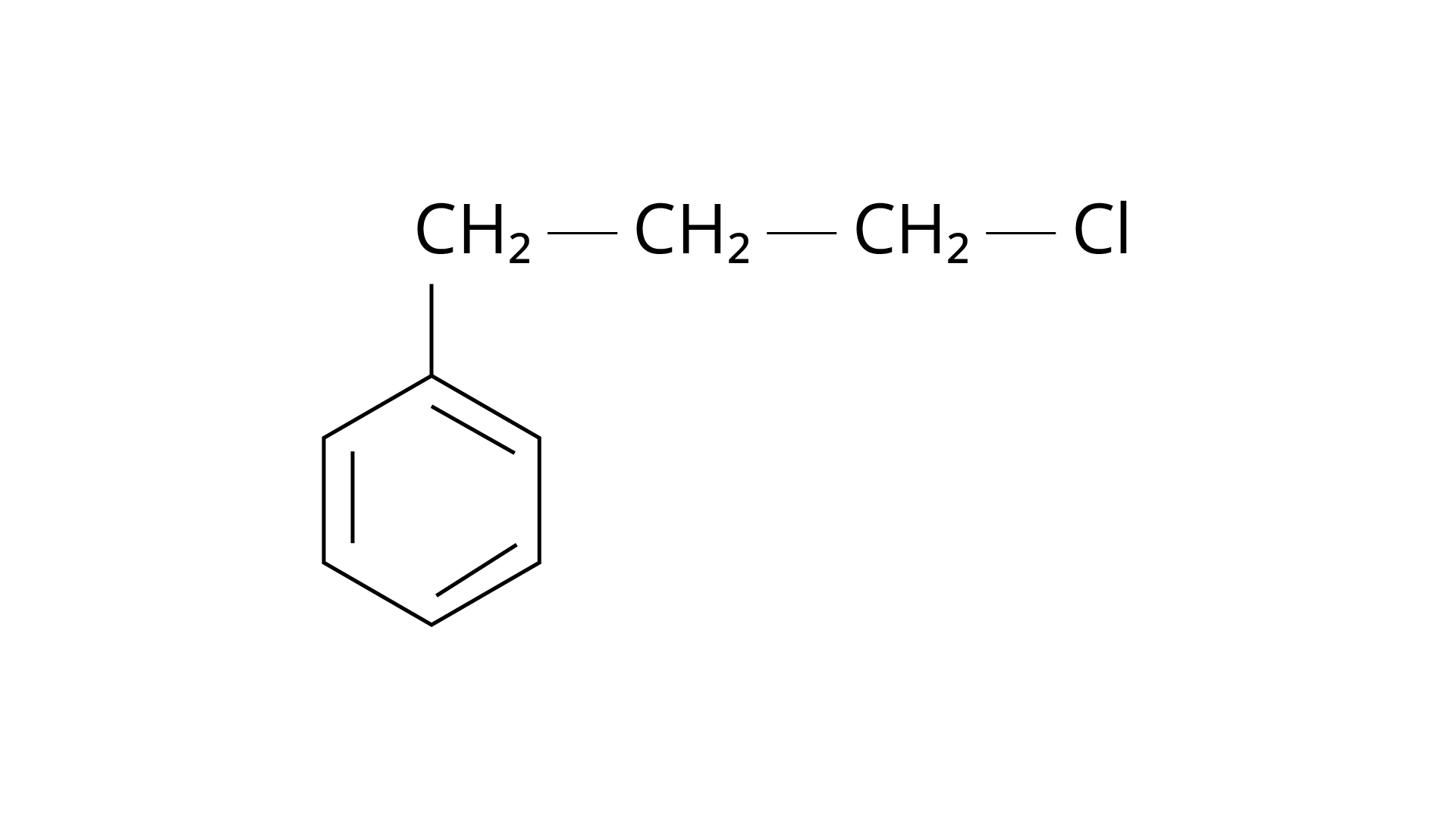
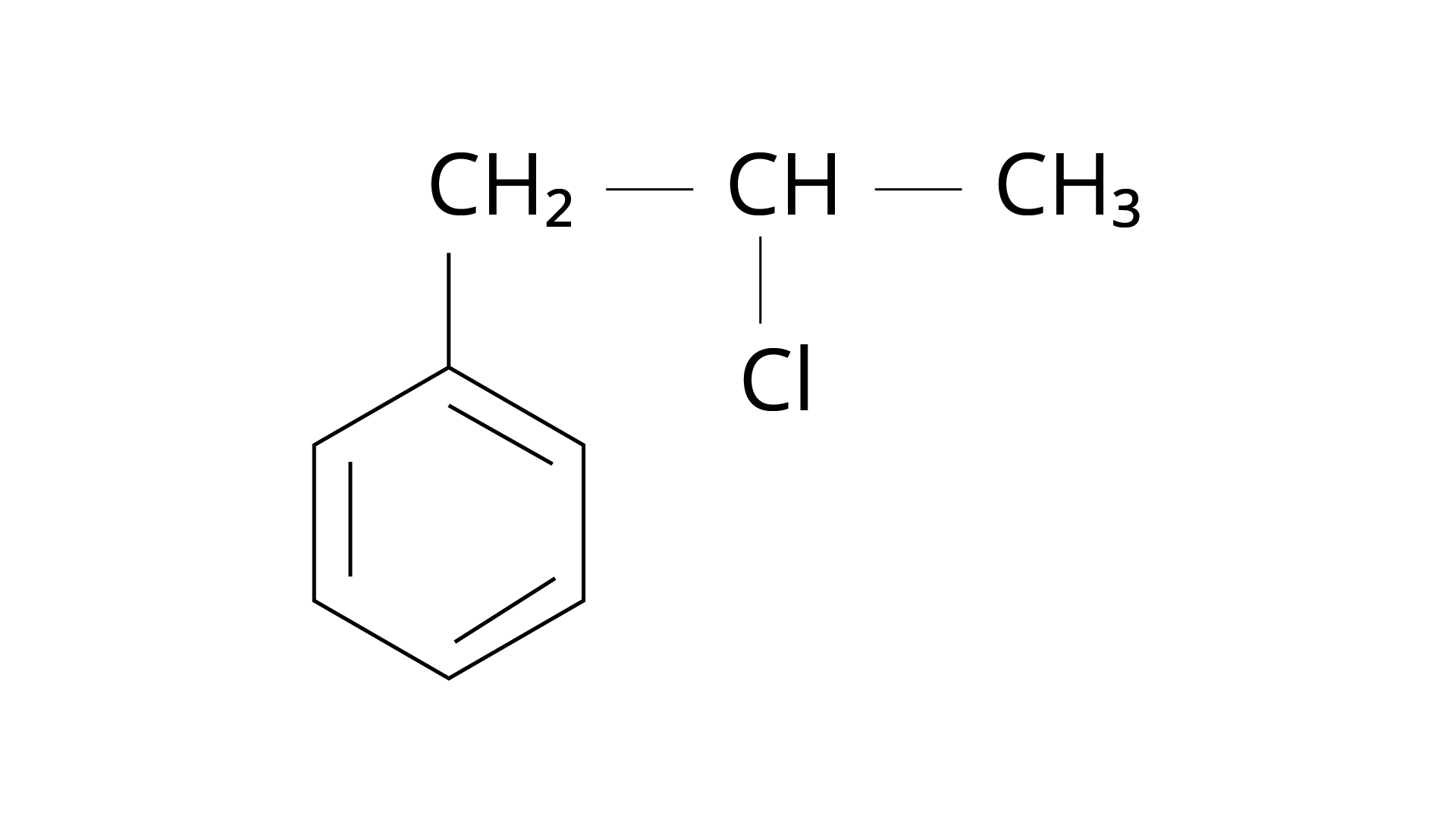
15. What is 'A' in the following reaction?
(i)
(ii)
(iii)
(iv)
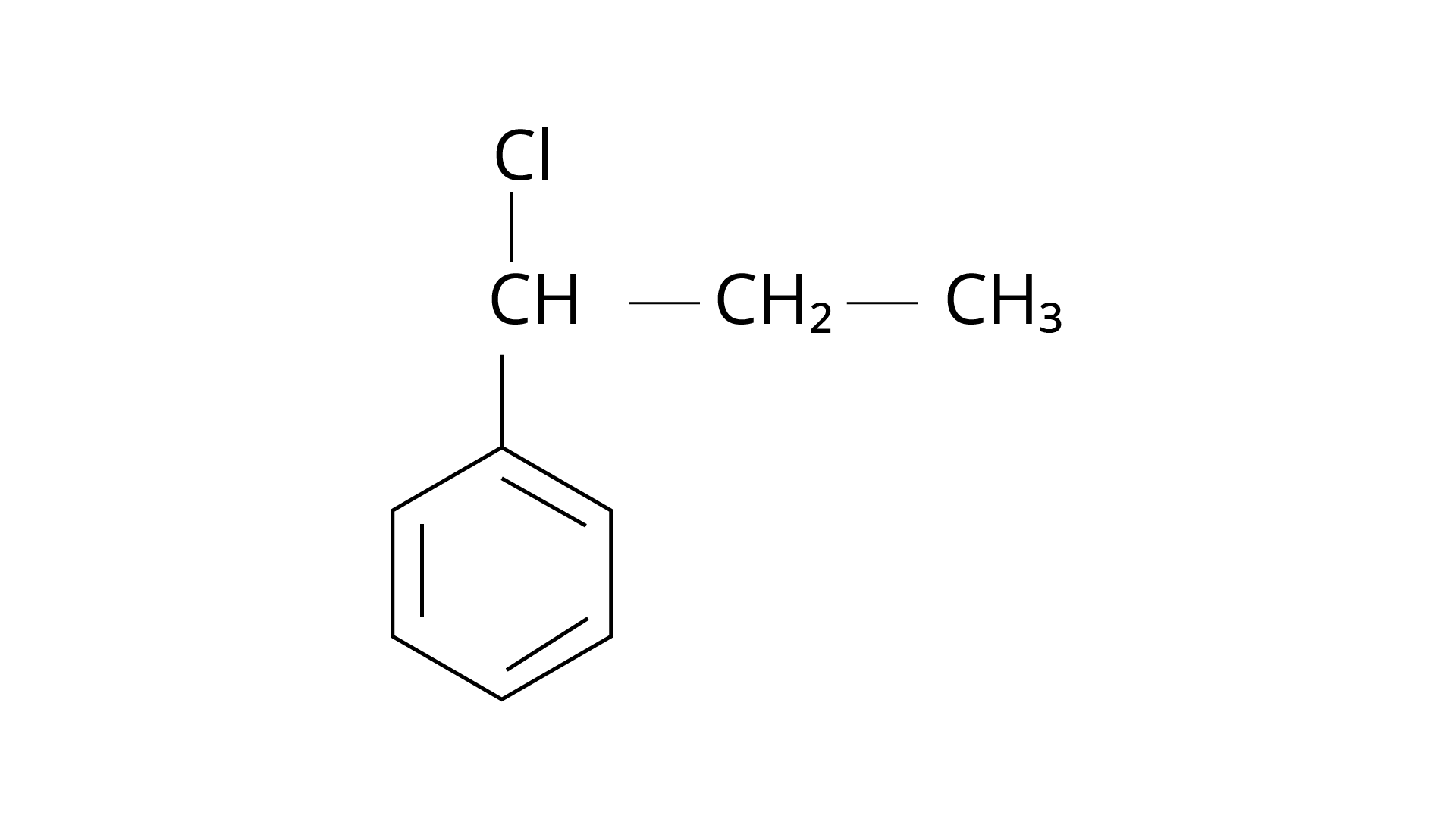
Ans: The correct answer is Option (iii).
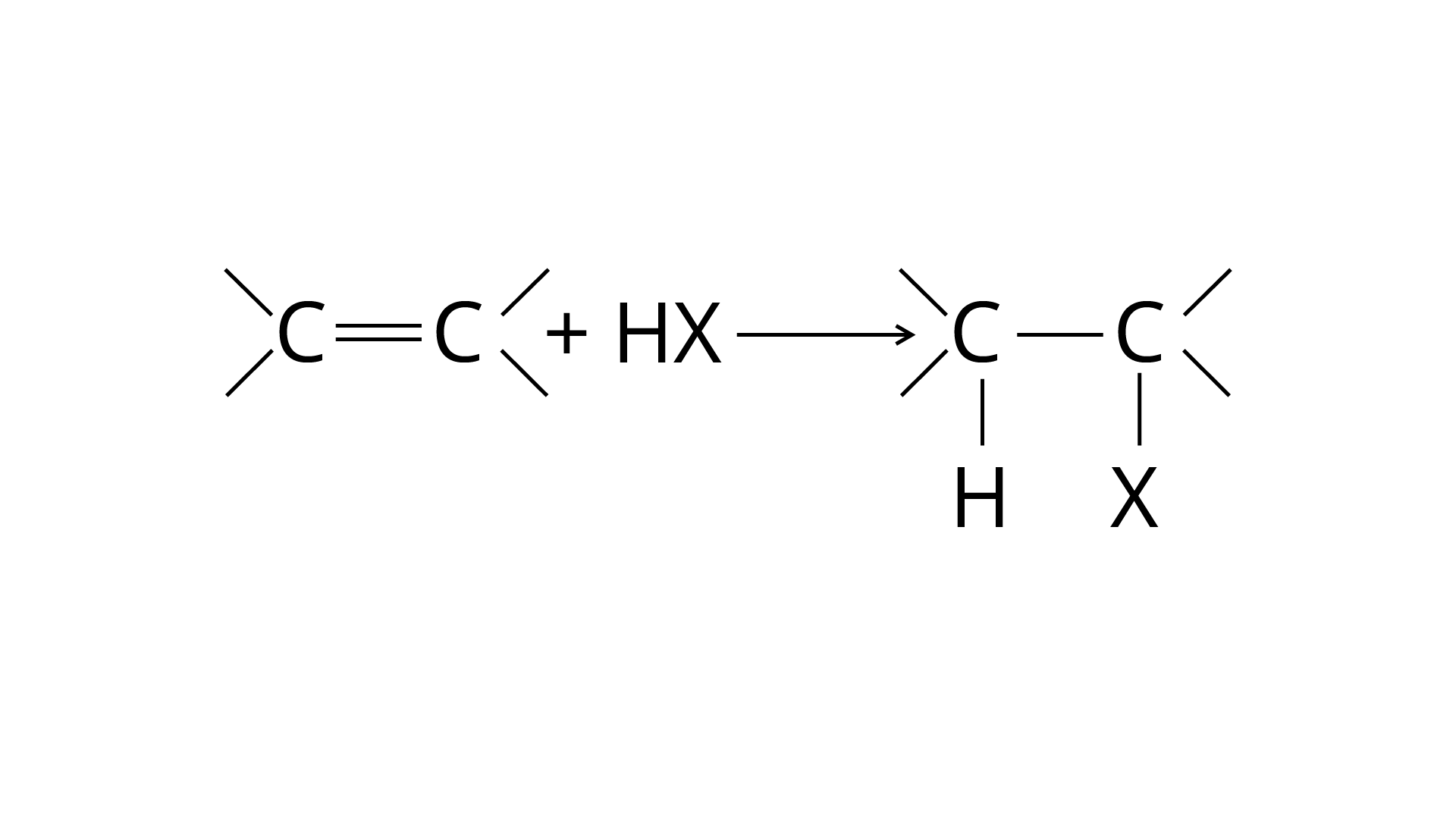
Markonikov's rule can be used to explain the results. The hydrogen from \[{\text{HCl}}\] is added to the carbon immediately bonded to the most hydrogen atoms, whereas the \[{\text{--Cl}}\] is attached to the carbon directly bonded to the least hydrogens, according to the rule. As a result, the right response is (iii).
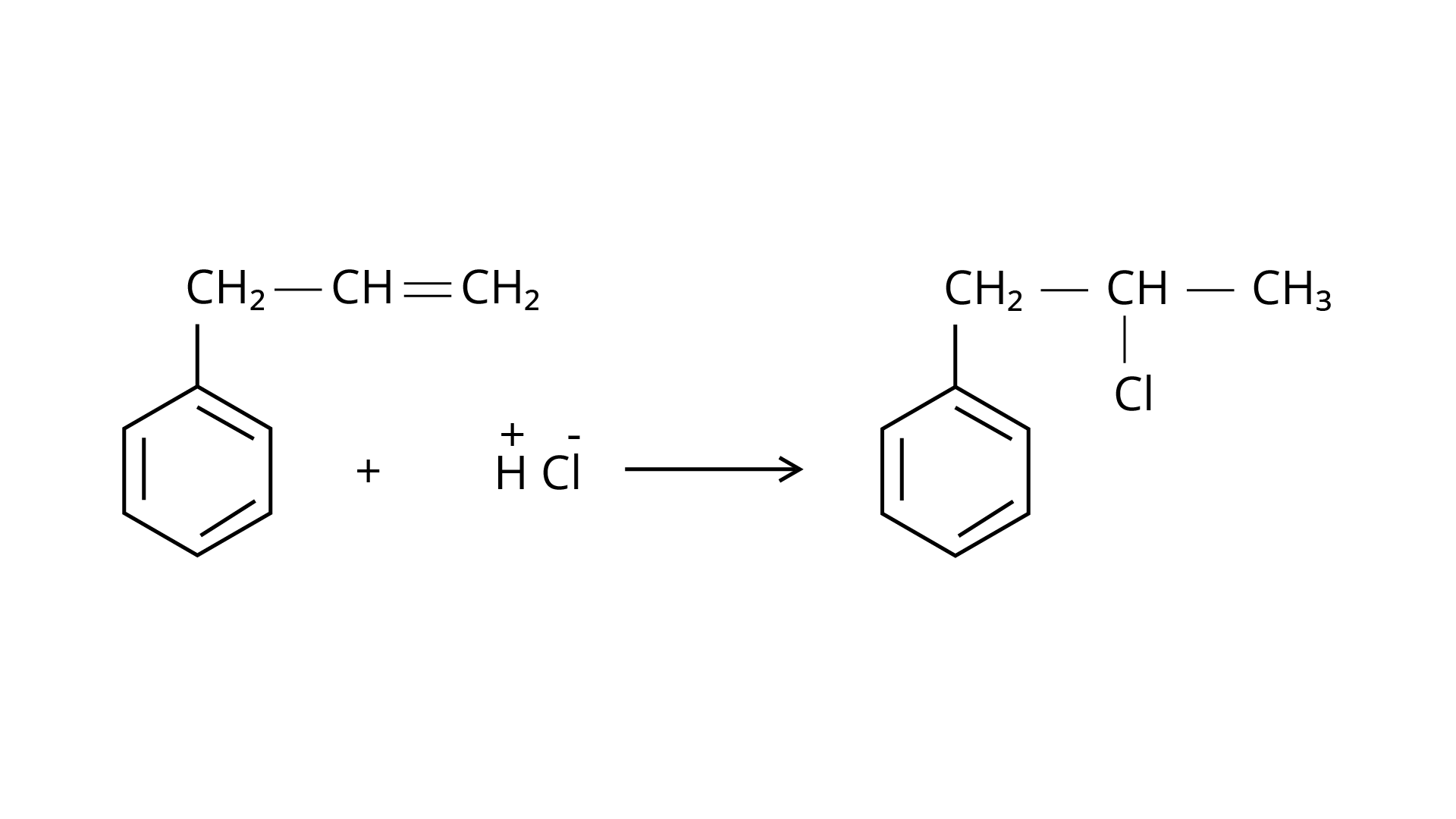
16. A primary alkyl halide would prefer to undergo
(i) \[{SN}^1\] reaction
(ii) \[{SN}^1\] reaction
(iii) \[\alpha \]-Elimination
(iv) Racemisation
Ans: The Correct Answer is Option (ii).
When an alkyl halide with -hydrogen atoms reacts with a base or a nucleophile, depending on the nature of the alkyl halide, the strength of the base or nucleophile, the size of the molecules, and the reaction circumstances, it can undergo a specific kind of reaction. A substitution or elimination process can be followed by an alkyl halide, as well as the two kinds of substitution, \[{SN}^1\] and \[{SN}^2\]. Because the entering nucleophile cannot engage with the bulky substituents on or near the carbon atom, the main alkyl halide prefers the \[{SN}^2\] reaction. As a result, primary alkyl halides and methyl halides have the greatest proclivity for \[{SN}^2\] reactions.
17. Which of the following alkyl halides will undergo \[{SN}^1\] reaction most readily?
(i) \[{\left( {C{H_3}} \right)_3}C - F\]
(ii) \[{\left( {C{H_3}} \right)_3}C - Cl\]
(iii) \[{\left( {C{H_3}} \right)_3}C - Br\]
(iv) \[{\left( {C{H_3}} \right)_3}C - I\]
Ans: The Correct Answer is Option (iv).
\[{S_N}1\] reactions occur mostly in polar protic solvents such as \[{{\text{H}}_{\text{2}}}{\text{O}}\] and follow first-order kinetics. This indicates that the reaction rate is solely determined by one reactant. Because of the great stability of the generated carbocation, this reaction favours tertiary alkyl halides. When a molecule is polarised in water, it generates a carbocation as well as a halide ion. The halides' reactivity is \[{\text{R--I > R--Br > R--Cl > > R--F}}\]. As a result, \[{\left( {{\text{C}}{{\text{H}}_{\text{3}}}} \right)_{\text{3}}}{\text{C---I}}\] will be the most likely to undergo the reaction.
18. Which is the correct IUPAC name for :
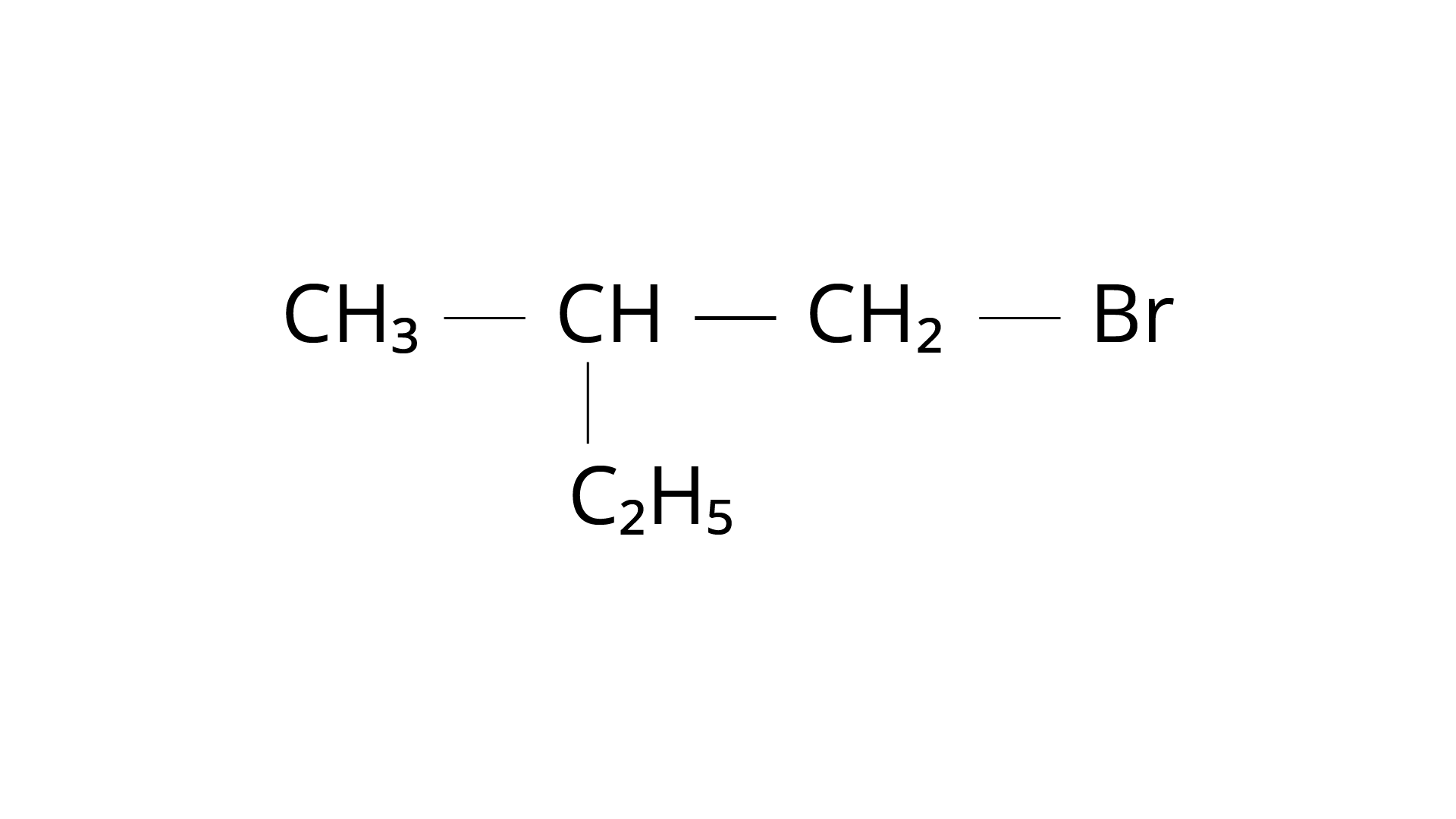
(i) 1-Bromo-2-ethylpropane
(ii) 1 -Bromo-2-ethyl-2-methylethane
(iii) 1 -Bromo-2-methylbutane
(iv) 2-Methyl-1-bromobutane
Correct Answer: Option (iii)
Ans: The correct answer is option iii.
First, we must determine which carbon chain is the longest. After that, we should have \[{\text{C}}{{\text{H}}_{\text{3}}}{\text{---C}}{{\text{H}}_{\text{2}}}{\text{---CH}}\left( {{\text{C}}{{\text{H}}_{\text{3}}}} \right){\text{---C}}{{\text{H}}_{\text{2}}}{\text{---Br}}\] as the real structure. \[{\text{--Br}}\], because the functional halide group is linked to the first carbon atom, we begin numbering there. The methyl group branch is joined to the chain's second carbon atom. Butane is named from the number of carbons in the unbranched parent chain, which is four. 1-Bromo-2-methylbutane is the name of the molecule. Option (iii) is the right answer.
19. What should be the correct IUPAC name for diethylbromomethane?
(i) 1 -Bromo- 1,1 -diethylmethane
(ii) 3-Bromopentane
(iii) 1-Bromo-1-ethylpropane
(iv) 1-Bromopentane
Ans: The Correct Answer is Option (ii).
We may conclude from the given compound's common name that there are two \[{\text{--}}{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}\] groups and one \[{\text{--Br}}\] group linked to a \[{\text{C}}{{\text{H}}_{\text{4}}}\] molecule. Bromomethane would be represented by the symbol \[{\text{C}}{{\text{H}}_{\text{3}}}{\text{---Br}}\]. Two ethyl groups can be replaced for the hydrogens in the methyl molecule to make diethylbromomethane \[\left( {{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}} \right){\text{CH}}\left( {{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}} \right){\text{Br}}\]. The longest parent chain must be identified in order to offer an IUPAC name. The parent chain is \[{\text{C}}{{\text{H}}_{\text{3}}}{\text{---C}}{{\text{H}}_{\text{2}}}{\text{---CH}}\left( {{\text{Br}}} \right){\text{---C}}{{\text{H}}_{\text{2}}}{\text{---C}}{{\text{H}}_{\text{3}}}\], with the \[{\text{--Br}}\] group from either side connected to the third carbon atom. Because the parent chain has five carbons, the molecule is termed 3-bromopentane.
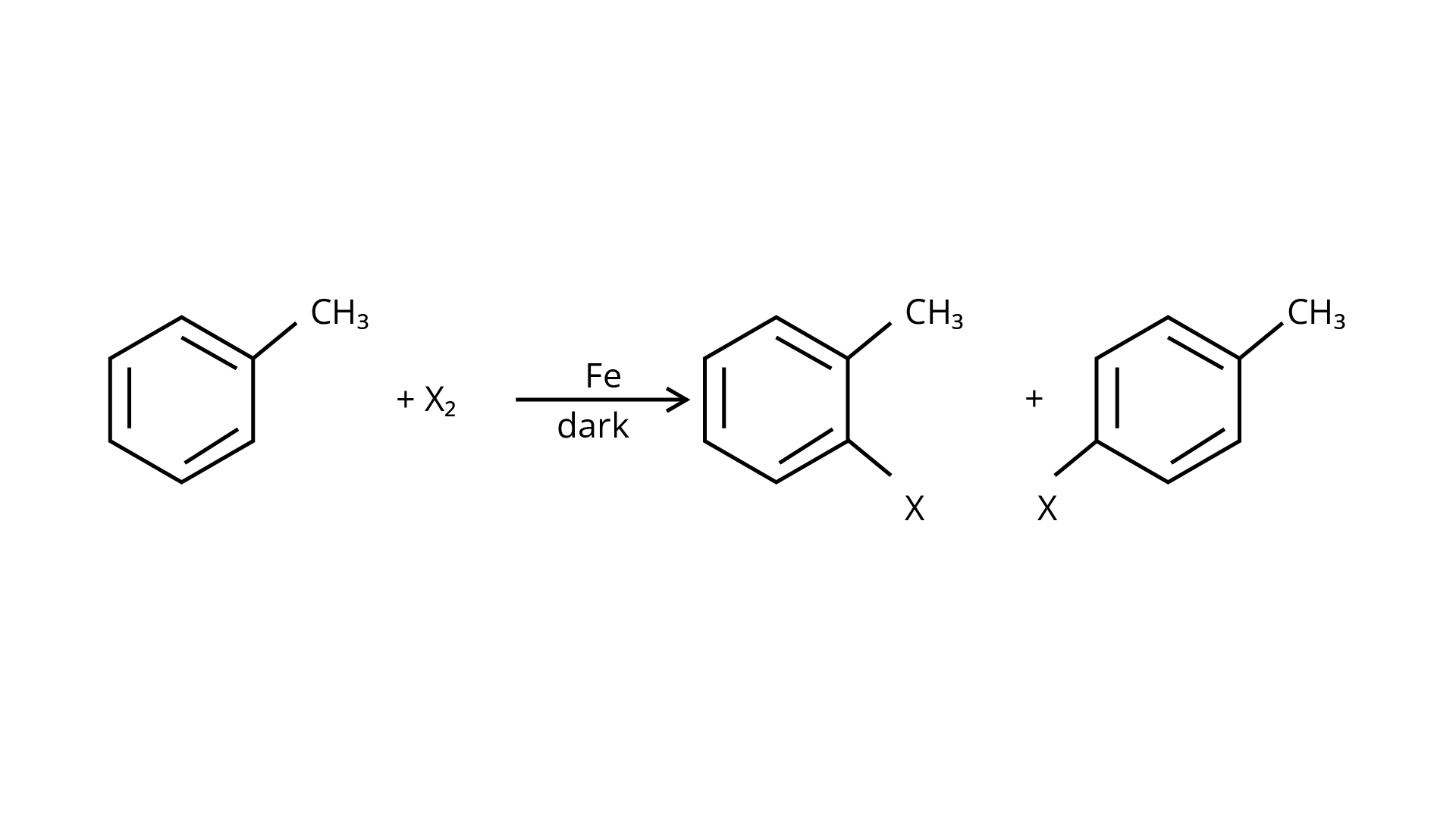
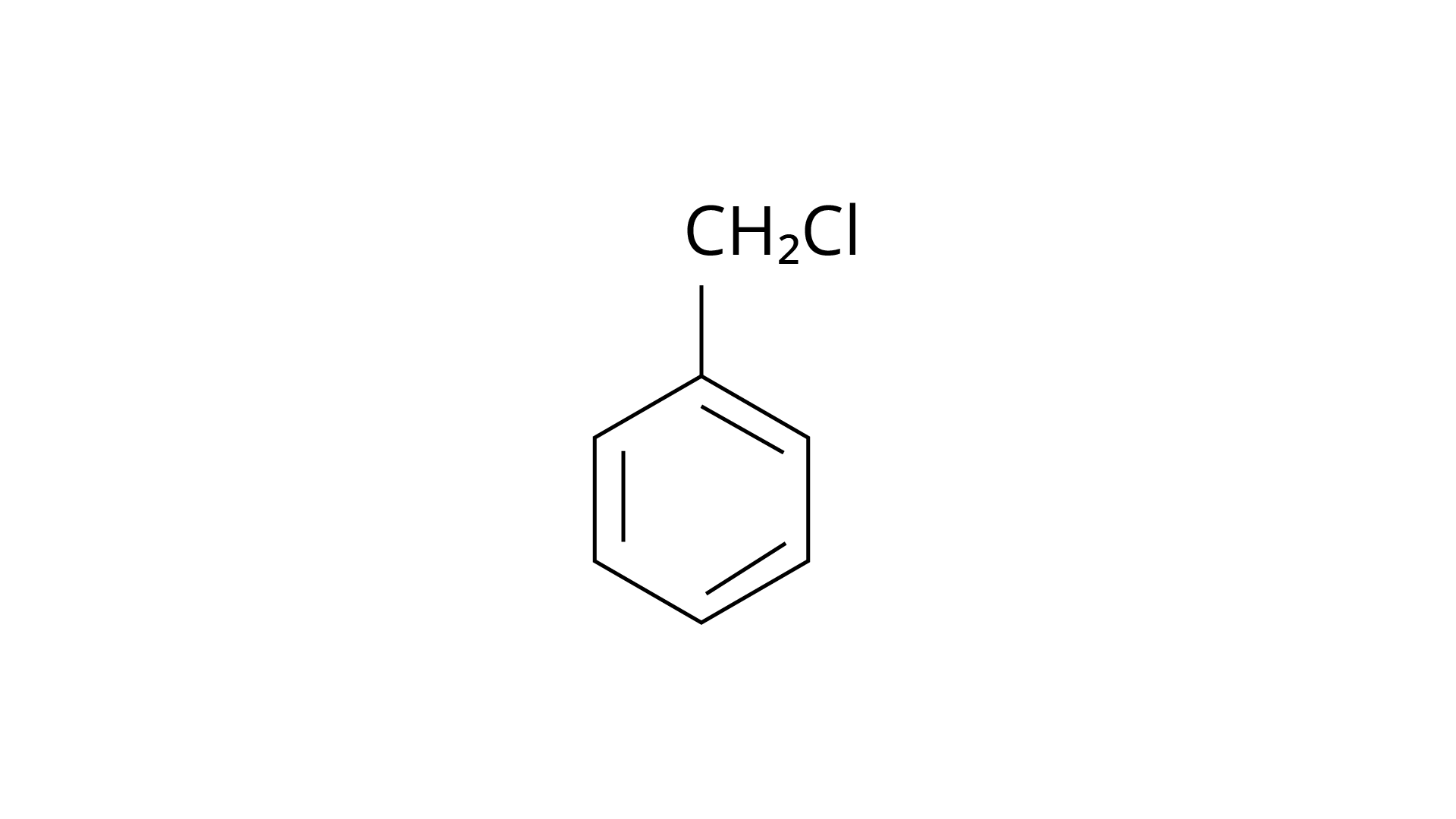
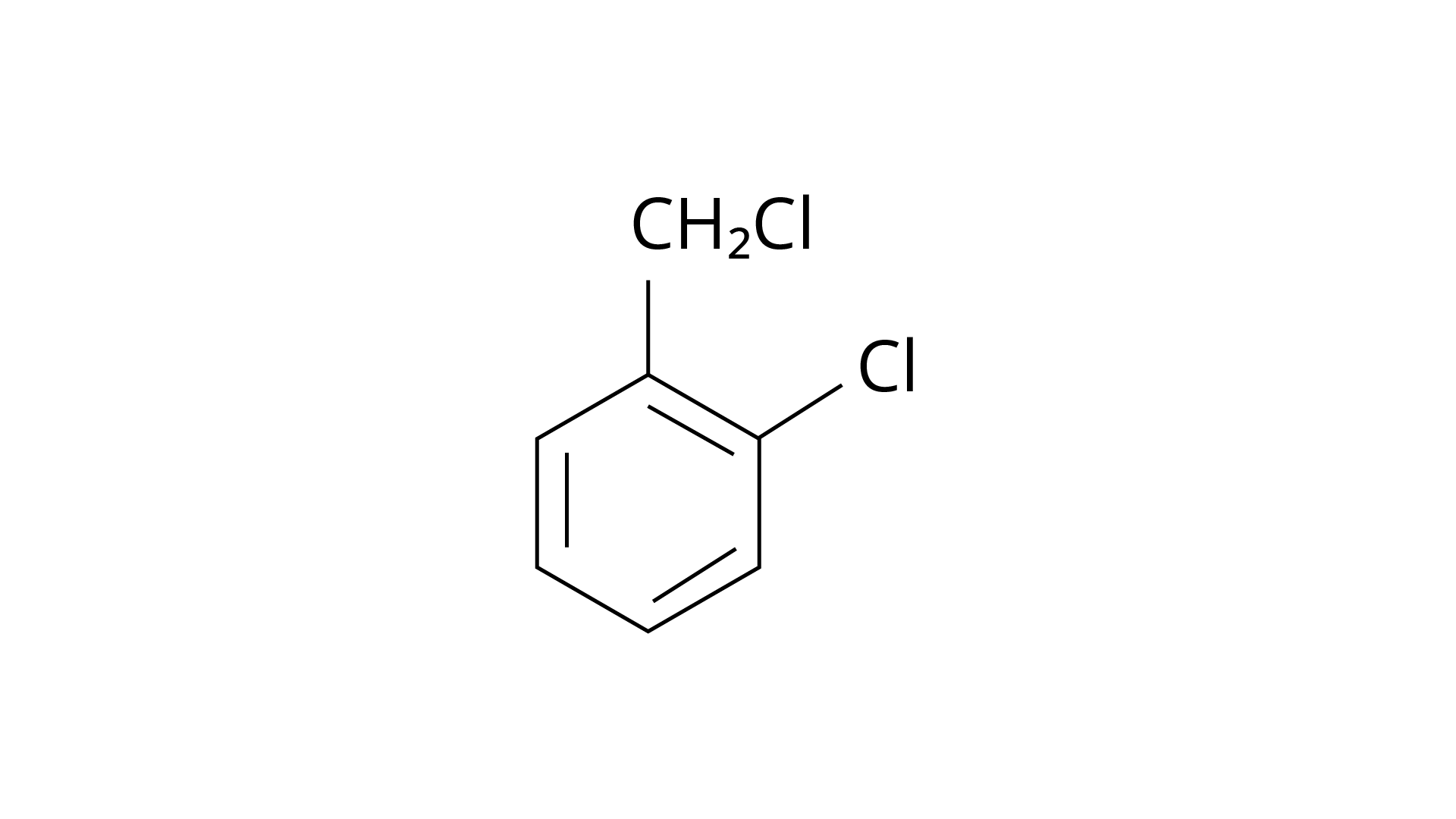
20. The reaction of toluene with chlorine in the presence of iron and in the absence of light yields:
(i)
(ii)
(iii)
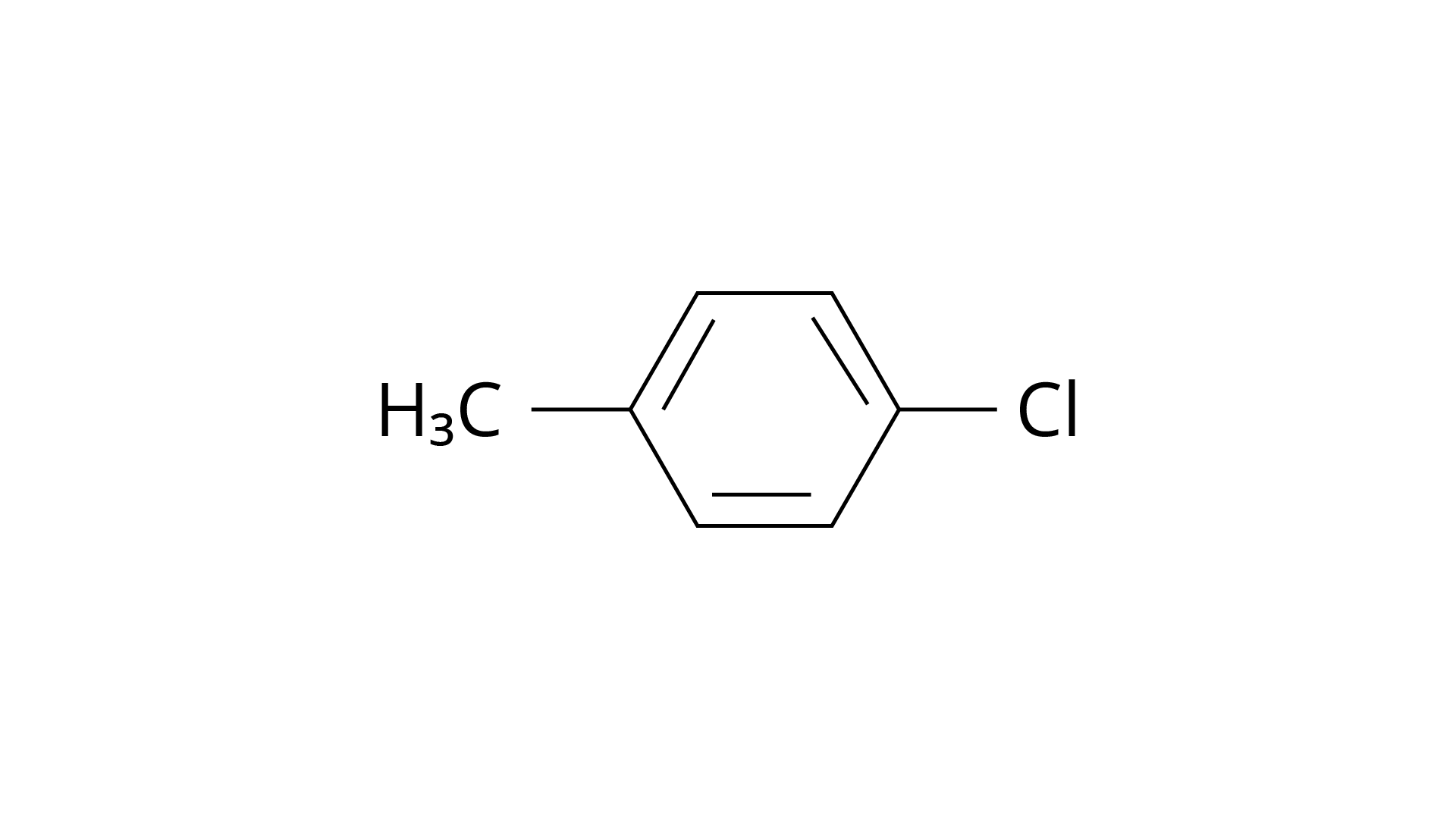
(iv) Mixture of (ii) and (iii)
Ans: The Correct Answer is Option (iv).
By electrophilic substitution, aromatic arenes react with chlorine in the presence of Lewis acid catalysts such as iron (III) chloride, yielding ortho and para isomers of haloarenes. (ii) and (iii) are both products of the reaction. \[{\text{C}}{{\text{l}}_{\text{2}}}\] forms a coordination complex with \[{\text{FeC}}{{\text{l}}_{\text{3}}}\], creating the \[{\text{C}}{{\text{l}}^{\text{ + }}}{\text{FeC}}{{\text{l}}_4}^{\text{ - }}\]complex, which has a small positive charge on \[{\text{Cl}}\] and a negative charge on \[{\text{FeC}}{{\text{l}}_4}^{\text{ - }}\]. This \[{\text{C}}{{\text{l}}^{\text{ + }}}\] then interacts with the aromatic double bonds of the toluene molecule to create an addition product, which is subsequently deprotonated to form a mixture of o-, p-, and m- chlorotoluene isomers. Because the m- isomer is highly unstable, the product is not accessible in the o- and p- forms.
21. Chloromethane on treatment with excess of ammonia yields mainly
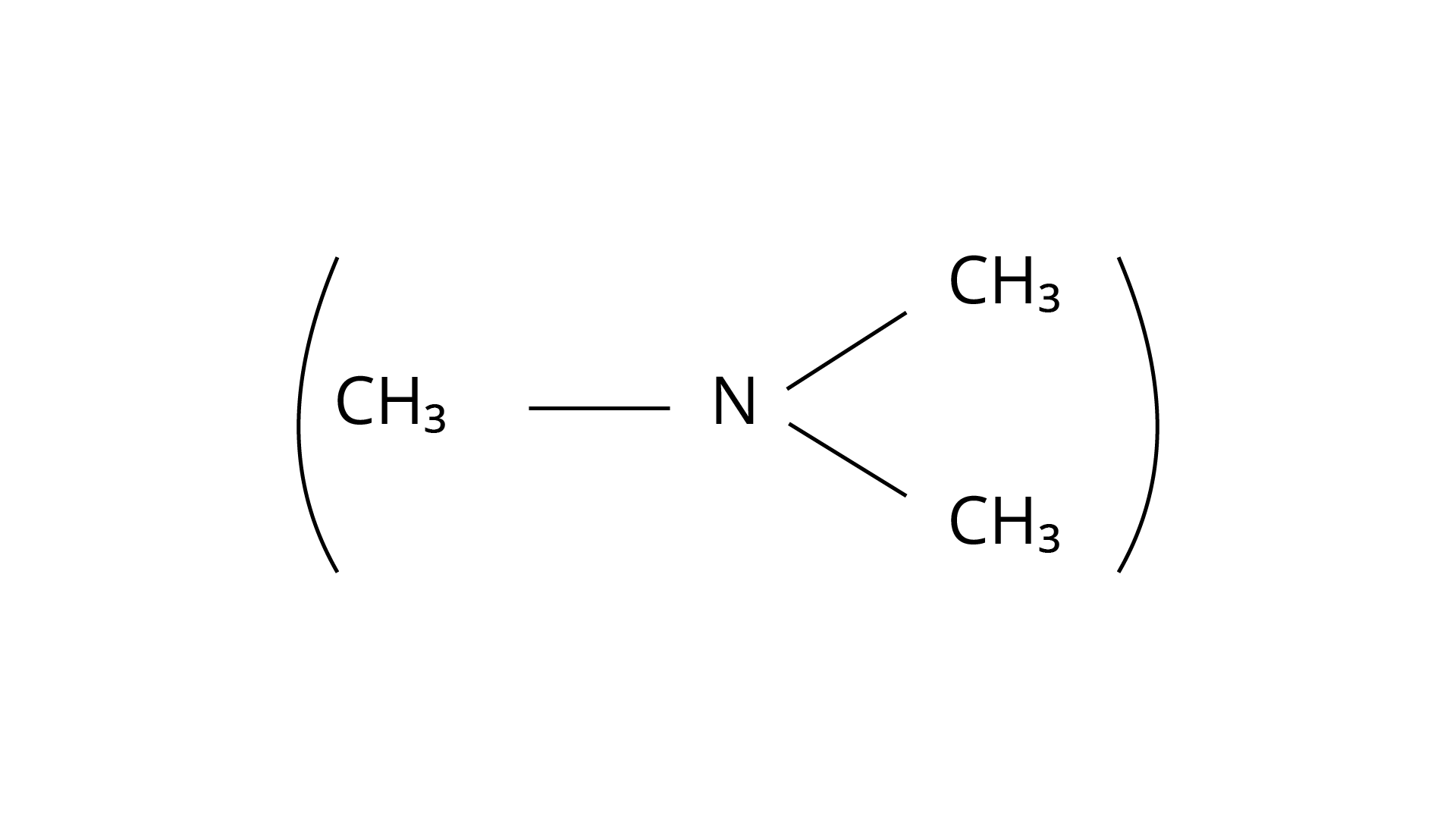
(i) N, N-Dimethylmethanamine
(ii) \[N - methylmethanamine\left( {C{H_3} - NH - C{H_3}} \right)\]
(iii) Methanamine \[\left( {C{H_3}N{H_2}} \right)\]
(iv) Mixture containing all these in equal proportion
Ans: The Correct Answer is Option (iii).
Because it possesses unpaired electrons, the ammonia molecule is a nucleophile in nature. By nucleophilic substitution, this nucleophile attacks the chloromethane molecule and forms methyl amine or methanamine. The carbon atom in the molecule is partially positive due to the electronegativity of the linked halide, which is partially negative. The positive ion is attacked by the electron-rich nucleophile, causing the halide ion to be detached from the molecule. The proper response is (iii).
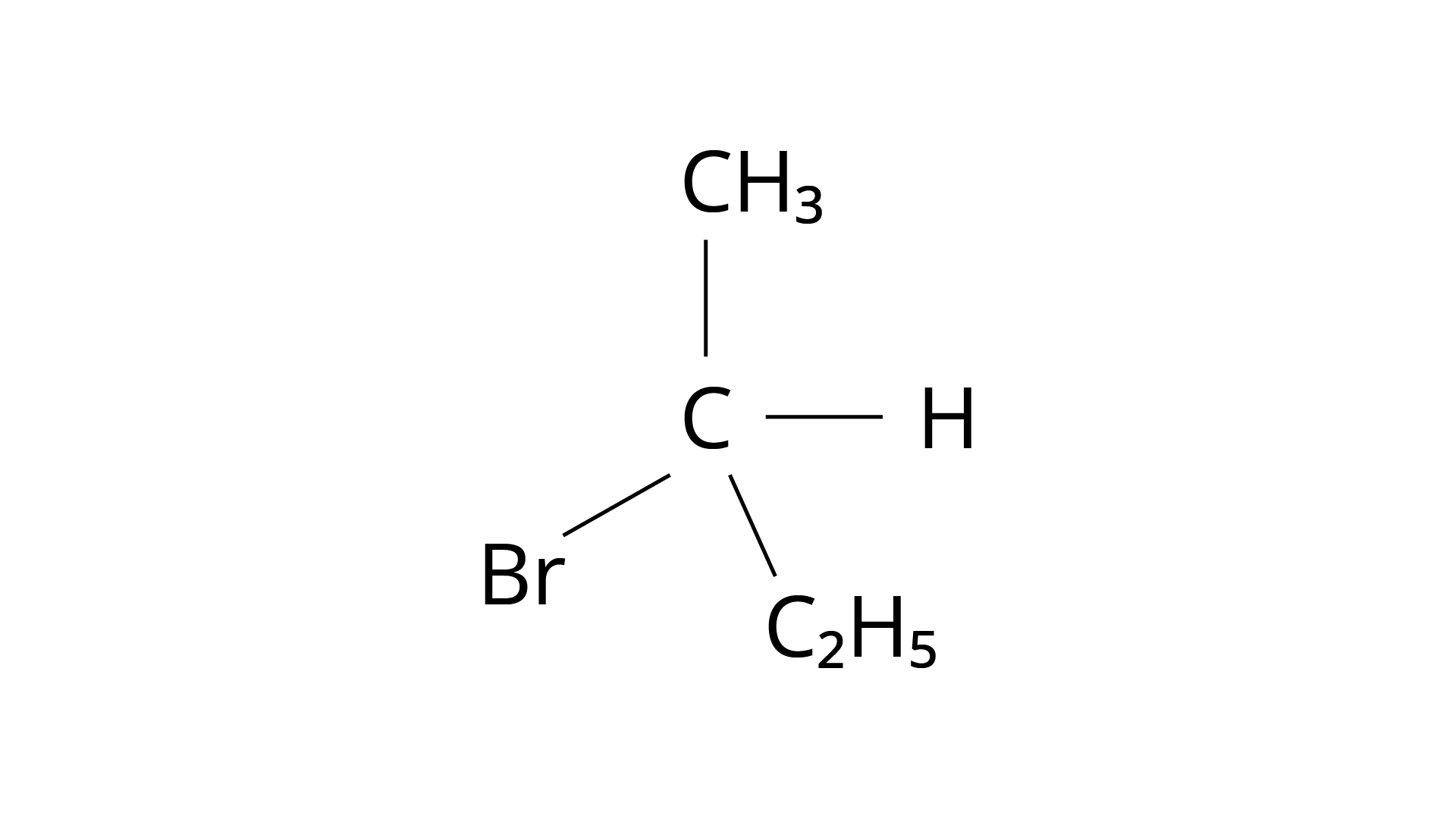
22. Molecules whose mirror image is non super-imposable over them are known as chiral. Which of the following molecules is chiral in nature?
(i) 2-Bromobutane
(ii) 1-Bromobutane
(iii) 2-Bromopropane
(iv) 2-Bromopropan-2-ol
Ans: The Correct Answer is Option (i).
The structure of 2-Bromobutane is depicted in the diagram below. Because all of the groups connected to the central carbon atom differ, the mirror image of the molecule cannot be superimposed on the original molecule.
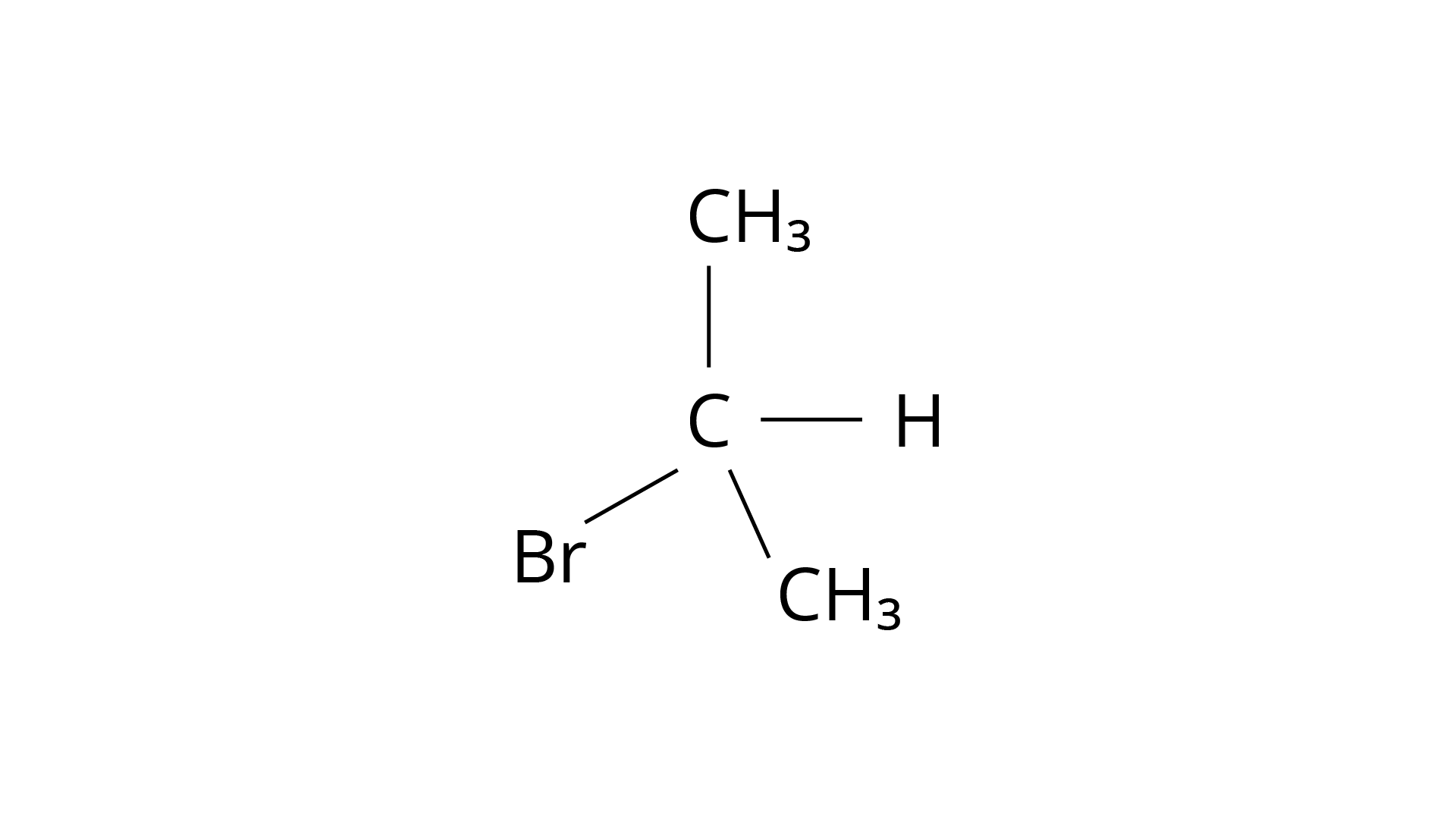
The structure of 1-Bromobutane is shown in the diagram below. Because there are just three groups that vary from one another, the molecule is not chiral in nature.
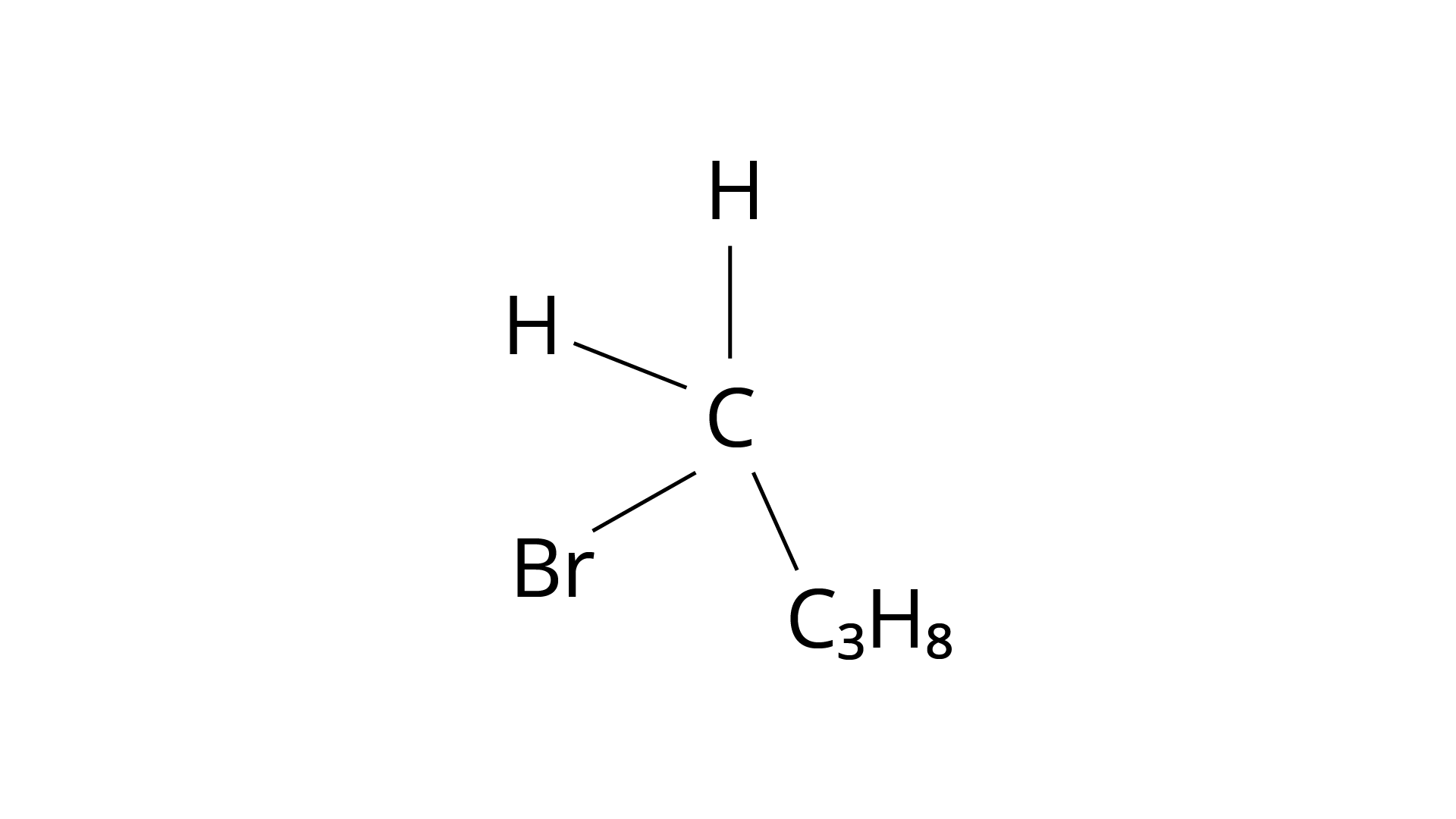
The structure of 1-Bromopropane is shown in the diagram below. Again, just three groups vary from one another, and the molecule is not chiral.
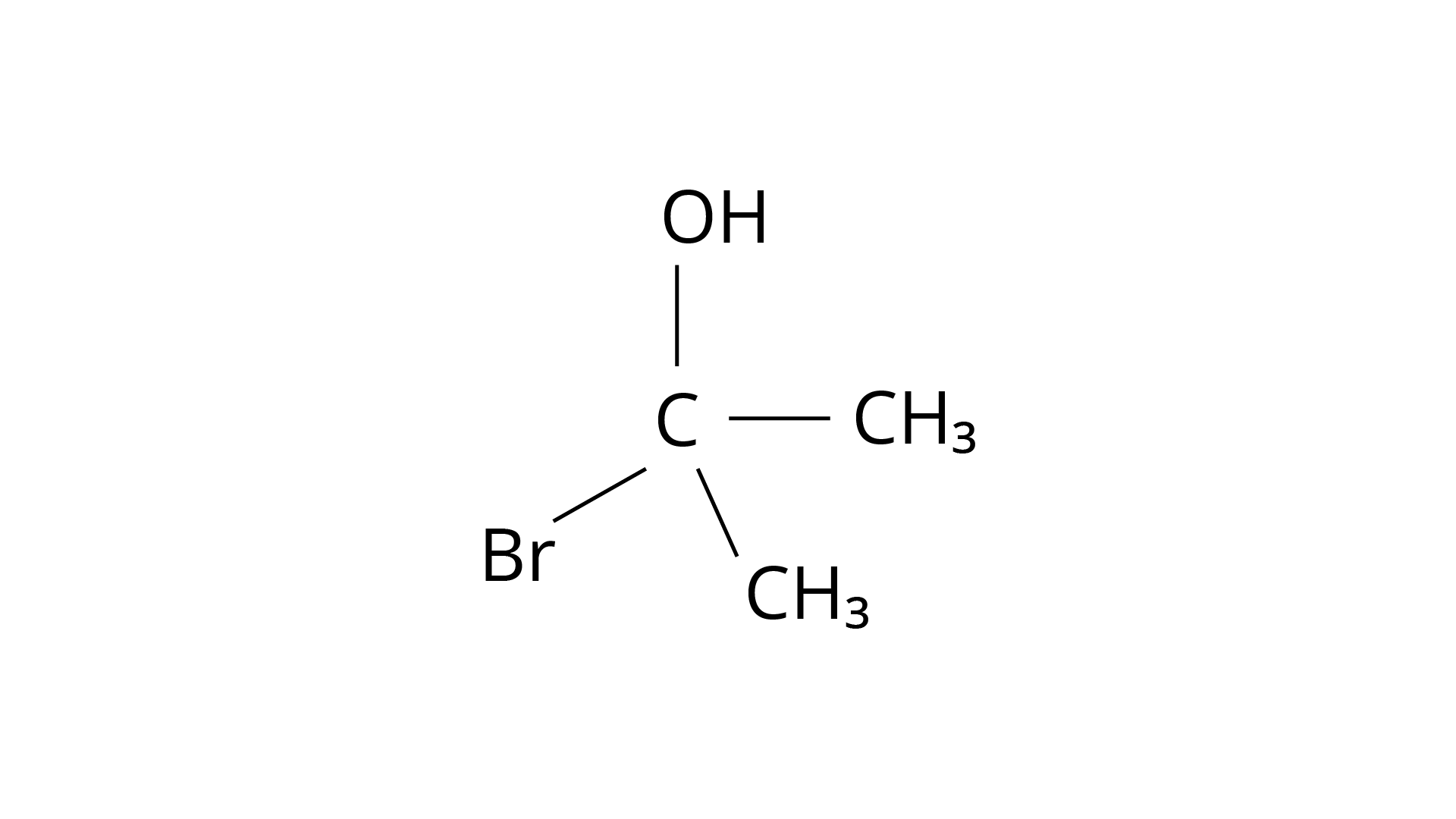
The structure of 2-Bromopropan-2-ol, shown below, has two identical species and hence cannot be chiral.
23. Reaction of \[{C_6}{H_5}C{H_2}Br\] with aqueous sodium hydroxide follows
(i) \[{SN}^1\] mechanism
(ii) \[{SN}^1\] mechanism
(iii) Any of the above two depending upon the temperature of reaction
(iv) Saytzeff rule
Ans: The Correct Answer is Option (i).
When benzyl chloride is treated with aqueous sodium hydroxide, where \[{\text{--OH}}\] is the nucleophile, a nucleophilic substitution reaction occurs, resulting in the formation of benzyl alcohol. The benzene ring is resonance stabilised here, and this stability is extended to the connected methylene group, giving a positive charge to \[{\text{--C}}{{\text{H}}_{\text{2}}}\], making the whole carbocation stable when the link between benzyl and bromide is broken. This is an \[{{\text{S}}_{\text{N}}}{\text{1}}\] reaction with two stages that is followed due to the stability of the carbocation. This is a coordinated reaction that takes place in two phases. The halide group first exits the carbocation, and then the nucleophile binds to the cation, producing alcohol. The right answer is (i).
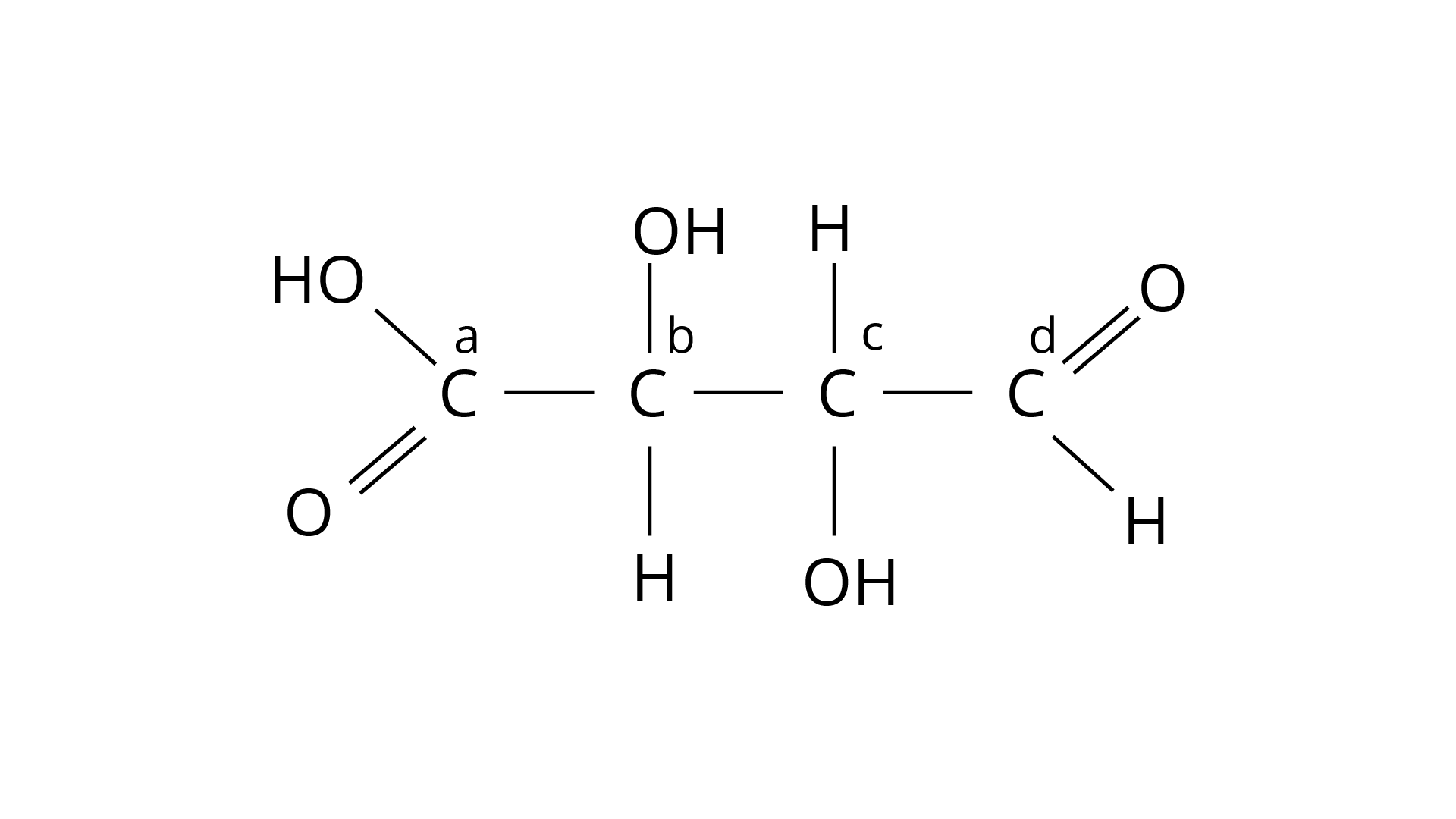
24. Which of the carbon atoms present in the molecule given below are asymmetric?
(i) \[a,b,c,d\]
(ii) \[b,c\]
(iii) \[a,d\]
(iv) \[a,b,c\]
Ans: The Correct Answer is Option (ii).
Chiral molecules are made up of one carbon atom surrounded by four different species. Because of the presence of two or more identical groups, such as hydrogens, all straight chain molecules cannot be chiral. Even carbons with double or triple bonds to a group are not considered chiral. Through covalent connections, an asymmetric carbon must be surrounded by four distinct species. As a result, atoms b and c are asymmetric. The correct answer is option (ii).
25. Which of the following compounds will give racemic mixture on nucleophilic substitution by \[O{H^ - }\]ion?
(a)
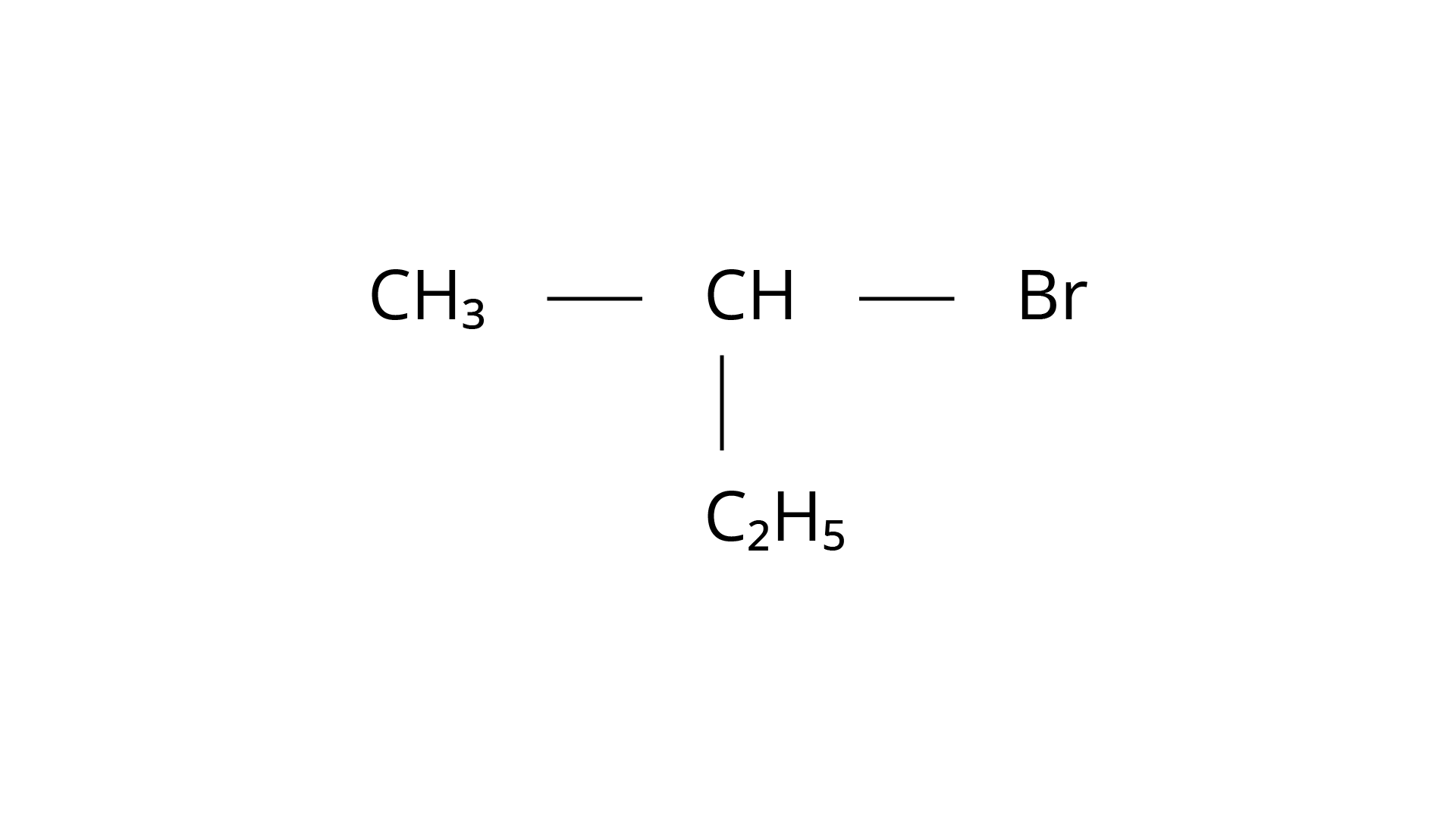
(b)
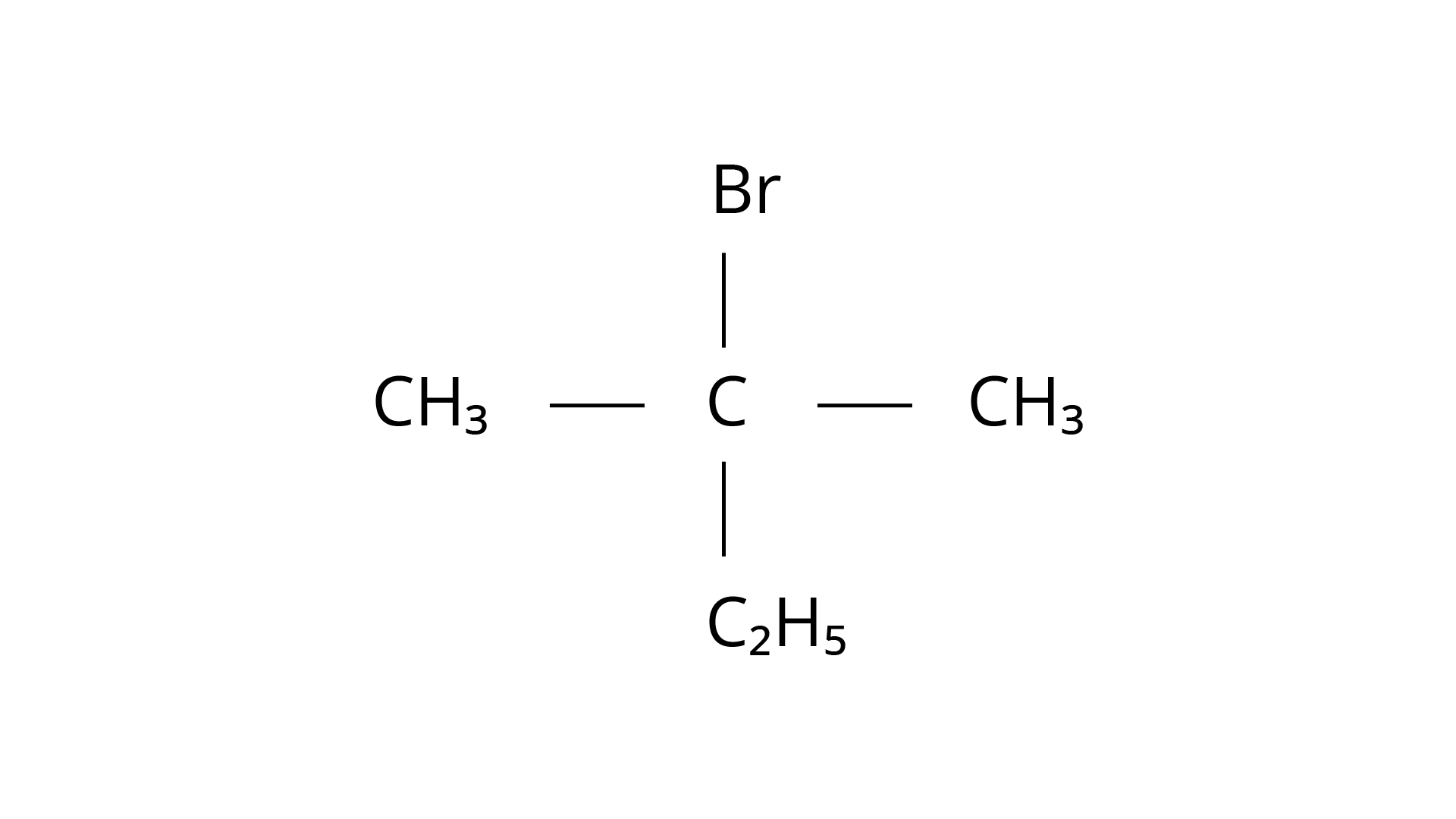
(c)
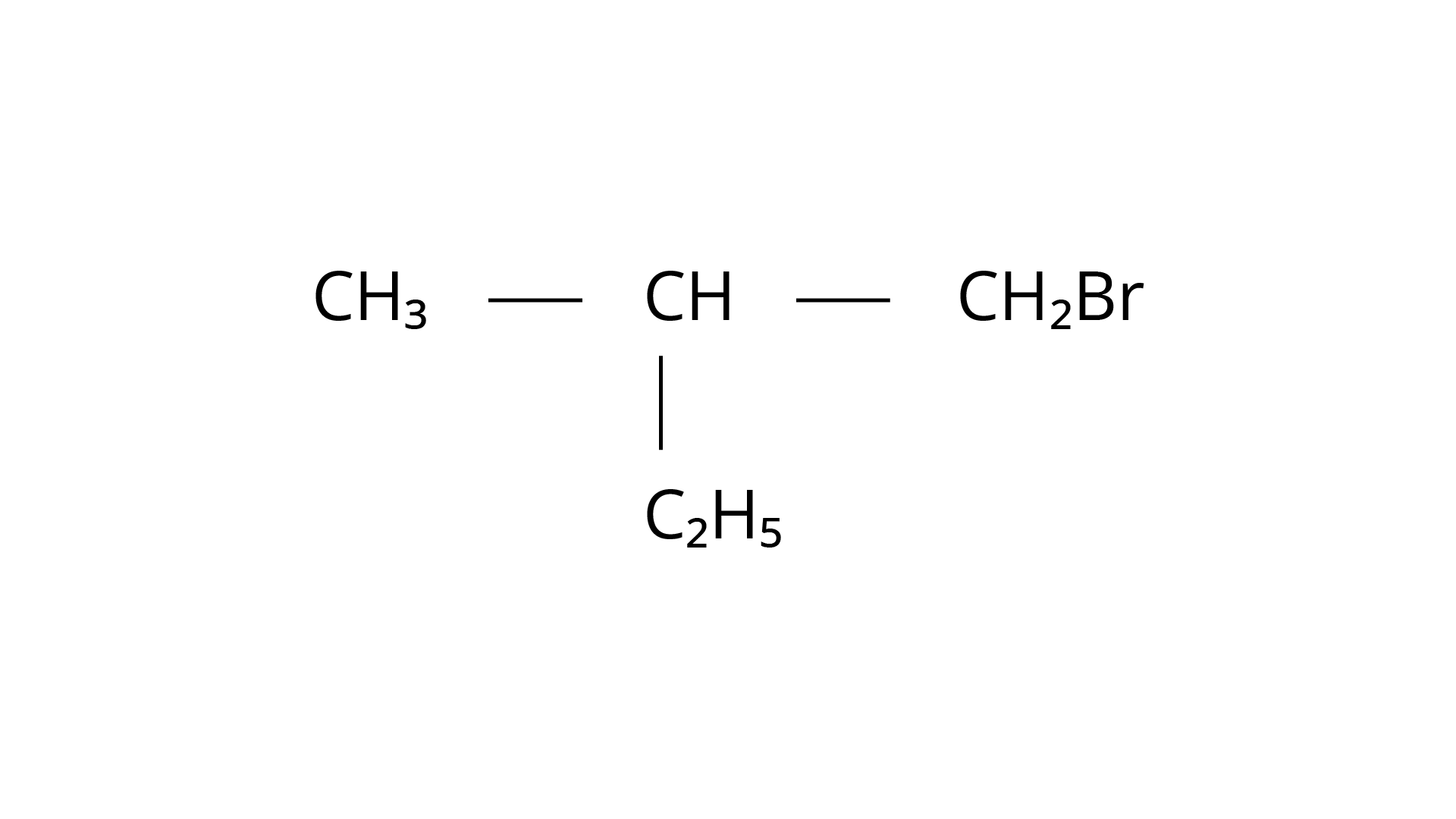
(i) (a)
(ii) (a), (b), (c)
(iii) (b), (c)
(iv) (a), (c)
Ans: The Correct Answer is Option (i).
A racemic mixture is one that contains two enantiomers in equal quantities but has no optical activity because the opposing optical rotations of the two enantiomers cancel each other out. The optically active reactant undergoes the \[{{\text{S}}_{\text{N}}}{\text{1}}\] reaction in order for a racemic mixture to form following nucleophilic substitution. Option (a) is a chiral carbon atom that will undergo the \[{{\text{S}}_{\text{N}}}{\text{1}}\] process, resulting in a racemic mixture. Option (b) does not include an asymmetric carbon, but option (c) has a secondary carbon asymmetric atom that is less reactive to an \[{{\text{S}}_{\text{N}}}{\text{1}}\] substitution. The right answer is (i).
Note : In the questions 26 to 29 arrange the compounds in increasing order
of rate of reaction towards nucleophilic substitution.
26.
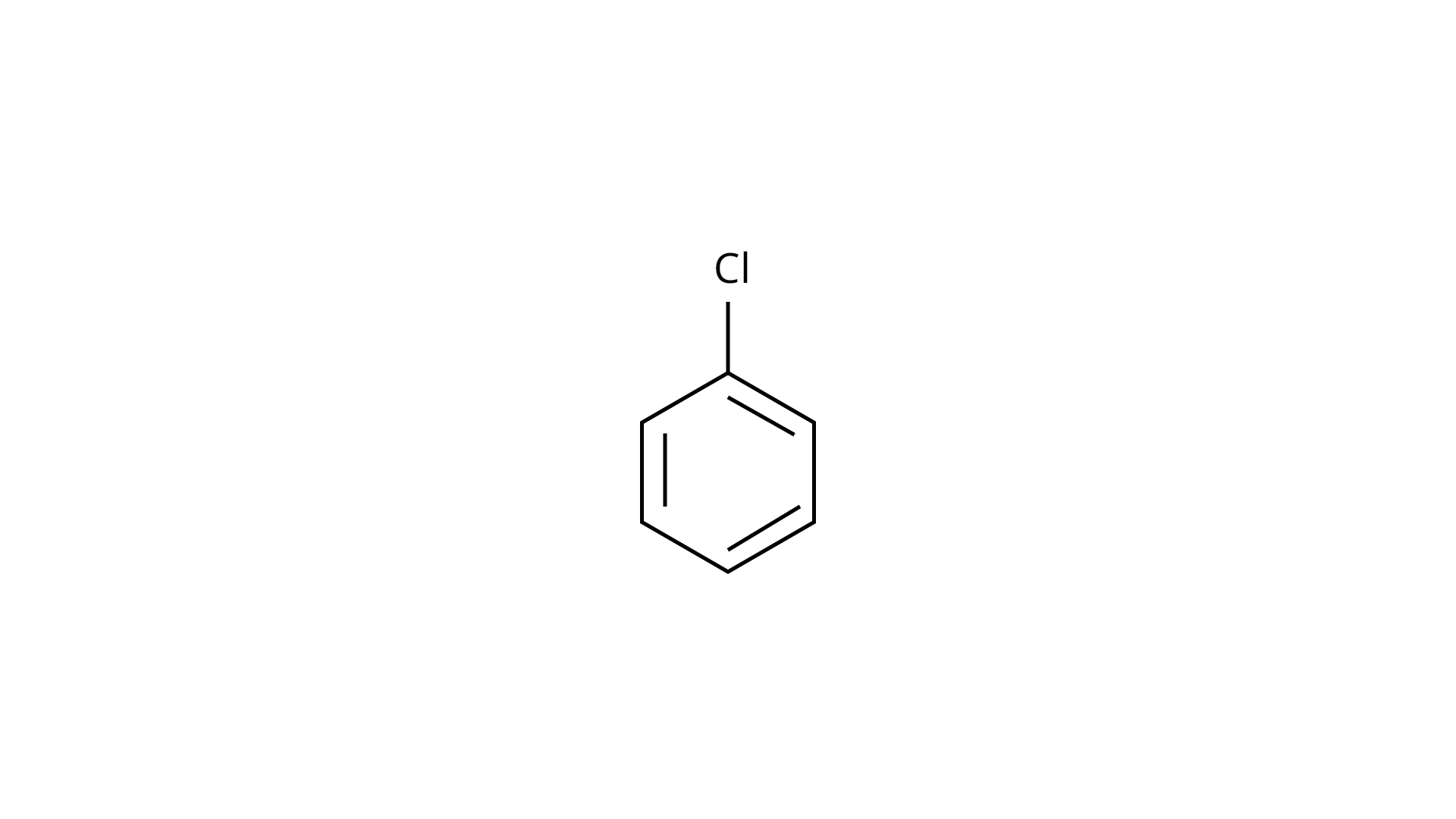
(a)
(b)
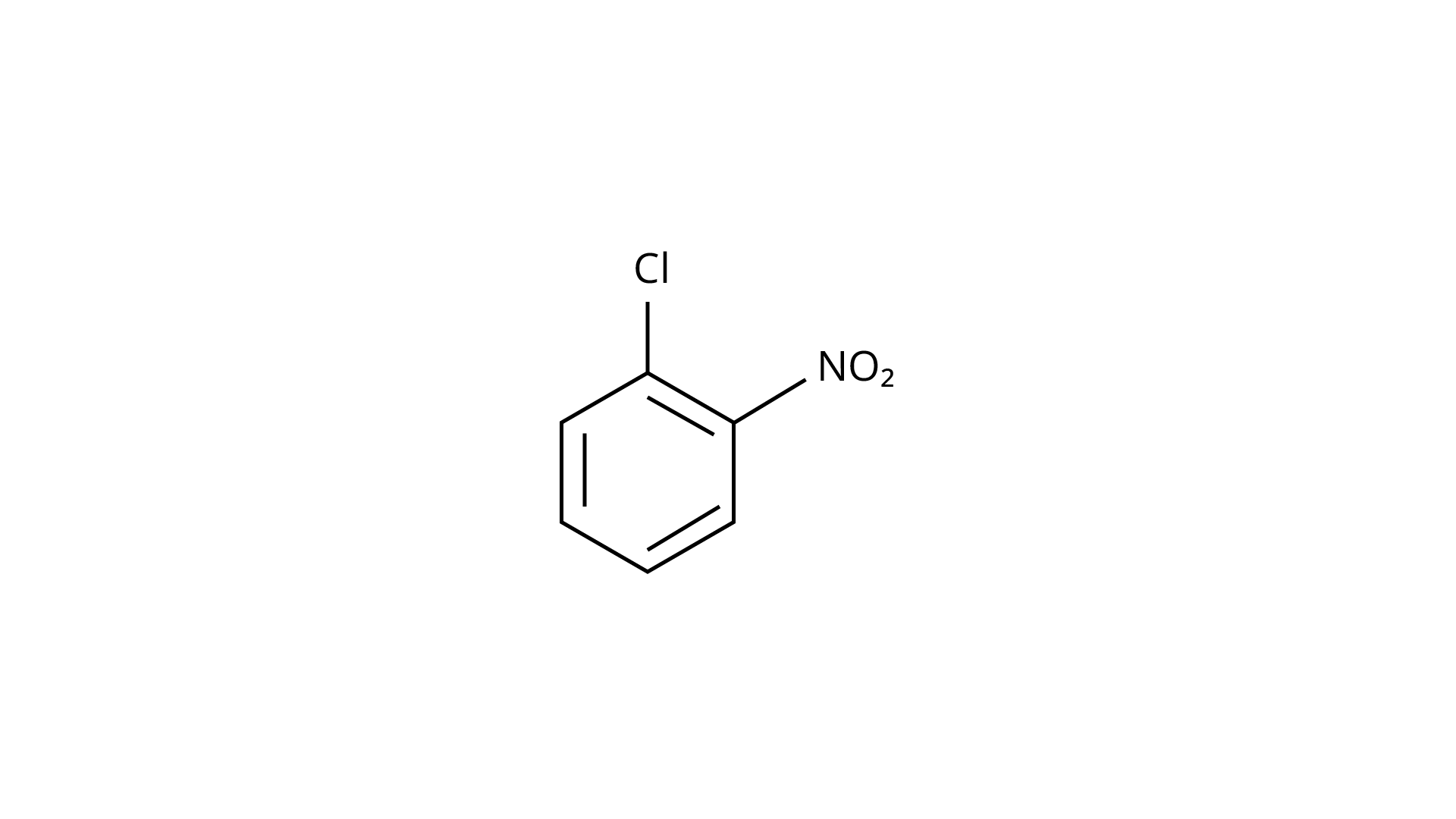
(c)
(i) \[(a) < (b) < (c)\]
(ii) \[(c) < (b) < (a)\]
(iii) \[(a) < (c) < (b)\]
(iv) \[(c) < (a) < (b)\]
Ans: The Correct Answer is Option (iii).
Due to the resonance stabilisation of the benzene ring, the reactivity of aryl halides to nucleophilic substitution is exceedingly low. Because of resonance, the \[{\text{--Cl}}\] bond gains a partial double bond. The presence of an electron withdrawing \[{\text{--N}}{{\text{O}}_{\text{2}}}\] group at ortho or para positions on the ring enhances the reactivity of aryl halides. The presence of \[{\text{--N}}{{\text{O}}_{\text{2}}}\] near the \[{\text{C---Cl}}\] makes the molecule more reactive. The order of reactivity should be (b) > (c) > (a). The right option is (iii).
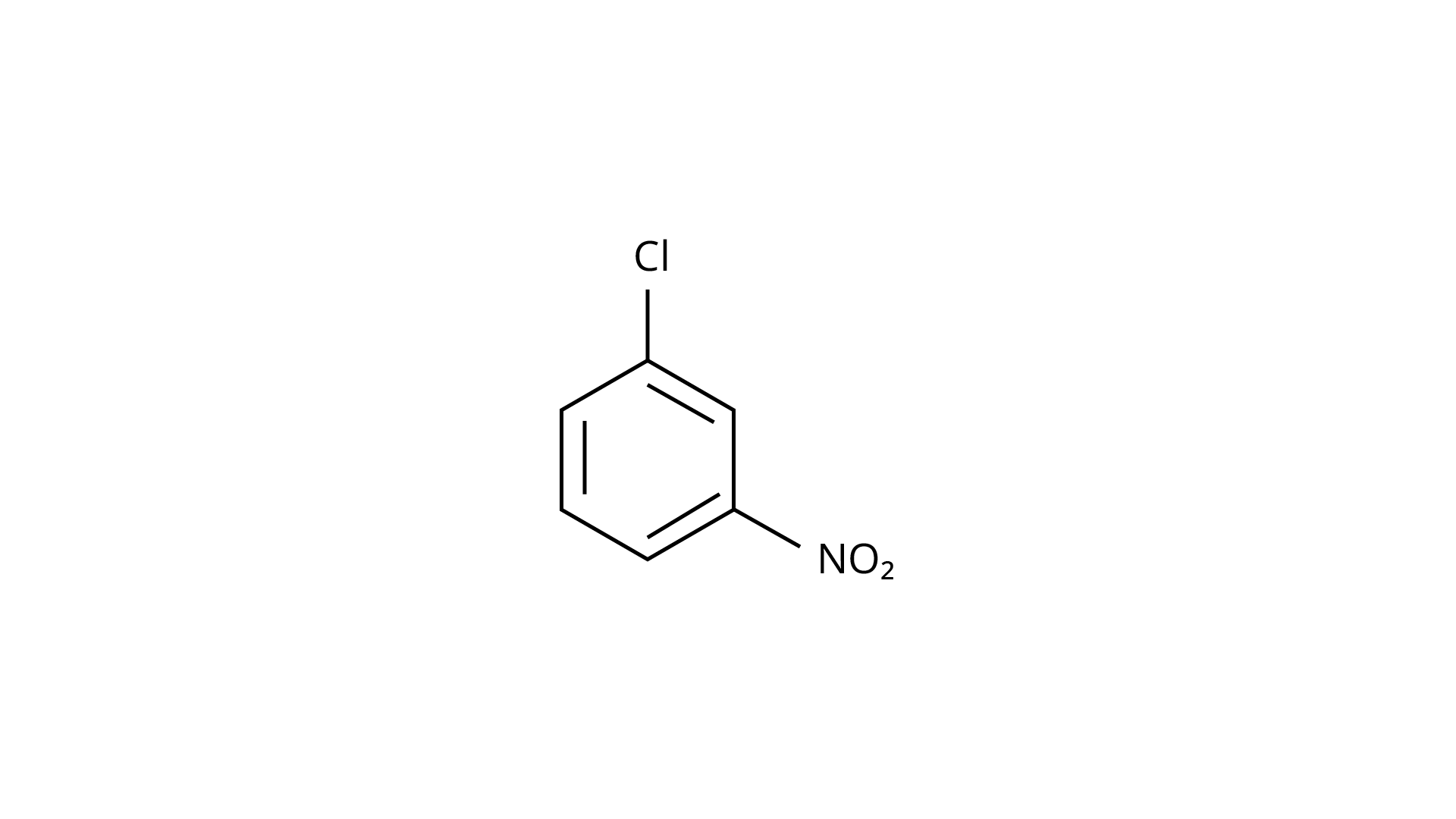
27.
(a)
(b)
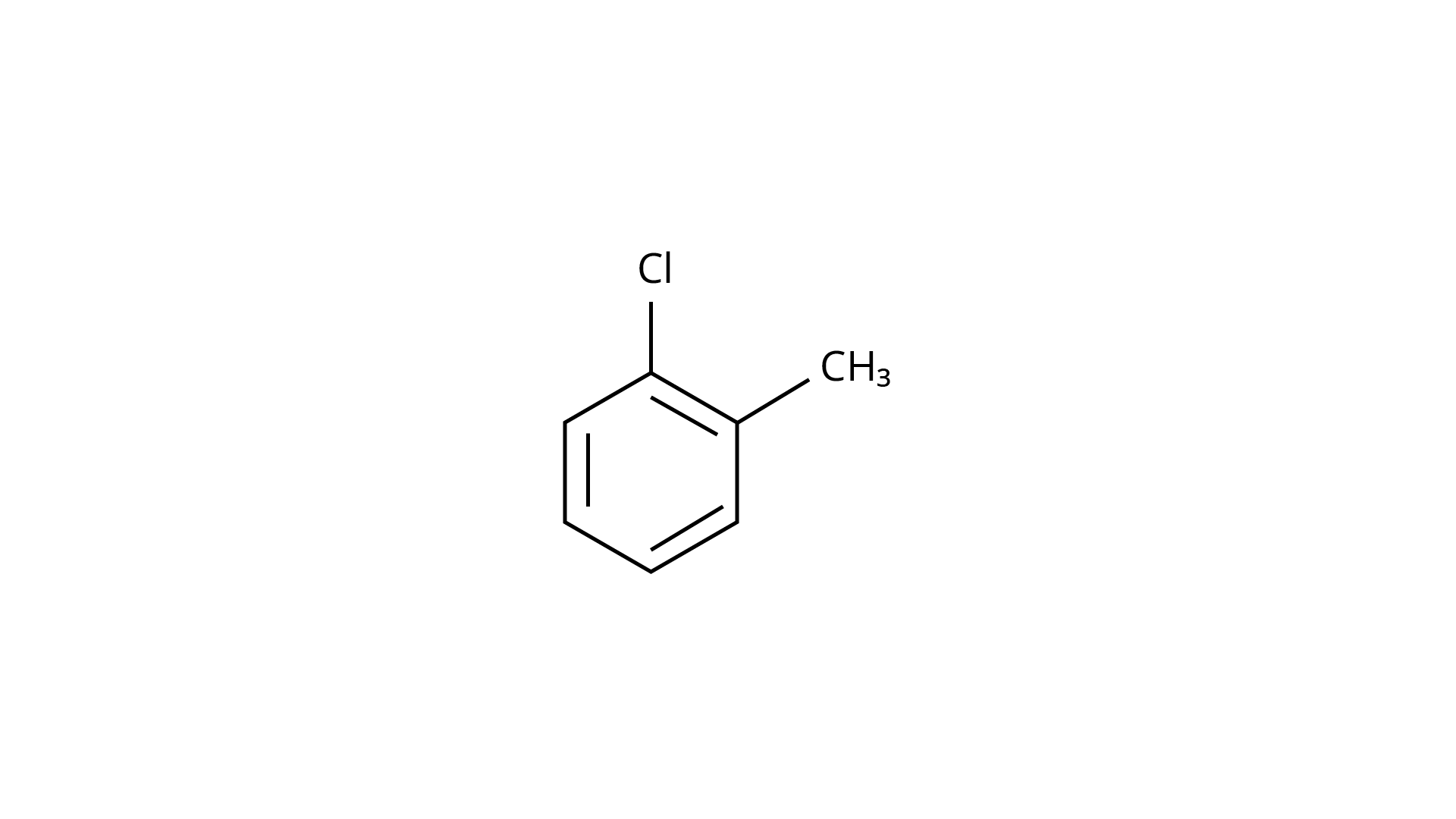
(c)
(i) \[(a) < (b) < (c)\]
(ii) \[(a) < (c) < (b)\]
(iii)\[(c) < (b) < (a)\]
(iv) \[(b) < (c) < (a)\]
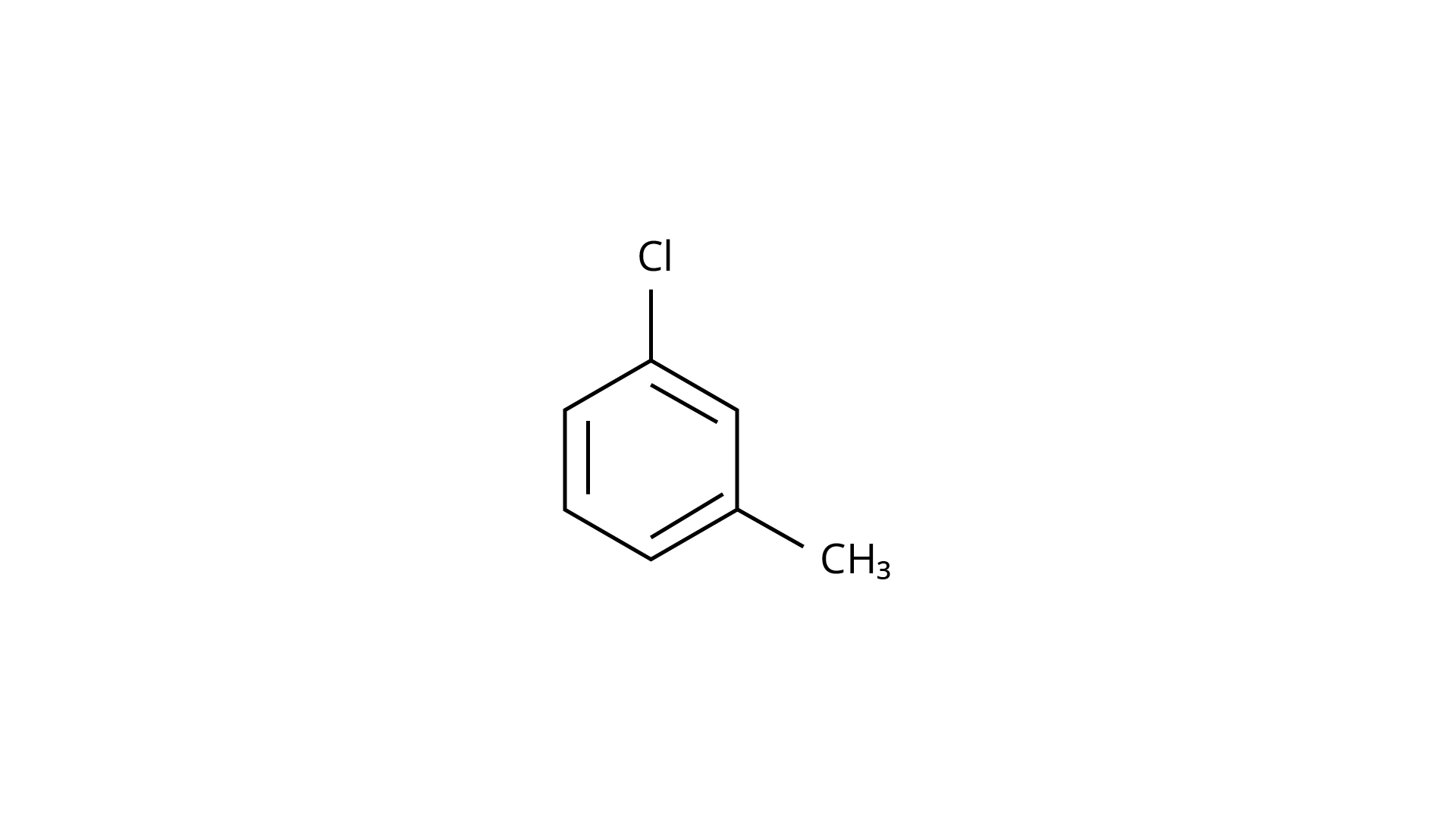
Ans: The Correct Answer is Option (iv).
Because the \[{\text{--C}}{{\text{H}}_{\text{3}}}\] group is an electron releasing group, it reduces the reactivity of aryl halides in the ortho and para locations. As a result, aryl halides without electron releasing groups are more reactive. As a result, the order of reactivity is (a) > (c) > (d) (b). The right answer is (iv).
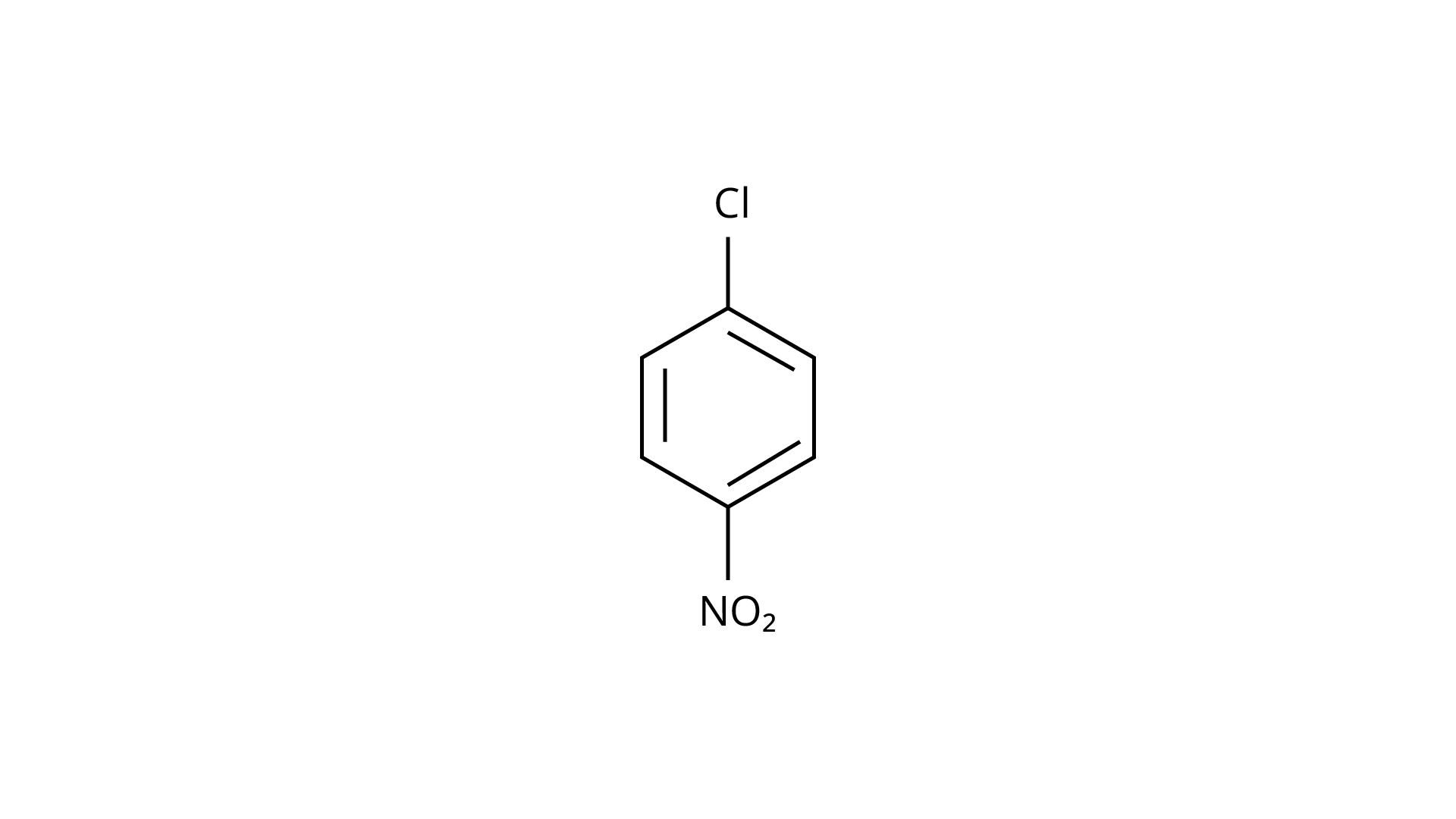
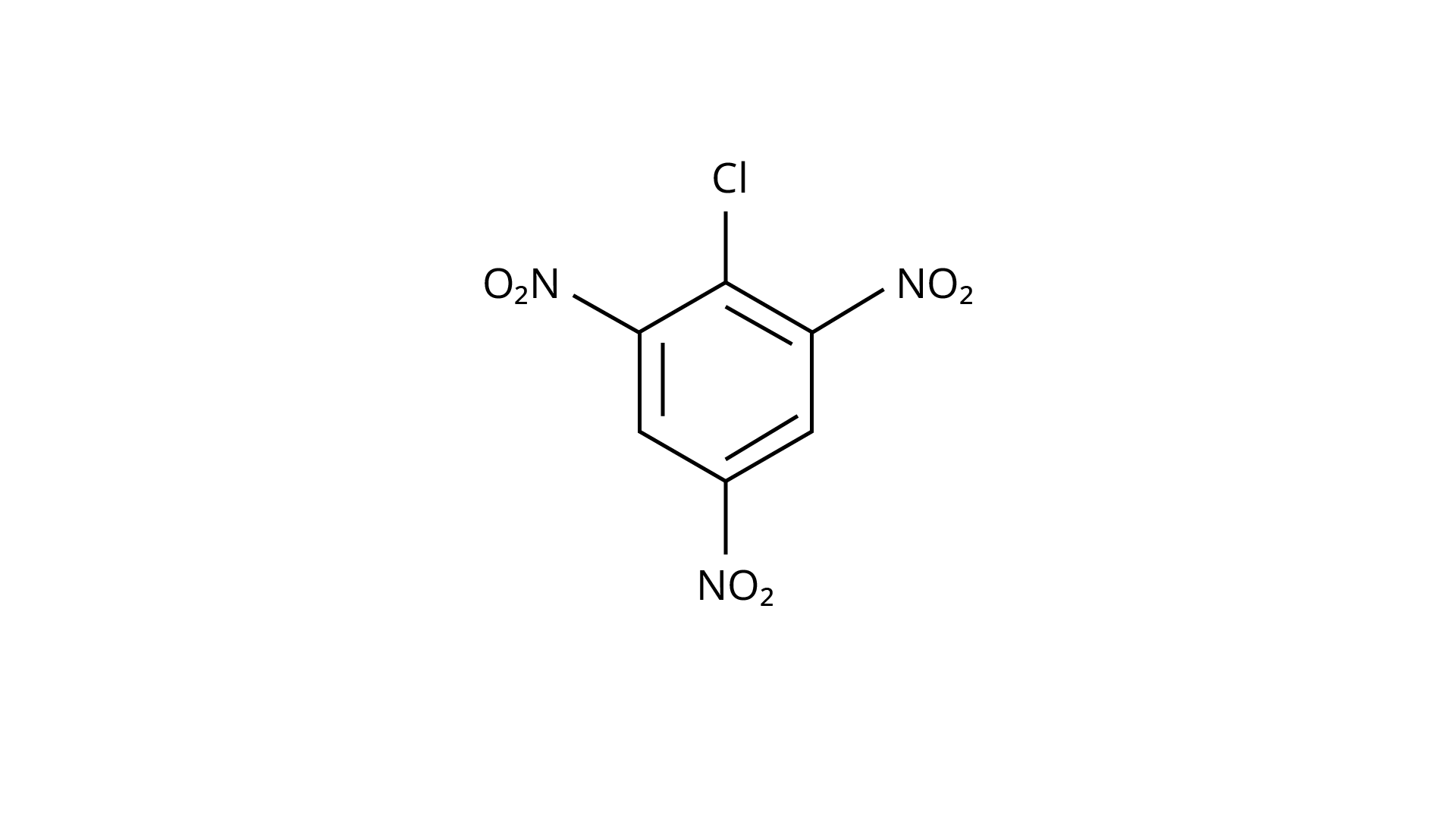
28.
(a)
(b)
(c)
(i) \[(c) < (b) < (a)\]
(ii) \[(b) < (c) < (a)\]
(iii) \[(a) < (c) < (b)\]
(iv) \[(a) < (b) < (c)\]
Ans: The Correct Answer is option (iv).
(\[{\text{ - N}}{{\text{O}}_{\text{2}}}\]) is an electron-withdrawing group that induces nucleophilic substitution when it is in the ortho and para positions. When this identical electron-drawing group is in the meta position, its impact is much reduced. The reactivity of aryl halides rises as the number of electron-withdrawing groups increases. As a result, the greater the number of electron-drawing groups, the greater the rate of nucleophilic substitution.
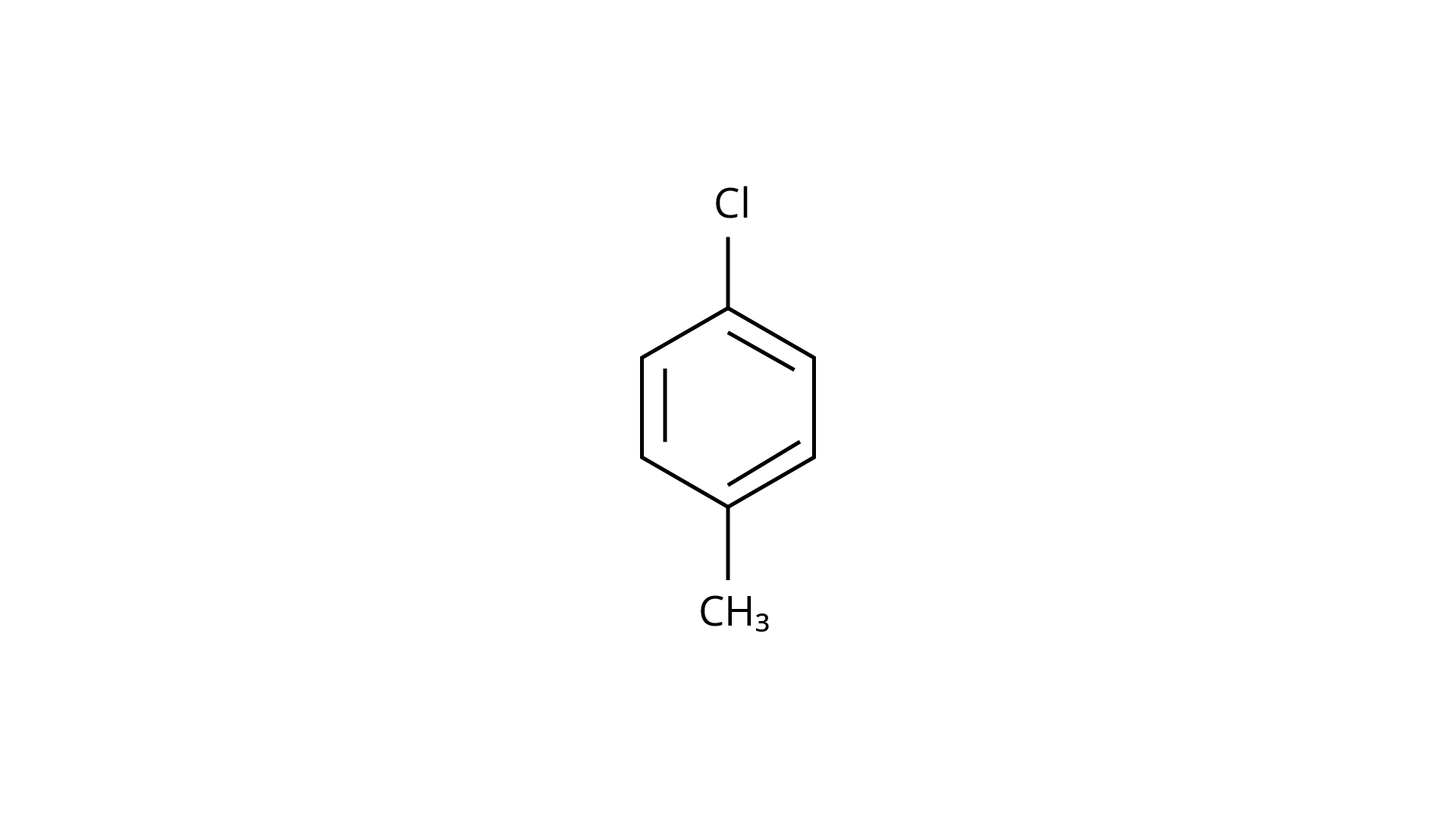
29.
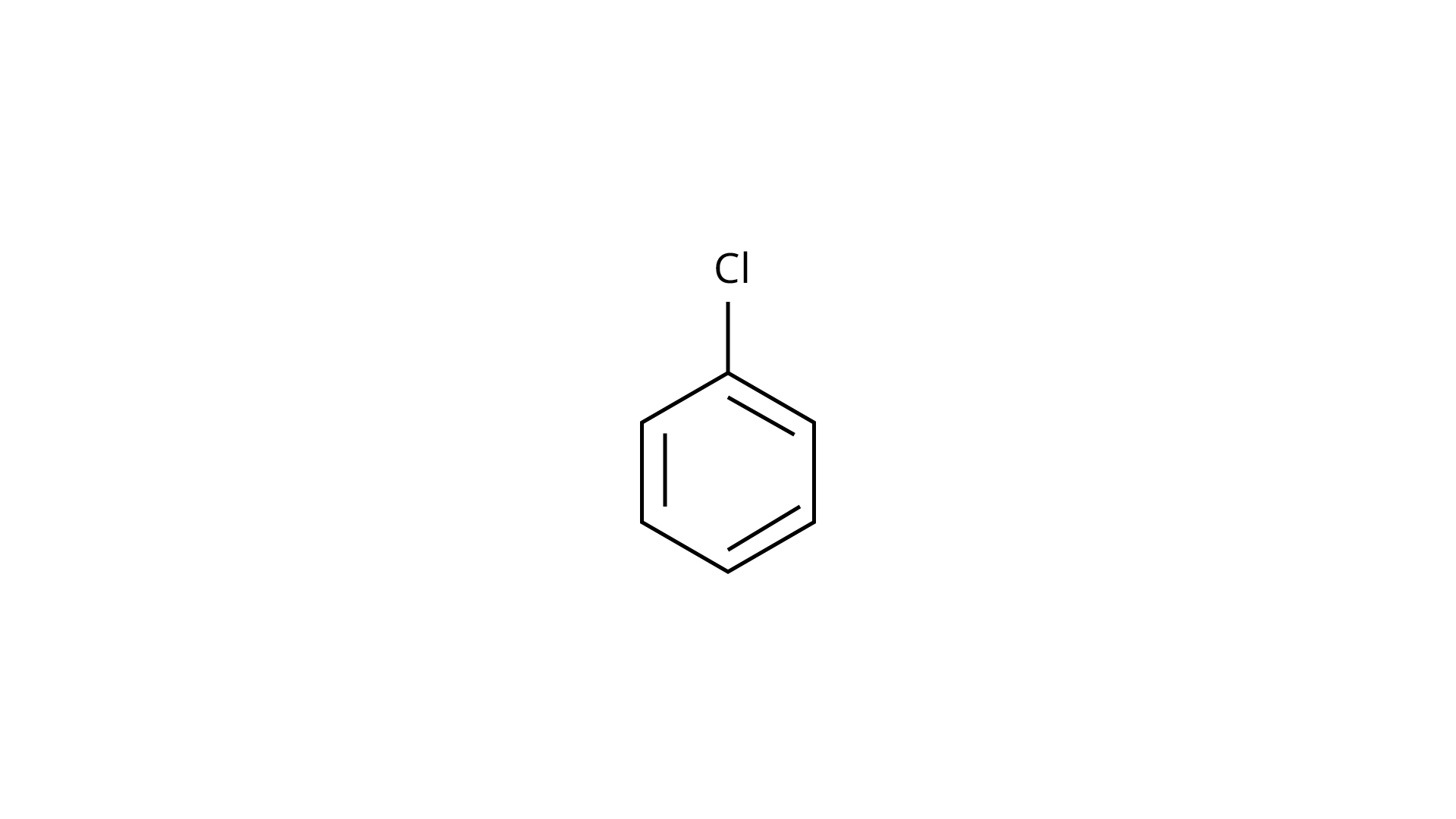
(a)
(b)
(c)
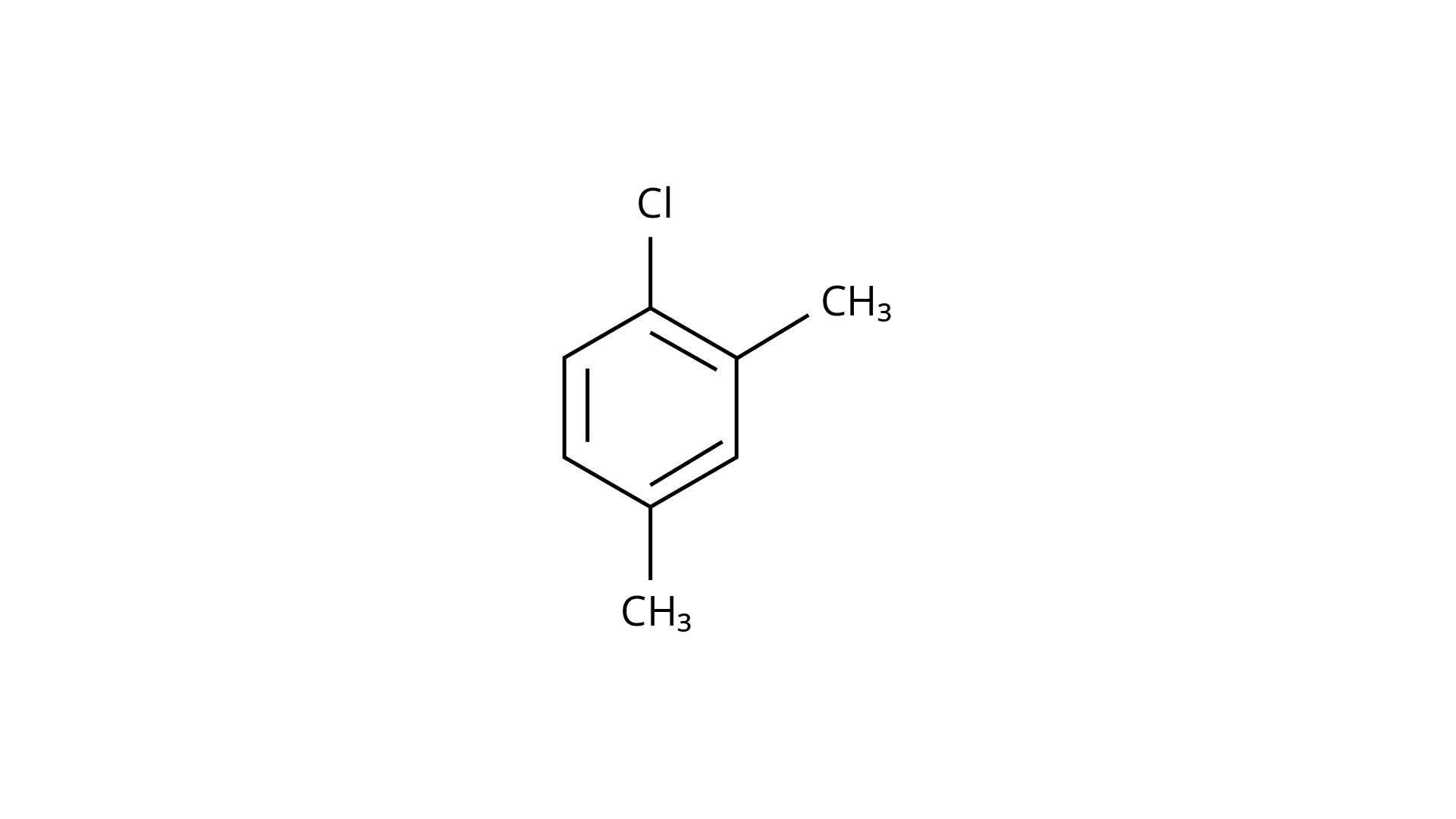
(i) \[(a) < (b) < (c)\]
(ii) \[(b) < (a) < (c)\]
(iii) \[(c) < (b) < (a)\]
(iv) \[(a) < (c) < (b)\]
Ans: The Correct Answer is Option (iii).
The presence of electron releasing groups increases the reactivity of aryl halides; the fewer the electron releasing groups, the slower the rate of nucleophilic substitution. As a result, an increase in methyl groups decreases reactivity. The sequence of reactivity is (a) > (b) > (c). The right answer is (iii).
30. Which is the correct increasing order of boiling points of the following compounds?
1-Iodobutane, 1-Bromobutane, 1-Chlorobutane, Butane
(i) \[Butane < 1 - Chlorobutane < 1 - Bromobutane < 1 - Iodobutane\]
(ii) \[1 - Iodobutane < 1 - Bromobutane < 1 - Chlorobutane < Butane\]
(iii) \[Butane < 1 - Iodobutane < 1 - Bromobutane < 1 - Chlorobutane\]
(iv) \[Butane < 1 - Chlorobutane < 1 - Iodobutane < 1 - Bromobutane\]
Ans: The Correct Answer is Option (i).
The intermolecular force of attraction will grow as the surface area increases. As a result, the boiling point rises as well. The boiling point of a comparable alkyl halide rises with increasing molecular mass. Iodine has the greatest atomic mass. As a result, 1-iodobutane has the greatest boiling point.
31. Which is the correct increasing order of boiling points of the following compounds?
1-Bromoethane, 1-Bromopropane, 1-Bromobutane, Bromobenzene
(i) \[Bromobenzene < 1 - Bromobutane < 1 - Bromopropane < 1 - Bromoethane\]
(ii) \[Bromobenzene < 1 - Bromoethane < 1 - Bromopropane < 1 - Bromobutane\]
(iii) \[1 - Bromopropane < 1 - Bromobutane < 1 - Bromoethane < Bromobenzene\]
(iv) \[1 - Bromoethane < 1 - Bromopropane < 1 - Bromobutane < Bromobenzene\]
Ans: The Correct Answer is Option (iv).
If the halogen atom in all of the supplied alkyl halides is the same kind, the boiling point of a compound rises as the number of alkyl groups added increases. As the quantity and size of molecules and electrons grow, so do their attractions. As a result, the greater the boiling point, the larger the molecular weight of the alkyl halide. The boiling points of the following compounds are listed in ascending order: 1-Bromoethane 1-Bromopropane 1-Bromobutane Bromobenzene The right answer is (iv).
II Multiple Choice Questions (Type-II)
Note : In the following questions two or more options may be correct.
Consider the following reaction and answer the questions no. 32-34.
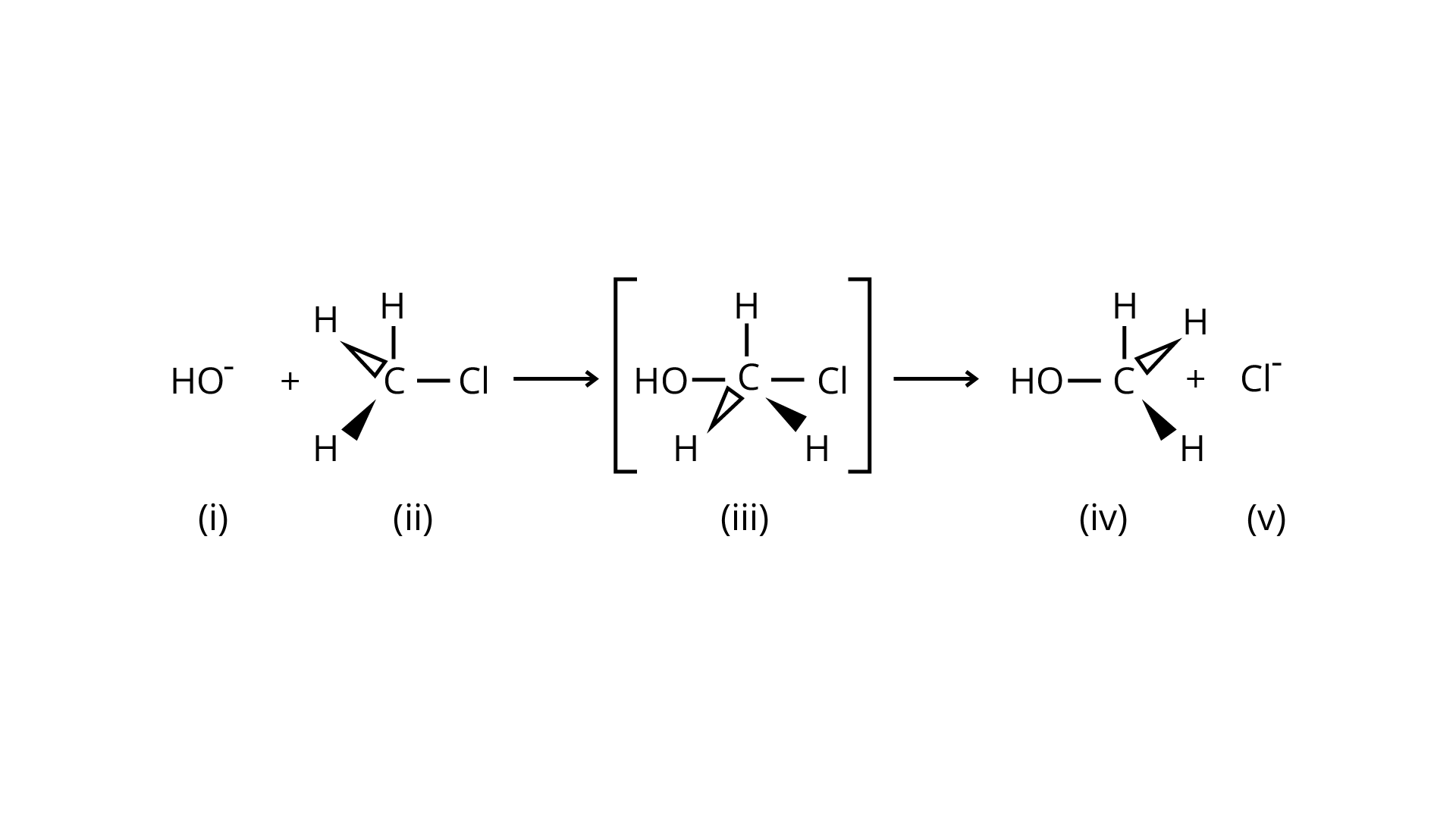
32. Which of the statements are correct about above reaction?
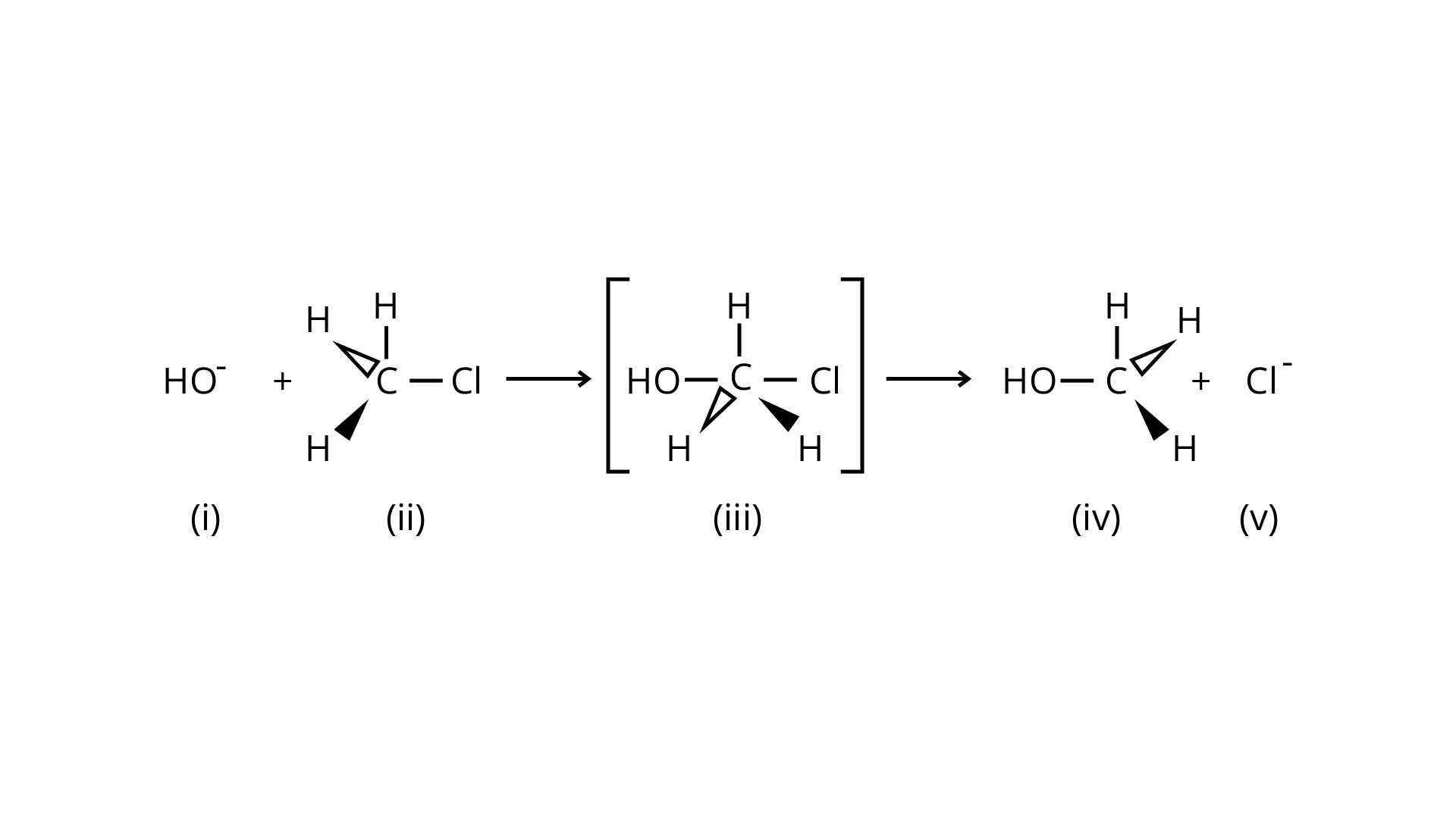
(A) (i) and (v) both are nucleophiles.
(B) In (iii) carbon atom is \[s{p^3}\]hybridised.
(C) In (iii) carbon atom is \[s{p^2}\]hybridised.
(D) (i) and (v) both are electrophiles.
Ans: An \[{{\text{S}}_{\text{N}}}2\] nucleophilic substitution reaction occurs when \[{\text{C}}{{\text{H}}_{\text{3}}}{\text{Cl}}\] interacts with the hydroxide ion to produce \[{\text{C}}{{\text{H}}_{\text{3}}}{\text{OH}}\]. Both \[{\text{O}}{{\text{H}}^{\text{ - }}}\] and \[{\text{C}}{{\text{l}}^{\text{ - }}}\]are nucleophiles with an excess of electrons in this reaction. Because the binding of \[{\text{O}}{{\text{H}}^{\text{ - }}}\] and the leaving of \[{\text{C}}{{\text{l}}^{\text{ - }}}\]occurs simultaneously in the transition state (c), the carbon atom is linked to just three hydrogens. As a result, the carbon atom becomes \[{\text{s}}{{\text{p}}^{\text{2}}}\] hybridised. Aand (C) are the right statements.
Correct Answer: Option (A) and (C)
33. Which of the following statements are correct about this reaction?
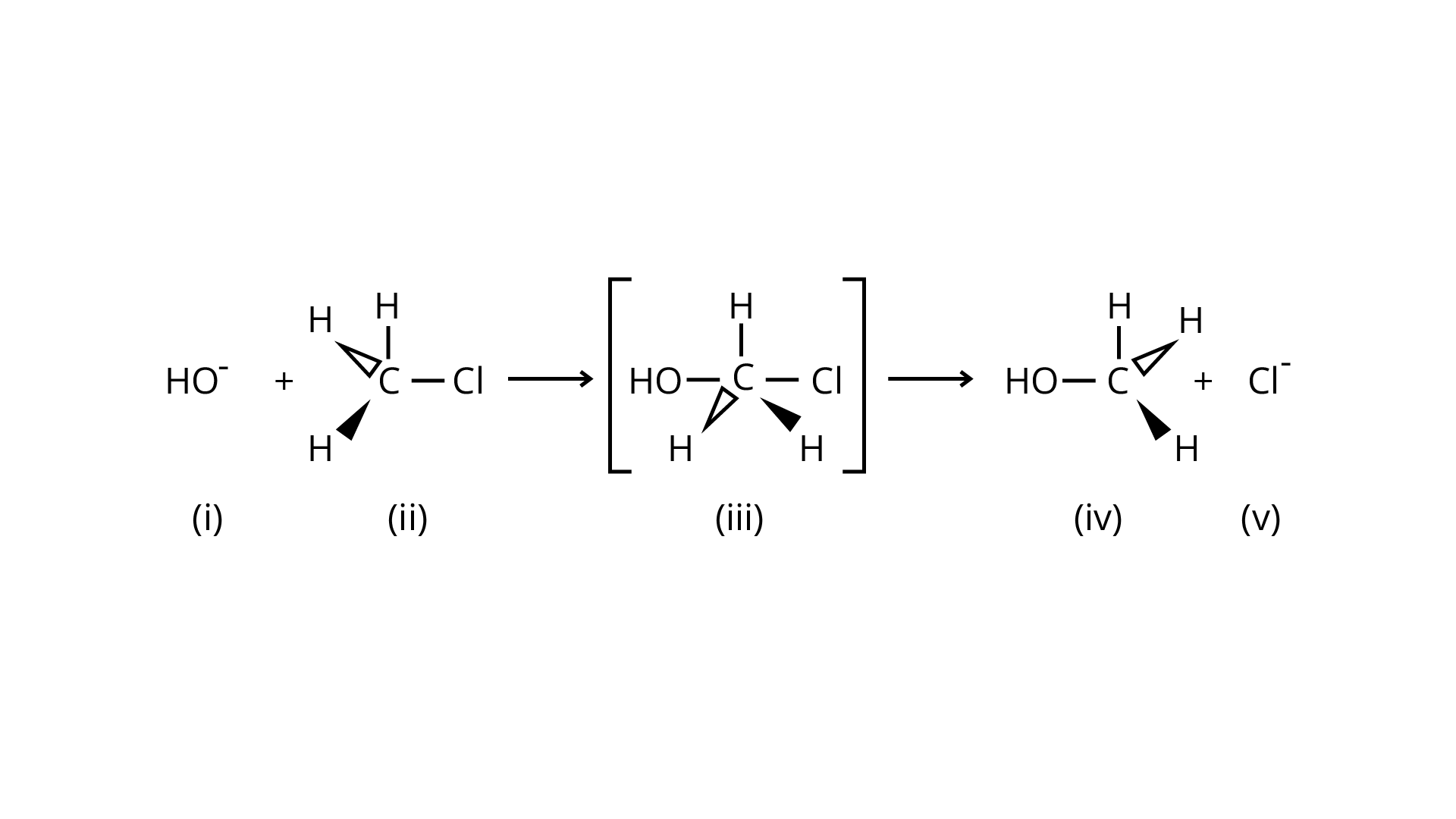
(A) The given reaction follows \[{S_N}2\] mechanism.
(B) (ii) and (v) have opposite configuration.
(C) (ii) and (v) have same configuration.
(D) The given reaction follows \[{S_N}1\]mechanism.
Ans: An \[{{\text{S}}_{\text{N}}}2\] reaction, also known as a nucleophilic substitution, is the reaction in question. The entering nucleophile causes the groups surrounding the carbon atom to migrate in the opposite direction of the nucleophile, causing the configuration of alkyl halides to invert. As a result, (b) and (d) will be in the opposite order. This reaction is also \[{{\text{S}}_{\text{N}}}2\] since there is just one phase in which \[{\text{O}}{{\text{H}}^{\text{ - }}}\]is added and \[{\text{C}}{{\text{l}}^{\text{ - }}}\] is removed at the same time. (i) and (ii) are the right answers.
Correct Answer: Option (A) and (B)
34. Which of the following statements are correct about the reaction intermediate?
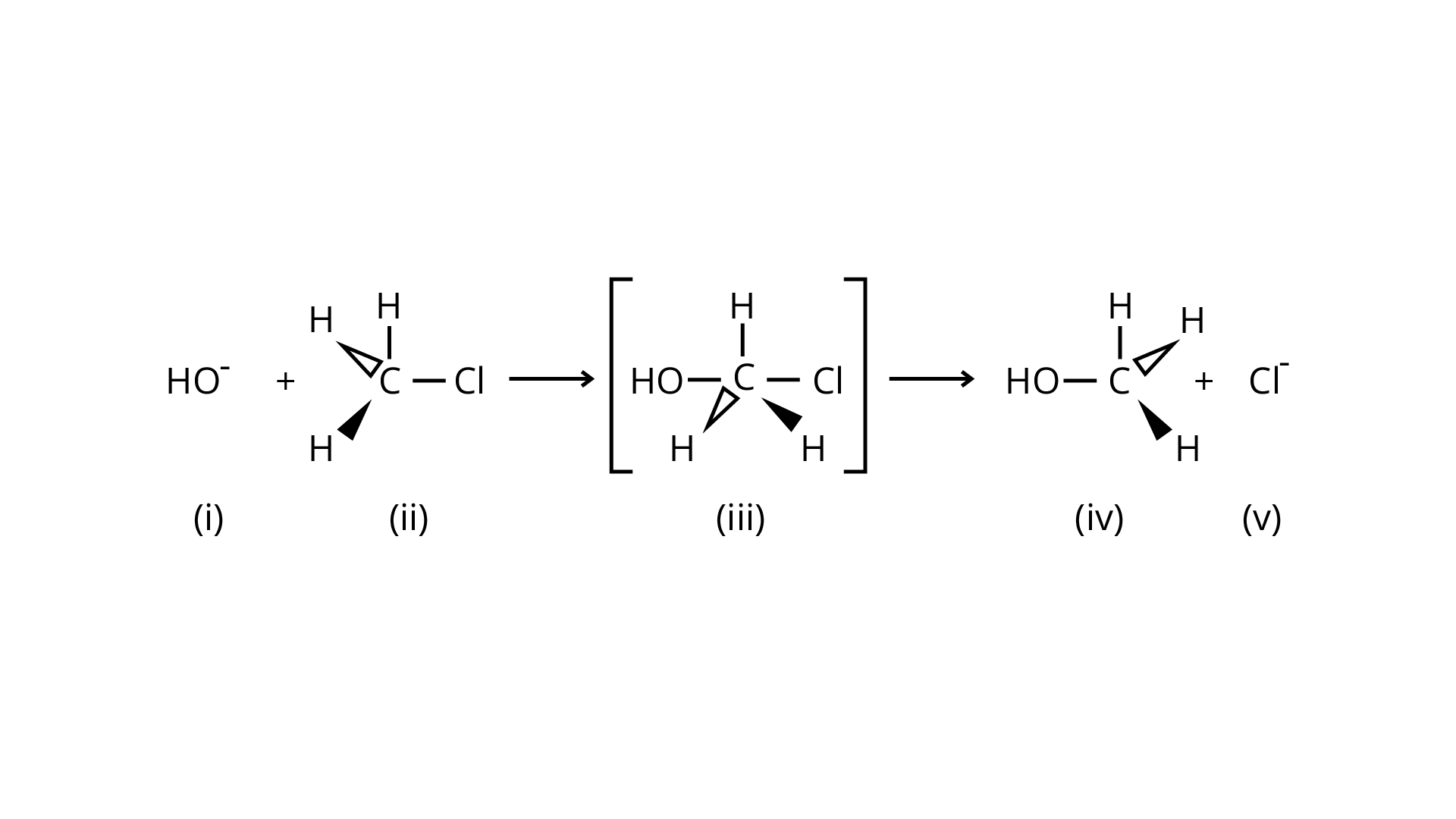
(A) Intermediate (iii) is unstable because in this carbon is attached to 5 atoms.
(B) Intermediate (iii) is unstable because carbon atom is \[s{p^2}\]hybridised.
(C) Intermediate (iii) is stable because carbon atom is \[s{p^2}\] hybridised.
(D) Intermediate (iii) is less stable than the reactant (ii).
Ans: Because the carbon atom is transiently linked to five atoms, the intermediate (iii) is less stable than reactant (ii), which is coupled to four groups.
Answer \[{\text{Q}}{\text{.No}}{\text{.35}}\]and \[{\text{36}}\] on the basis of the following reaction.
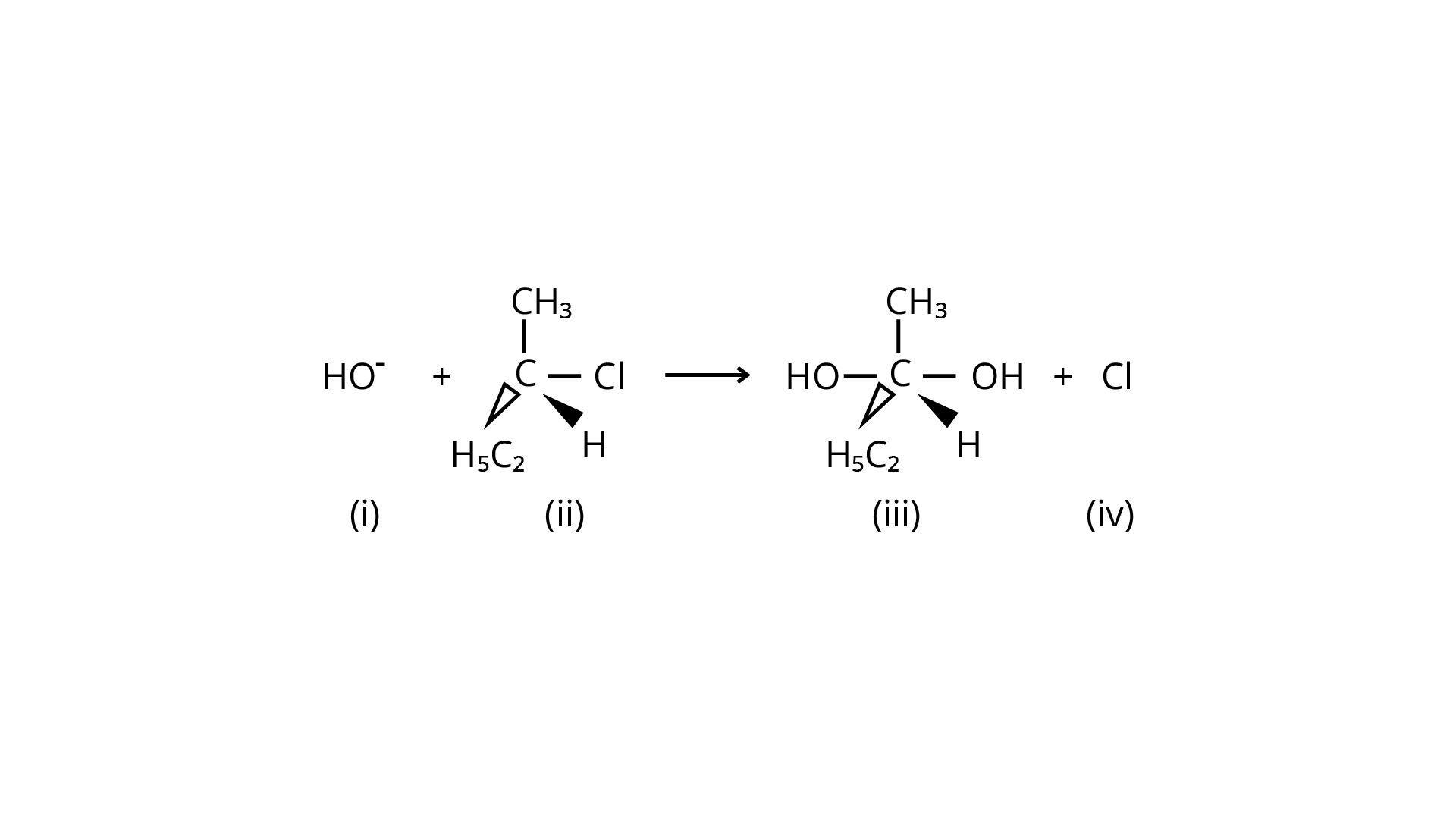
Correct Answer: option (A) and (D)
35. Which of the following statements are correct about the mechanism of this reaction?

(A) A carbocation will be formed as an intermediate in the reaction.
(B) \[O{H^ - }\] will attach the substrate (ii) from one side and \[C{l^ - }\] will leave it simultaneously from other side.
(C) An unstable intermediate will be formed in which \[O{H^ - }\] and \[C{l^ - }\] will be attached by weak bonds.
(D) Reaction proceeds through \[{{\text{S}}_{\text{N}}}1\]mechanism.
Ans: Because it is a tertiary alkyl halide, the described reaction is a nucleophilic substitution that follows the \[{{\text{S}}_{\text{N}}}1\] mechanism. It's a two-step process. The first step is the gradual breaking of a polarised \[{\text{C---Br}}\] bond, which results in the formation of a carbocation and a bromide ion. To complete the substitution, the stable intermediate carbocation is attacked by the nucleophile \[{\text{O}}{{\text{H}}^ - }\]. (i) and (iv) are the right statements.
Correct Answer: option (A) and (iv)
36. Which of the following statements are correct about the kinetics of this reaction?
(A) The rate of reaction depends on the concentration of only (ii).
(B) The rate of reaction depends on concentration of both (i) and (ii).
(C) Molecularity of reaction is one.
(D) Molecularity of reaction is two.
Ans: The reaction is of the type \[{{\text{S}}_{\text{N}}}1\] nucleophilic substitution. This sort of reaction is governed by second order kinetics, in which the rate of reaction is determined only by the concentration of the reactant. A reaction's molecularity is defined as the number of molecules involved in the rate-determining phase. Because of second order kinetics, the moleculeity of this reaction is one. (i) and (iii) are the right answers .
Correct Answer: Option (A) and (C)
37. Haloalkanes contain halogen atom (s) attached to the \[s{p^3}\] hybridised carbon atom of an alkyl group. Identify haloalkane from the following compounds.
(i) 2 -Bromopentane
(ii) Vinyl chloride (chloroethene)
(iii) 2-chloroacetophenone
(iv) Trichloromethane
Ans: Option (i) and (iv) have a halogen atom linked to an \[{\text{s}}{{\text{p}}^{\text{3}}}\] hybridised carbon that is single-bonded to other groups. The other possibilities are \[{\text{s}}{{\text{p}}^2}\] hybridised because they are linked to carbon atoms, which are coupled to other groups in double bonds. Option I and (iv) are both correct.
Correct Answer: Option (i) and (iv)
38. Ethylene chloride and ethylidene chloride are isomers. Identify the correct statements.
(i) Both the compounds form same product on treatment with alcoholic \[KOH\].
(ii) Both the compounds form same product on treatment with aq. \[NaOH\].
(iii) Both the compounds form same product on reduction.
(iv) Both the compounds are optically active.
Ans: Isomers are ethylene chloride \[{\text{ClC}}{{\text{H}}_{\text{2}}}{\text{---C}}{{\text{H}}_{\text{2}}}{\text{Cl}}\] and ethylidene dichloride \[{\text{C}}{{\text{H}}_{\text{3}}}{\text{---CHC}}{{\text{l}}_{\text{2}}}\]. Because the carbon atoms are not surrounded by distinct groups, neither molecule is optically active. When haloalkanes are treated with alcoholic \[{\text{KOH}}\], they undergo an elimination process in which a hydrogen atom from the -carbon atom and a halogen atom from the -carbon atom are eliminated. To form the ethyne molecule, each of these chemicals lose hydrogen and chlorine atoms. When exposed to aqueous \[{\text{NaOH}}\], the molecules undergo nucleophilic substitution, with the –Cl groups being replaced by \[{\text{--OH}}\] molecules. The results will differ due to the locations of the halides on various carbon atoms.
Correct Answer: Option (i) and (iii)
39. Which of the following compounds are gem-dihalides?
(i) Ethylidene chloride
(ii) Ethylene dichloride
(iii) Methylene chloride
(iv) Benzyl chloride
Ans:Dihaloalkanes with two halogen atoms linked to the same carbon atom are known as gem-dihalides. Alkylidene halides are another name for them. The chemical ethylene dichloride is a vicinal dihalide, meaning that chlorine atoms are linked to neighbouring carbon atoms. There is only one chlorine atom in benzoyl chloride. According to the molecule's nomenclature, ethylidene chloride is a gem-dihalide. The IUPAC terminology for methylene chloride is dichloromethane. Two chlorine atoms are joined to a single carbon atom in this compound.
Correct Answer: Option (i) and (iii)
40. Which of the following are secondary bromides?
(i) \[{\left( {C{H_3}} \right)_2}CHBr\]
(ii) \[{\left( {C{H_3}} \right)_3}CC{H_2}Br\]
(iii) \[C{H_3}CH(Br)C{H_2}C{H_3}\]
(iv) \[{\left( {C{H_3}} \right)_2}CBrC{H_2}C{H_3}\]
Ans: Checking with the carbon atom it is connected to is one way to see if a compound has a secondary bromide. In a molecule, a secondary carbon group is linked to one hydrogen atom and three non-hydrogen groups. As a result of the foregoing possibilities, secondary bromides are formed when the \[{\text{--Br}}\] group is linked to the \[{\text{--CH}}\] group. (i) and (iii) are the right answers.
Correct Answer: Option (i) and (iii)
41. Which of the following compounds can be classified as aryl halides?
(i) \[p - Cl{C_6}{H_4}C{H_2}CH{\left( {C{H_3}} \right)_2}\]
(ii) \[p - C{H_3}CHCl\left( {{C_6}{H_4}} \right)C{H_2}C{H_3}\]
(iii) \[o - Br{H_2}C - {C_6}{H_4}CH\left( {C{H_3}} \right)C{H_2}C{H_3}\]
(iv) \[{C_6}{H_s} - Cl\]
Ans: Aryl halides are halides in which the halogen atom is directly linked to the aromatic ring's \[{\text{s}}{{\text{p}}^{\text{2}}}\] hybridised carbon atom. Only (i) and (iv) contain halogen atoms directly linked to aryl rings among the choices. As a result, they are known as aryl halides. (i) and (ii) are the right answers (iv).
Correct Answer: Option (i) and (iv)
42. Alkyl halides are prepared from alcohols by treating with
(i) \[HCl + ZnC{l_2}\]
(ii) \[RedP + B{r_2}\]
(iii) \[{H_2}S{O_4} + KI\]
(iv) All the above
Ans:
(i) $R - OH\xrightarrow{{HCl + ZnCl}}R - C{l_2} + {H_2}O$
(ii) $R - OH\xrightarrow{{\operatorname{Re} d,P + }}B{r_2}$
Alcohols produce matching alkyl halides when they are treated with various reagents. Alcohols, red phosphorous, and bromine treated with alcohol produce alkyl chlorides and alkyl bromide, respectively, when treated with \[{\text{HCl}} + {\text{ZnC}}{{\text{l}}_2}\]. \[{{\text{H}}_2}{\text{S}}{{\text{O}}_4} + {\text{KI}}\] cannot be used to convert an alcohol to alkyl iodide because they will convert \[{\text{KI}}\] to the equivalent acid, \[{\text{HI}}\], which will then be oxidised to \[{{\text{I}}_{\text{2}}}\]. As a result, the right statements are (i) and (ii).
Correct Answer: Option (i) and (ii)
43. Alkyl fluorides are synthesised by heating an alkyl chloride/bromide in presence of
(i) \[Ca{F_2}\]
(ii) \[Co{F_2}\]
(iii) \[H{g_2}{F_2}\]
(iv) \[NaF\]
Ans: Heat an alkyl chloride/bromide in the presence of a metallic fluoride such as \[{\text{AgF}}\], \[{\text{H}}{{\text{g}}_{\text{2}}}{{\text{F}}_{\text{2}}}\], \[{\text{Co}}{{\text{F}}_2}\], or \[{\text{Sb}}{{\text{F}}_{\text{3}}}\] to produce alkyl fluorides. Swarts reaction is the name given to this reaction. \[{\text{Co}}{{\text{F}}_2}\] and \[{\text{H}}{{\text{g}}_{\text{2}}}{{\text{F}}_{\text{2}}}\] are the only choices that work as reagents. NaF is not suitable because the end products of \[{\text{NaF + RCl}} \to {\text{ RF + NaCl}}\] are soluble in the solvent, i.e. water, making separation difficult. As a result, the right alternatives are (ii) and (iii).
Correct Answer: Option (ii) and (iii)
III. Short Answer Type:
44. Aryl chlorides and bromides can be easily prepared by electrophilic substitution of arenes with chlorine and bromine respectively in the presence of Lewis acid catalysts. But why does preparation of aryl iodides requires presence of an oxidising agent?
Ans: Because \[{\text{HI}}\] is produced throughout the process, iodination reactions are reversible in nature. To keep the reaction moving ahead, we must remove the \[{\text{HI}}\] by an oxidation process using oxidising agents such as \[{\text{HI}}{{\text{O}}_{\text{4}}}\].
45. Out of o -and p -dibromobenzene which one has higher melting point and why?
Ans:The melting point of p-dibromobenzene is greater because p-symmetry of bromobenzene allows it to fit better in a crystal lattice. As a result, breaking the bonds between the molecules requires a greater temperature, resulting in a higher melting point.
46. Which of the compounds will react faster in \[{S_N}1\]reaction with the \[OH\] ion? \[C{H_3} - C{H_2} - Cl\] or \[{C_6}{H_5} - C{H_2} - Cl\]
Ans:In an \[{{\text{S}}_{\text{N}}}1\] reaction with the \[{\text{OH}}\] ion, \[{{\text{C}}_6}{{\text{H}}_5} - {\text{C}}{{\text{H}}_2} - {\text{Cl}}\] will react quicker. This is owing to the carbocation's stability in the compound. The \[{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}\] group is already stable owing to resonance, and the \[{\text{C}}{{\text{H}}_{\text{2}}}\] attached will receive that stability after the cleavage in the first stage of the \[{{\text{S}}_{\text{N}}}1\] reaction, resulting in a stable \[{C_6}{H_5}C{H_2}^ + \] carbocation. \[{\text{C}}{{\text{H}}_{\text{3}}}{\text{---C}}{{\text{H}}_{\text{2}}}{\text{---Cl}}\] does not have the same sort of carbocation.
47. Why iodoform has appreciable antiseptic property?
Ans: Triiodomethane is the chemical name for iodoform. Because of its ability to liberate free iodine, it has antibacterial properties.
48. Haloarenes are less reactive than haloalkanes and haloalkenes. Explain.
Ans: The resonance stabilisation of the aryl ring is the main reason haloarenes are less reactive than haloalkanes and haloalkenes. The electron pairs on the halogen atom, for example, are conjugated with the ring's -electrons in \[{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}{\text{ - Cl}}\]. The \[{\text{C---Cl}}\] link takes on a partial double bond character as a result of resonance, making it less reactive to nucleophilic substitution than haloalkanes and haloalkanes.
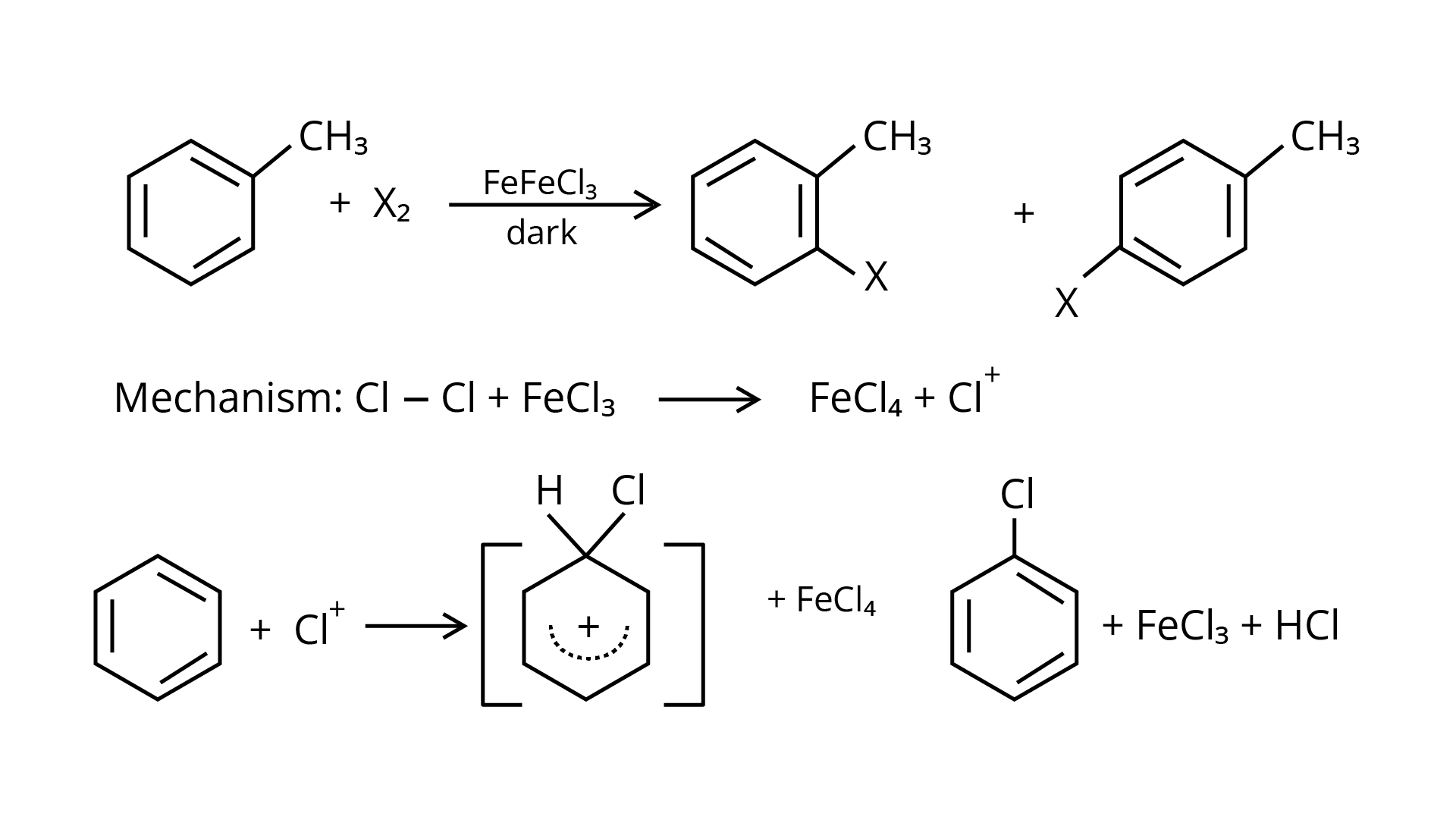
49. Discuss the role of Lewis acids in the preparation of aryl bromides and chlorides in the dark.
Ans: In the presence of Lewis acid catalysts (iron or iron chloride), aryl bromides and chlorides can be made by electrophilic replacement of arenas with bromine and chlorine, respectively.
50. Which of the following compounds (a) and (b) will not react with a mixture of \[NaBr\] and \[{H_2}S{O_4}.\]Explain why?
(a) \[C{H_3}C{H_2}C{H_2}OH\]
(b)
Ans: \[{\text{B}}{{\text{r}}_{\text{2}}}\] gas is produced when \[{\text{NaBr}}\] and \[{{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}\] are combined. Because of the stable molecule created as a result of resonance stabilisation, molecule (b) will not react with \[{\text{B}}{{\text{r}}_{\text{2}}}\] gas.
51. Which of the products will be major product in the reaction given below? Explain.
Ans:This addition reaction is carried out in accordance with Markovnikoff's rule, which states that in a double bond, the hydrogen from the hydrogen halide is added to the carbon atom with the most hydrogen atoms attached to it, while the halogen atom is attached to the carbon atom with the fewest hydrogens attached to it. The main product in the combination will be the molecule that follows this guideline. As a result, the molecule (B) will be the reaction's main product.
52. Why is the solubility of haloalkanes in water very low?
Ans: Because energy is required to overcome the attractions between the haloalkane molecules as well as to break the hydrogen bonds between water molecules in order to dissolve a haloalkane in water, haloalkanes are only weakly soluble in water. New attractions between the haloalkane and the water molecules, on the other hand, release less energy since they are weaker than the water's initial hydrogen bonds.
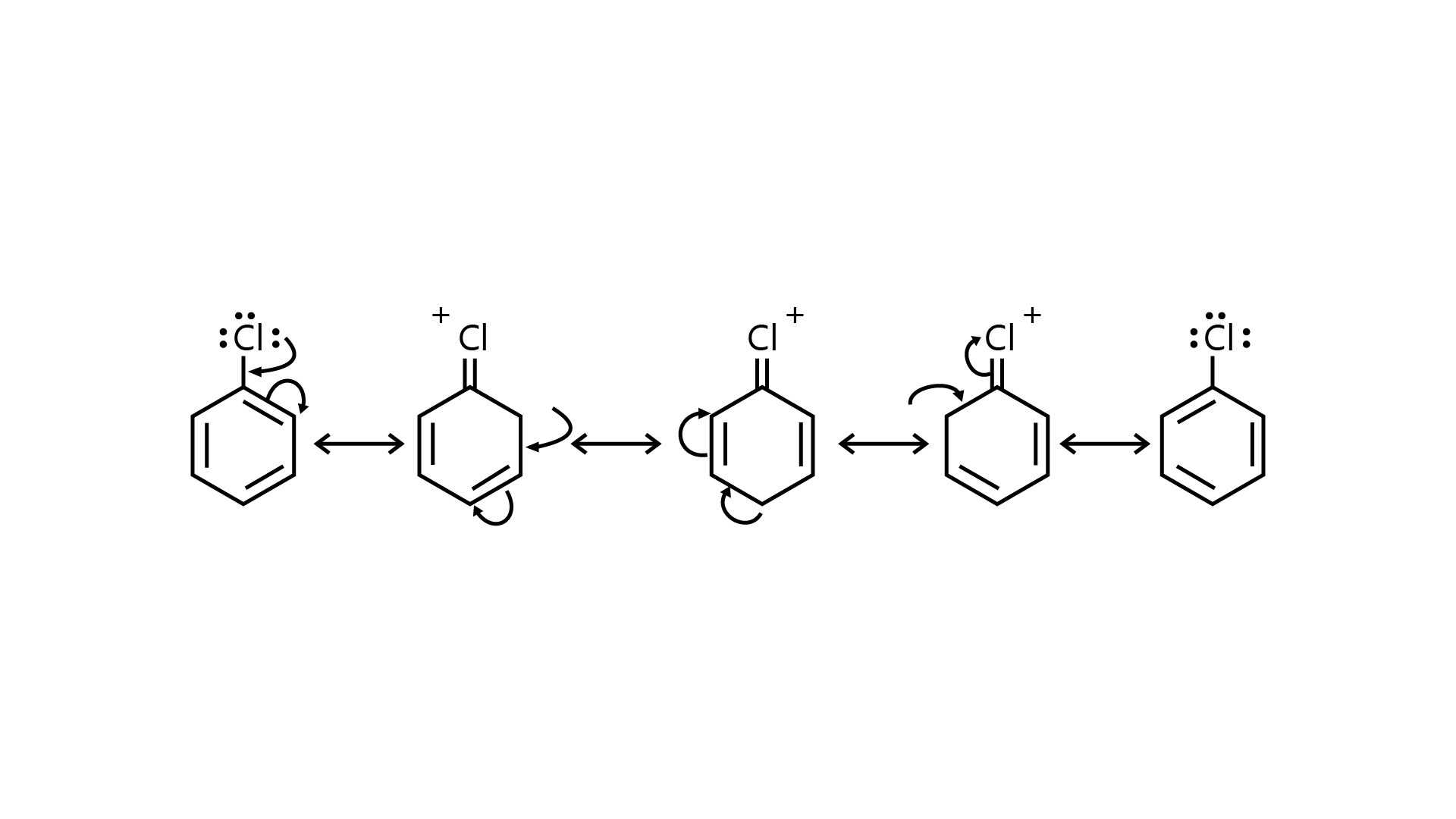
53. Draw other resonance structures related to the following structure and find out whether the functional group present in the molecule is ortho, para directing or meta directing.
Ans:
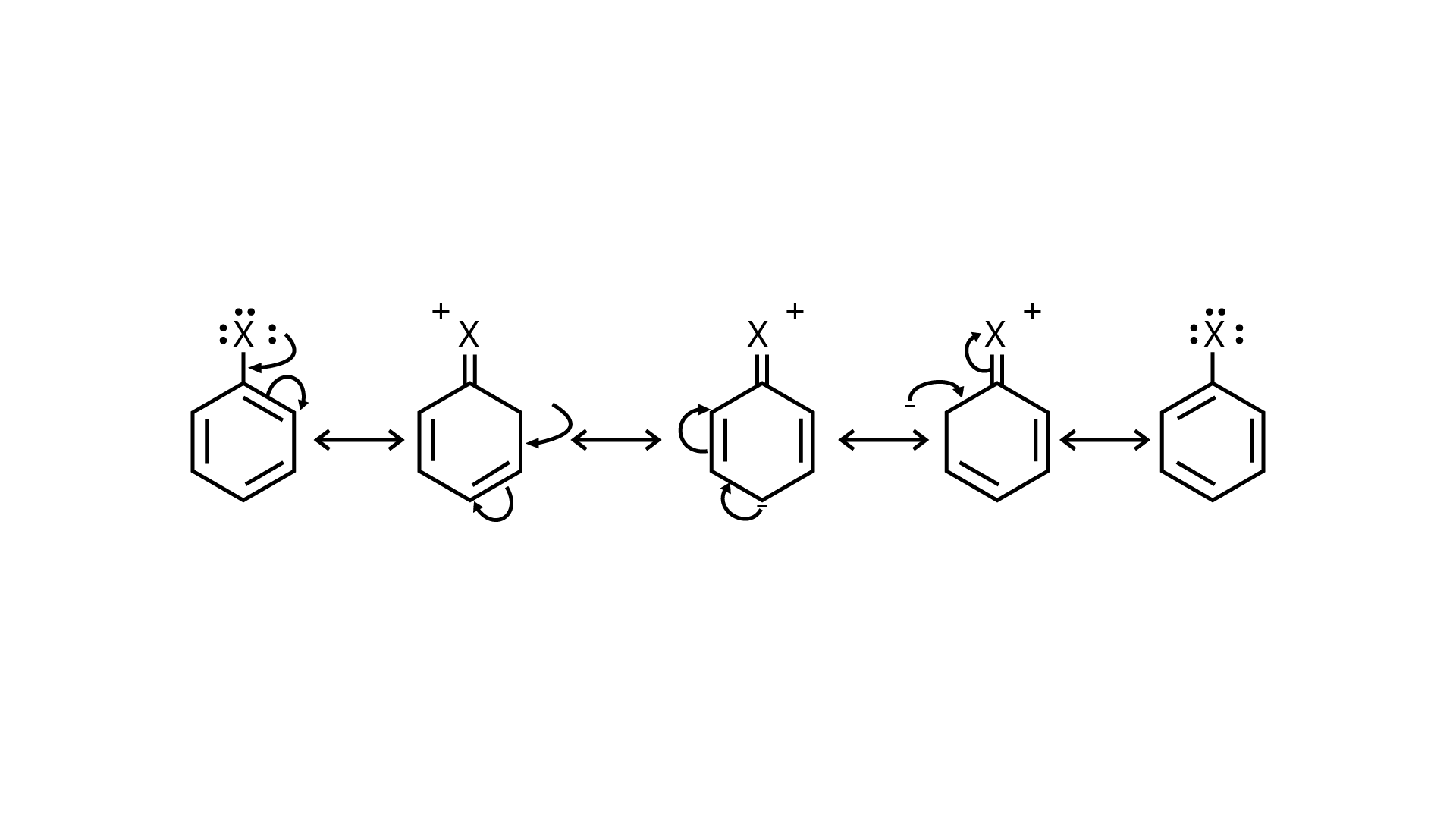
Because electron density is higher at ortho and para locations, the functional groups contained in these compounds are ortho-para directed.
54. Classify the following compounds as primary, secondary and tertiary halides.
(i) 1 -Bromobut-2-ene
Ans: 1-Bromobut-2-ene: It is a primary halide.
(ii) 4-Bromopent-2-ene
Ans: 4-Bromopent-2-ene: Here Bromine is attached to the secondary carbon. Hence, it is a secondary halide.
(iii) 2-Bromo-2-methylpropane
Ans: 2-Bromo-2-methylpropane: Here Bromine is attached to the tertiary carbon. Hence, it is a tertiary halide.
55. Compound 'A' with molecular formula \[{C_4}{H_9}Br\] is treated with aq. \[KOH\] solution. The rate of this reaction depends upon the concentration of the compound 'A' only. When another optically active isomer 'B' of this compound was treated with aq. \[KOH\] solution, the rate of reaction was found to be dependent on concentration of compound and \[KOH\] both.
(i) Write down the structural formula of both compounds ' \[{\text{A}}\]' and ' \[{\text{B}}\] '.
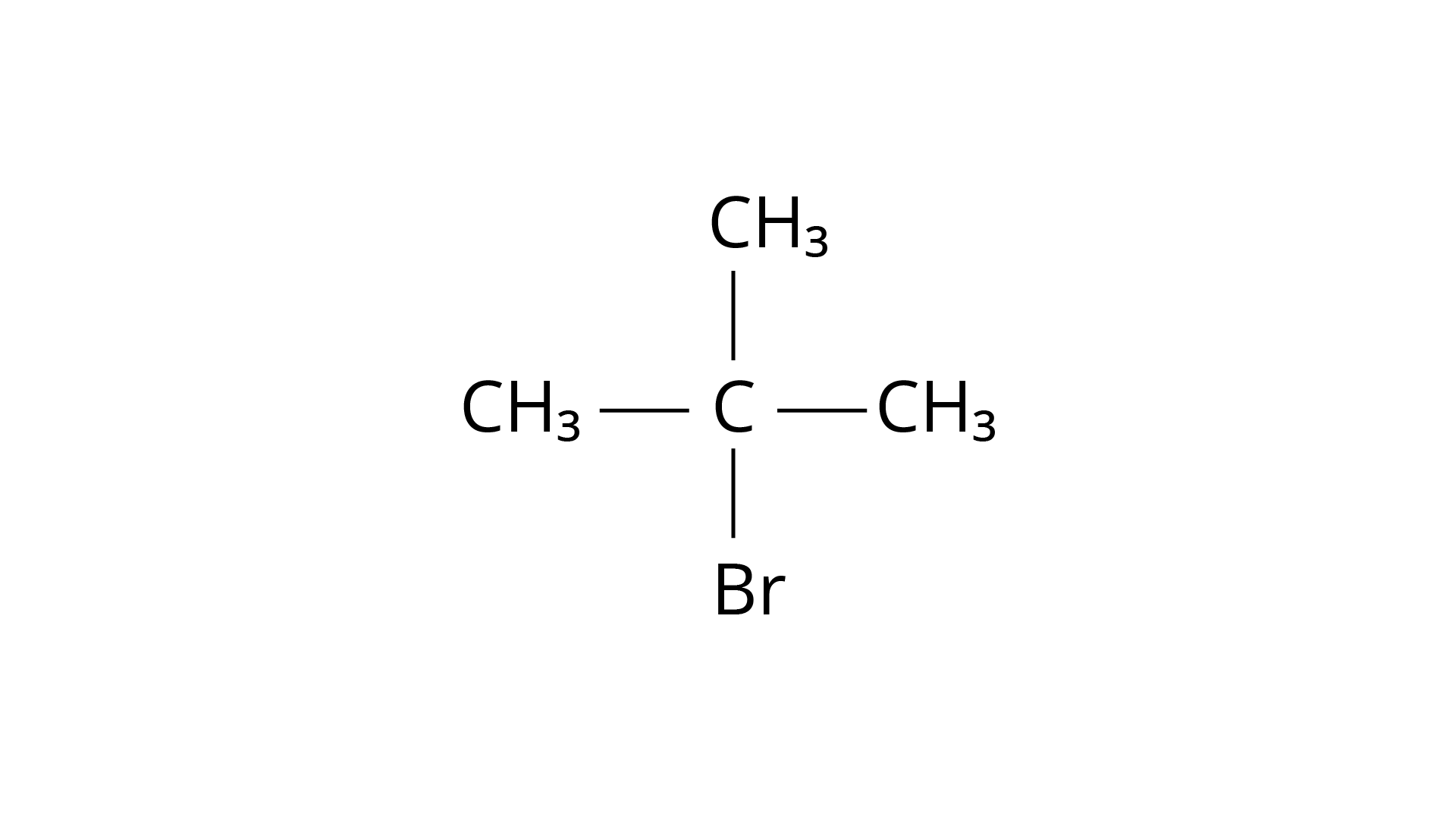
Ans: Because the rate of reaction of compound ‘A' with aqueous \[{\text{KOH}}\] is solely dependent on the concentration of ‘A,' the reaction mechanism is \[{{\text{S}}_{\text{N}}}1\]and ‘A' is 2-Bromo-2-methylpropane (tertiary bromide).
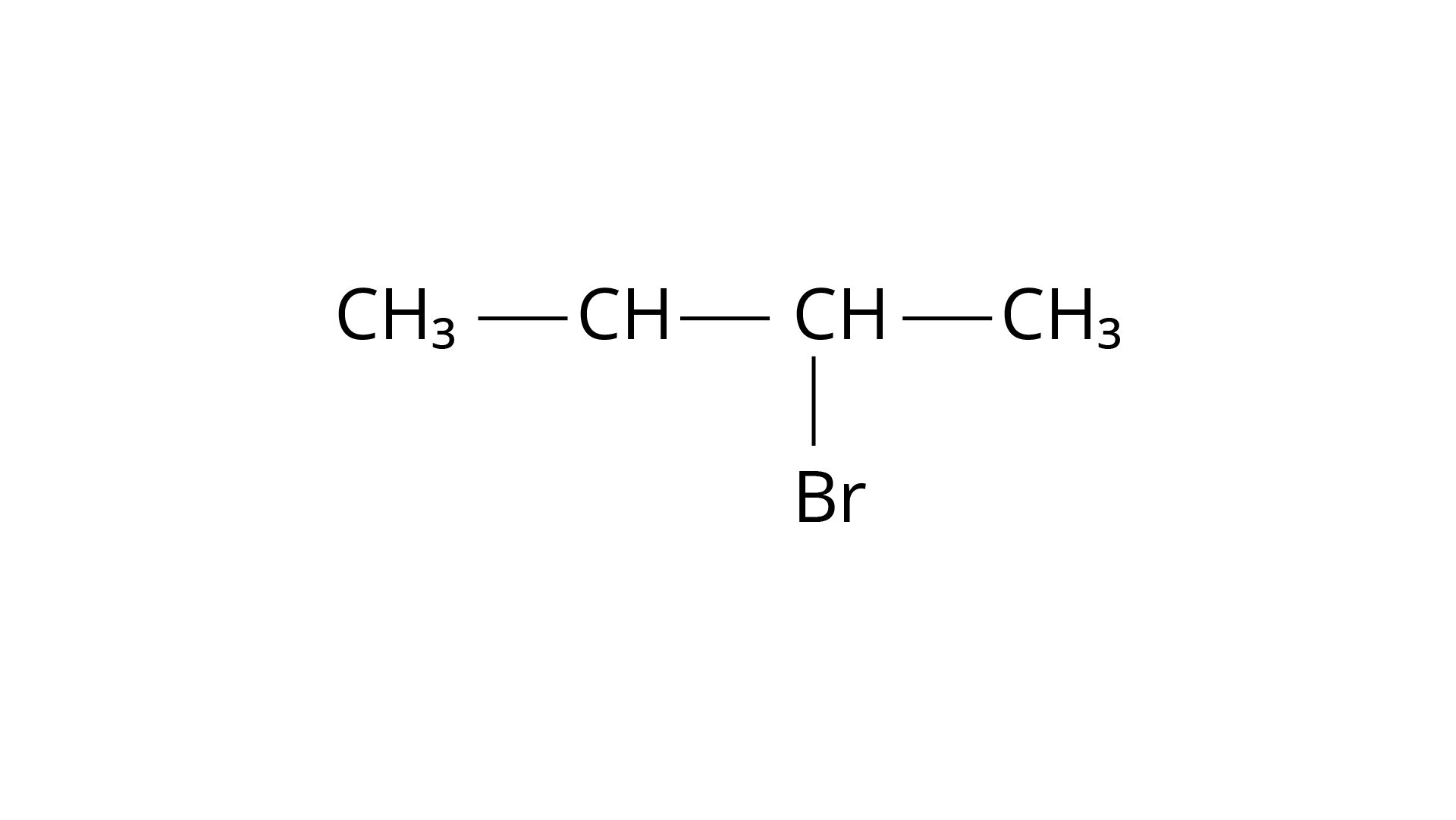
‘B', on the other hand, is an isomer of ‘A' and is optically active. As a result, ‘B' has to be 2-Bromobutane. Because the rate of reaction of ‘B' with aqueous \[{\text{KOH}}\] is determined by its concentration, the reaction mechanism is \[{{\text{S}}_{\text{N}}}2\].
(ii) Out of these two compounds, which one will be converted to the product with inverted configuration.
Ans: Compound ‘B' will have an inverted conformation as a result of the \[{{\text{S}}_{\text{N}}}2\]reaction, resulting in an inverted product.
Structure ‘A’ is and structure ‘B’ is
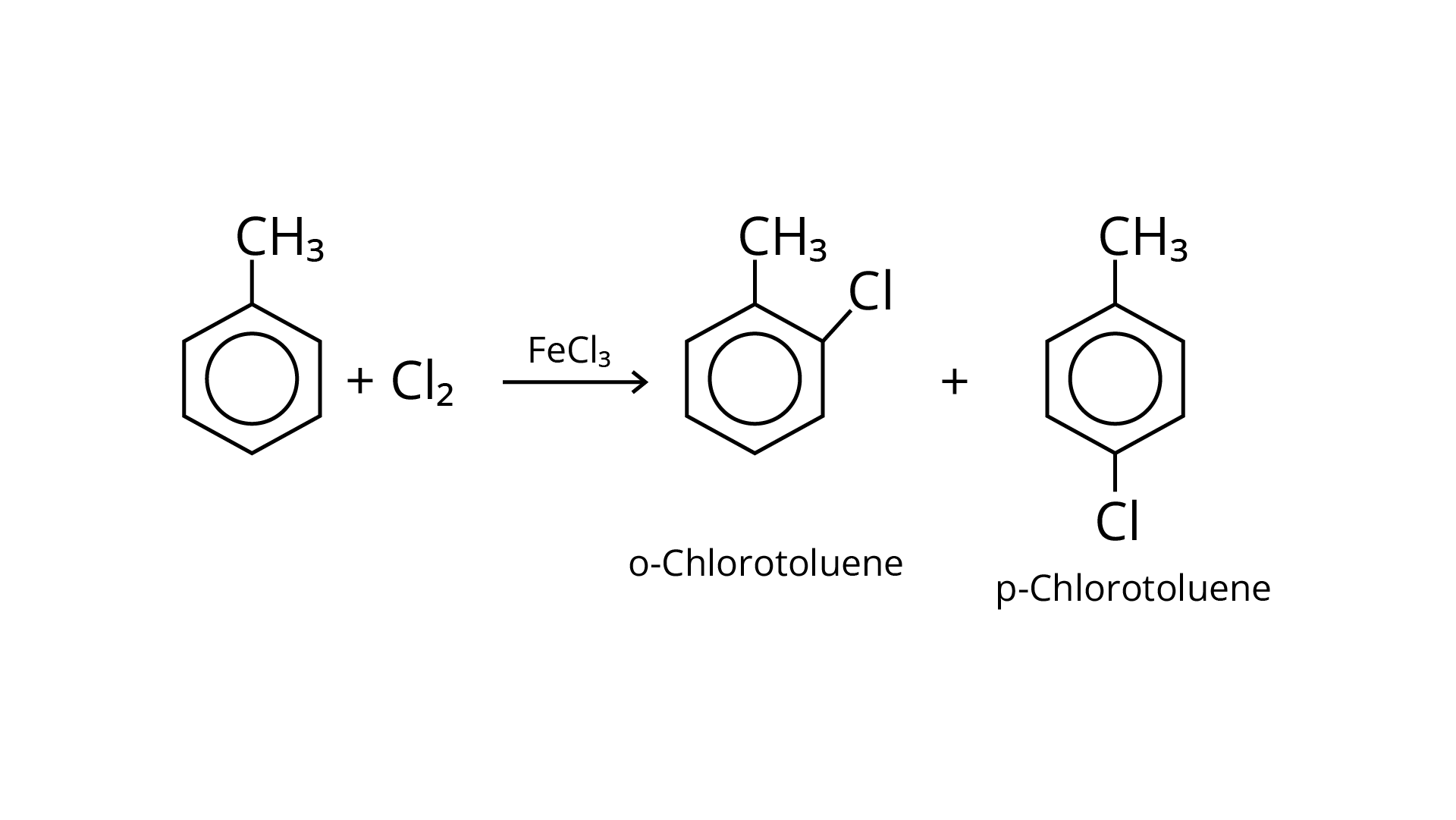
56. Write the structures and names of the compounds formed when compound "A' with molecular formula, \[{C_7}{H_8}\] is treated with \[C{l_2}\] in the presence of \[FeC{l_3}\].
Ans: When an arene is treated with \[{\text{C}}{{\text{l}}_2}\] in the presence of \[{\text{FeC}}{{\text{l}}_3}\], the electrophilic addition reaction chlorinates the arene to produce aryl chloride. As a result, the chemical provided is \[{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}{\text{---C}}{{\text{H}}_{\text{3}}}\], often known as toluene. o-chlorotoluene and p-chlorotoluene are the results of electrophilic substitution chlorination.
57. Identify the products A and B formed in the following reaction:
(a) $\mathrm{CH}_{3}-\mathrm{CH}_{2}-\mathrm{CH}=\mathrm{CH}-\mathrm{CH}_{3}+\mathrm{HCl} \longrightarrow \mathrm{A}+\mathrm{B}$
Ans: The addition reaction of the alkene produces two products, as indicated below.
\[{\text{C}}{{\text{H}}_3} - {\text{C}}{{\text{H}}_2} - {\text{CH}} = {\text{CH}} - {\text{C}}{{\text{H}}_3} + {\text{HCl}} \to A + B\]
\[(A)C{H_3} - C{H_2} - \mathop {CH}\limits_{\mathop |\limits_{Cl} } - C{H_2} - C{H_3}\]
(3- chloropentane)
\[(B)C{H_3} - C{H_2} - C{H_2} - \mathop {CH}\limits_{\mathop |\limits_{Cl} } - C{H_2} - C{H_3}\]
(2-chloropentance)
58. Which of the following compounds will have the highest melting point and why?
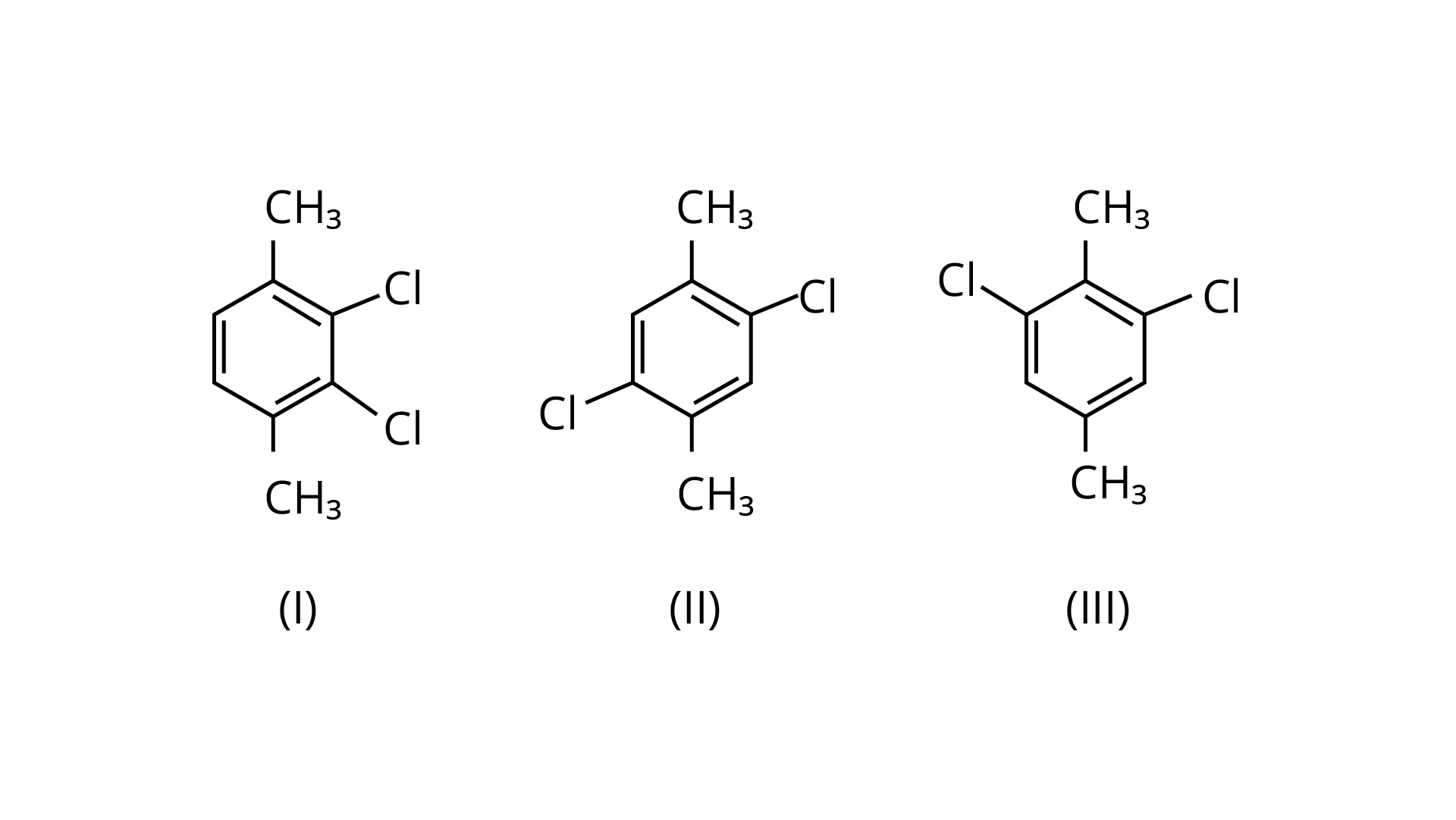
Ans: Because both methyl groups and chlorine atoms are symmetrically positioned at para-positions in compound (II), these molecules fit better in the crystal lattice than other isomers, and hence have the greatest melting point.
59. Write down the structure and IUPAC name for neo-pentylbromide.
Ans: The IUPAC name for this compound is 1-Bromo-2,2-dimethylpropane,
\[C{H_3} - \mathop C\limits_{\mathop |\limits_{C{H_3}} }^{\mathop |\limits^{C{H_3}} } - C{H_2} - Br\]
60. A hydrocarbon of molecular mass \[72gmo{l^{ - 1}}\] gives a single monochloro derivative and two dichloro derivatives on photo chlorination. Give the structure of the hydrocarbon.
Ans: The alkane \[{{\text{C}}_{\text{5}}}{{\text{H}}_{{\text{12}}}}\] is a hydrocarbon with a molecular mass of \[72{\text{g}}{\text{mo}}{{\text{l}}^{ - 1}}\]. On photo-chlorination, the tert-isomer of pentane yields a single monochloro derivative and two dichloro derivatives. The following is the structure of the alkane and its chloride derivatives.
\[C{H_3} - \mathop C\limits_{\mathop |\limits_{C{H_3}} }^{\mathop |\limits^{C{H_3}} } - C{H_3}\] \[C{H_3} - \mathop C\limits_{\mathop |\limits_{C{H_3}} }^{\mathop |\limits^{C{H_3}} } - C{H_2} - Cl\] \[C{H_3} - \mathop C\limits_{\mathop |\limits_{C{H_3}} }^{\mathop |\limits^{C{H_3}} } - CHC{l_2}\] \[C{H_3} - \mathop C\limits_{\mathop |\limits_{C{H_3}} }^{\mathop |\limits^{C{H_3} - Cl} } - C{H_2} - Cl\]
(Neopentane) (Monochloro derivative) (Dichloro derivative)
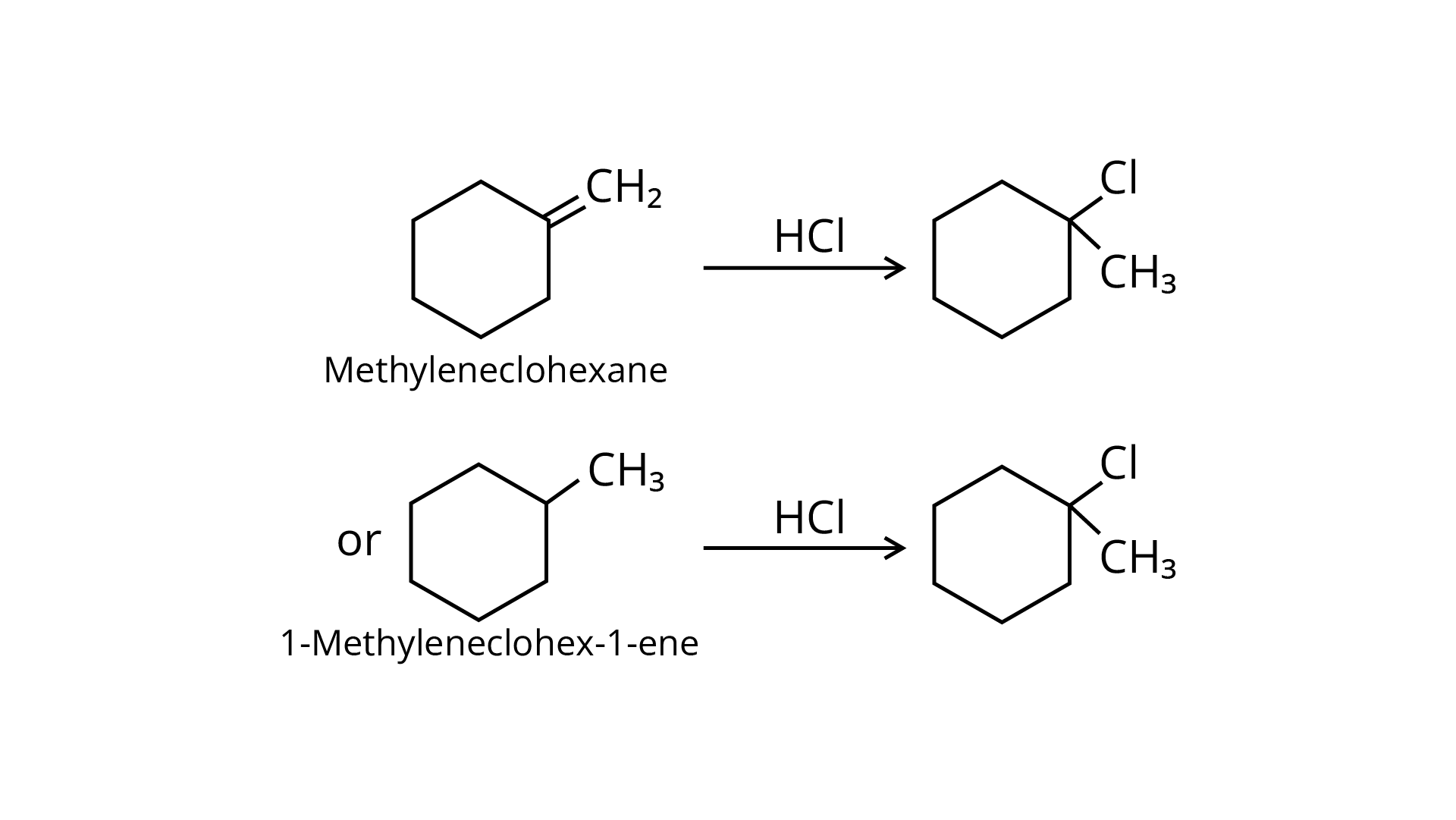
61. Name the alkene which will yield 1-chloro-1-methylcyclohexane by its reaction with \[HCl\]. Write the reactions involved.
Ans: Methylenecyclohexane and 1-methylcyclohex-1-ene are two chemicals that can produce 1-chloro-1-methylcyclohexane.
62. Which of the following haloalkanes reacts with aqueous \[KOH\] most easily? Explain giving reason.
(i) 1 -Bromobutane
(ii) 2 -Bromobutane
(iii) 2-Bromo-2-methylpropane
(iv) 2-Chlorobutane
Ans: Nucleophilic substitutions occur when haloalkanes react with aqueous \[{\text{KOH}}\]. The tertiary halide 2-Bromo-2-methylpropane will react with aq. \[{\text{KOH}}\] the easiest of the compounds because the initial step involves cleavage of the alkyl and halide groups, resulting in the production of the extremely stable carbocation.
63. Why can aryl halides not be prepared by reaction of phenol with \[HCl\] in the presence of \[ZnC{l_2}\] ?
Ans: Phenol will not react with \[{\text{HCl}}\] in the presence of \[{\text{ZnC}}{{\text{l}}_2}\]. This is owing to the presence of partial double bond properties between the benzene ring and \[{\text{O}}\], which will result in resonance between the benzene ring and the \[{\text{OH - }}\] group. As a result, no aryl halide will be made.
64. Which of the following compounds would undergo \[{S_N}1\] reaction faster and why?
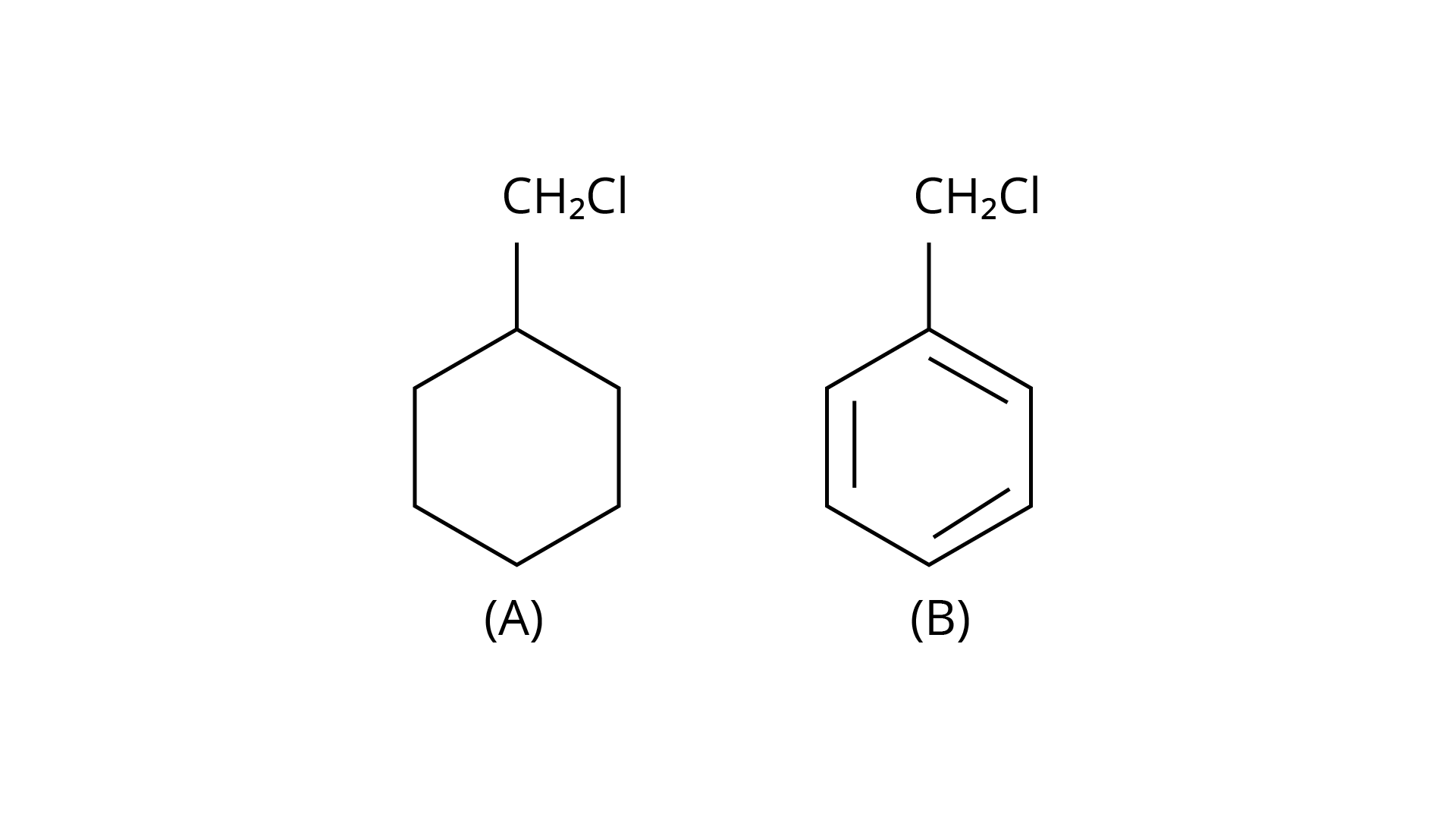
Ans: Due to the resonance stabilisation of the benzyl ring, compound (B) would conduct the \[{{\text{S}}_{\text{N}}}1\] reaction faster than compound (A). The carbocation \[{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{l}}^{\text{ + }}}\] is very stable when the \[{\text{--Cl}}\] from the ring is cleaved, and it is driven to carry out the \[{{\text{S}}_{\text{N}}}1\] reaction.
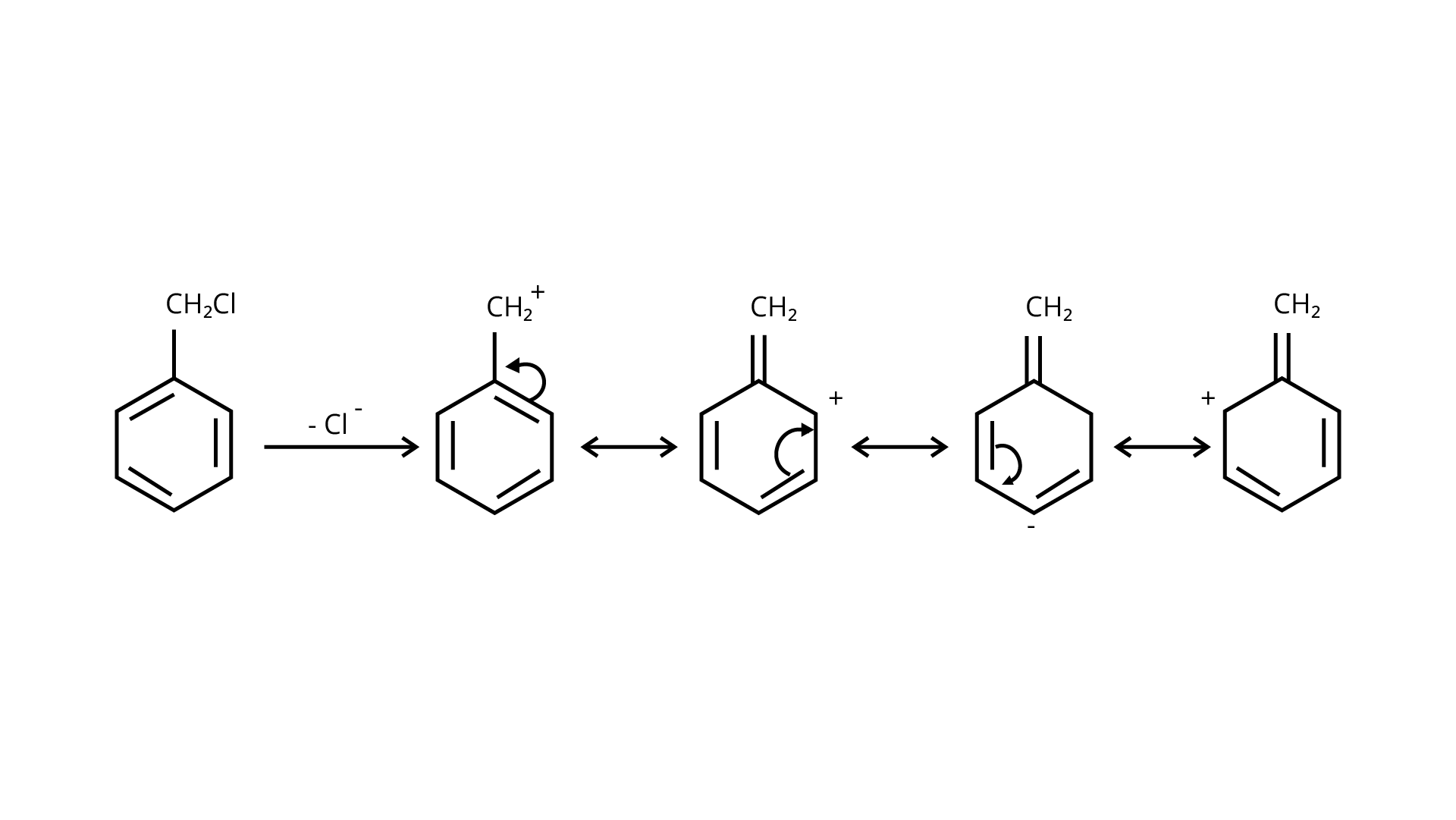
65. Allyl chloride is hydrolysed more readily than n -propyl chloride. Why?
Ans: In nature, allyl chloride becomes very reactive due to the production of carbocation, which is extremely stable due to resonance. Due to carbocation, there will be no such stability in the case of n-propyl chloride.
\[C{H_2} = CH - C{H_2}Cl + O{H^ - } \to C{H_2} = CH - C\mathop {{H_2}}\limits^ + \leftrightarrow C\mathop {{H_2} - CH = C{H_2}}\limits^ + \]
(Resonance stabilization)
66. Why is it necessary to avoid even traces of moisture during the use of a Grignard reagent?
Ans:\[{\text{RMgX}} + {{\text{H}}_2}{\text{O}} \to {\text{RH}} + {\text{Mg}}({\text{OH}}){\text{X}}\]
Because the Grignard reagents are very reactive in nature, even residues of moisture must be avoided. Even minute amounts of water can cause these reagents to generate hydrocarbons.
67. How do polar solvents help in the first step in \[{S_N}1\] mechanism?
Ans: The \[{{\text{S}}_{\text{N}}}1\] mechanism advances through the production of carbocation. The breakage of the C-halogen bond occurs during this phase. The solution of the halide ions is done with the protons of the protic solvent as a result. In this approach, polar solvents aid in the ionisation process by solvating the ions and stabilising them.
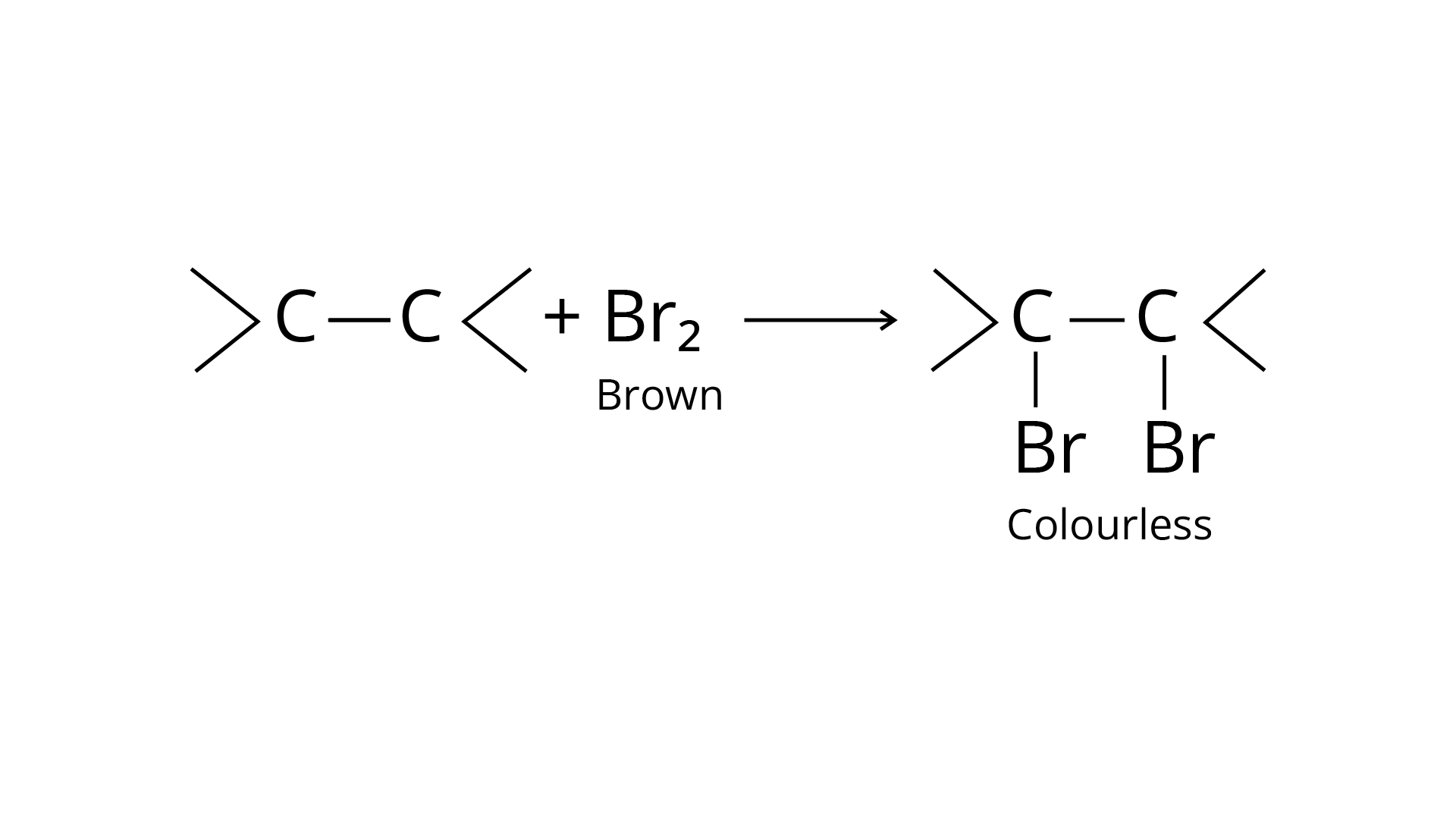
68. Write a test to detect the presence of double bond in a molecule.
Ans: The bromine water test and Bayer's test are two methods for detecting the presence of an unsaturated double bond in an organic molecule.
Bromine is added to the carbon atoms across the double bond in the bromine water test when the molecule is introduced to bromine water. Bromine water changes colour from orange to brown to colourless at the end of the process.
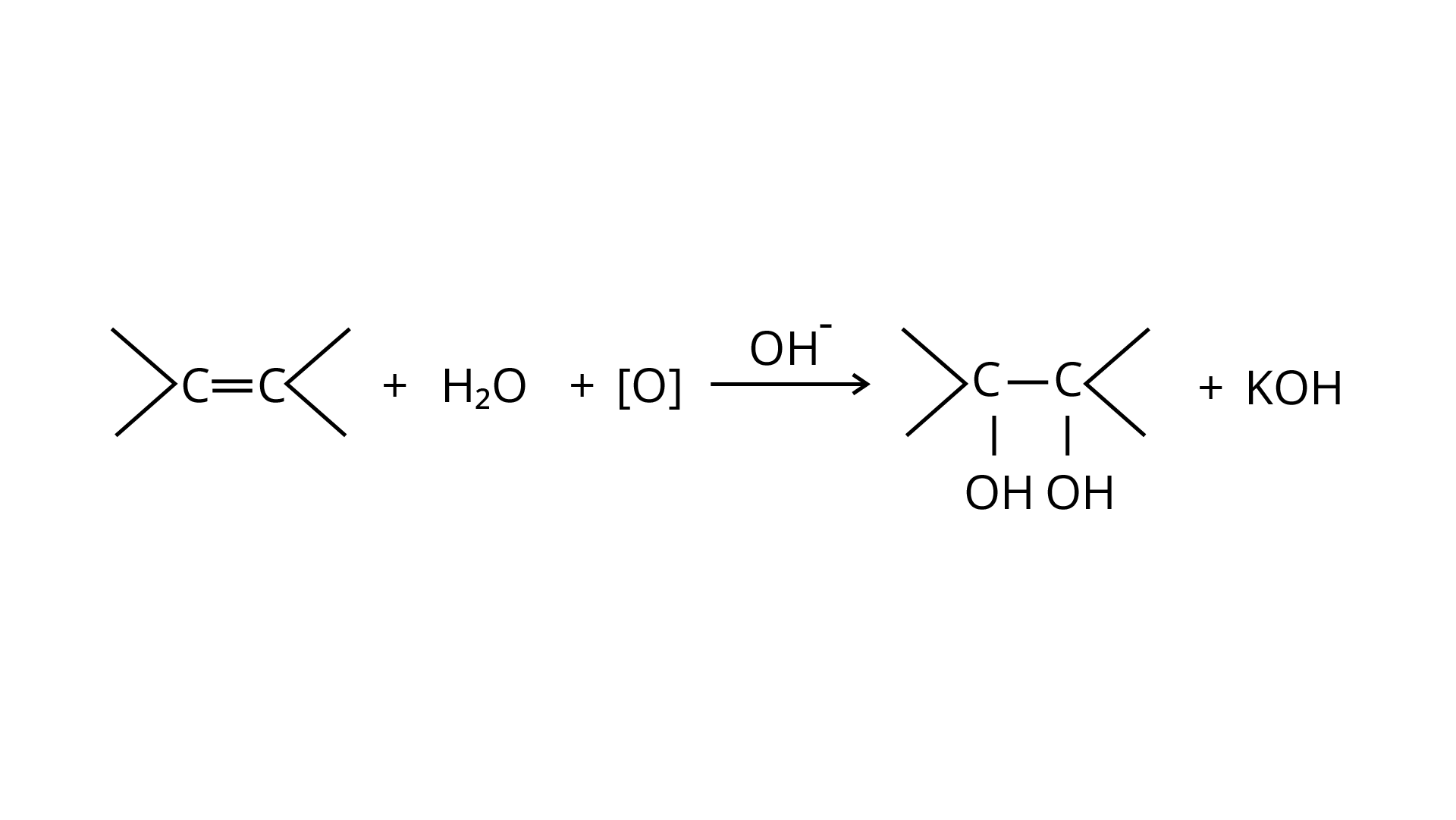
The alkaline \[{\text{KMn}}{{\text{O}}_{\text{4}}}\] is employed in Bayer's test to detect unsaturated carbon with a double bond. The chemical produces \[\left[ {\text{O}} \right]\], which hydrolyzes the carbons across the double bond, resulting in a purple-to-colorless solution containing the two molecules.
\[2{\text{KMn}}{{\text{O}}_4} + {{\text{H}}_2}{\text{O}} \to 2{\text{KOH}} + 2{\text{Mn}}{{\text{O}}_2} + 3[{\text{O}}]\]
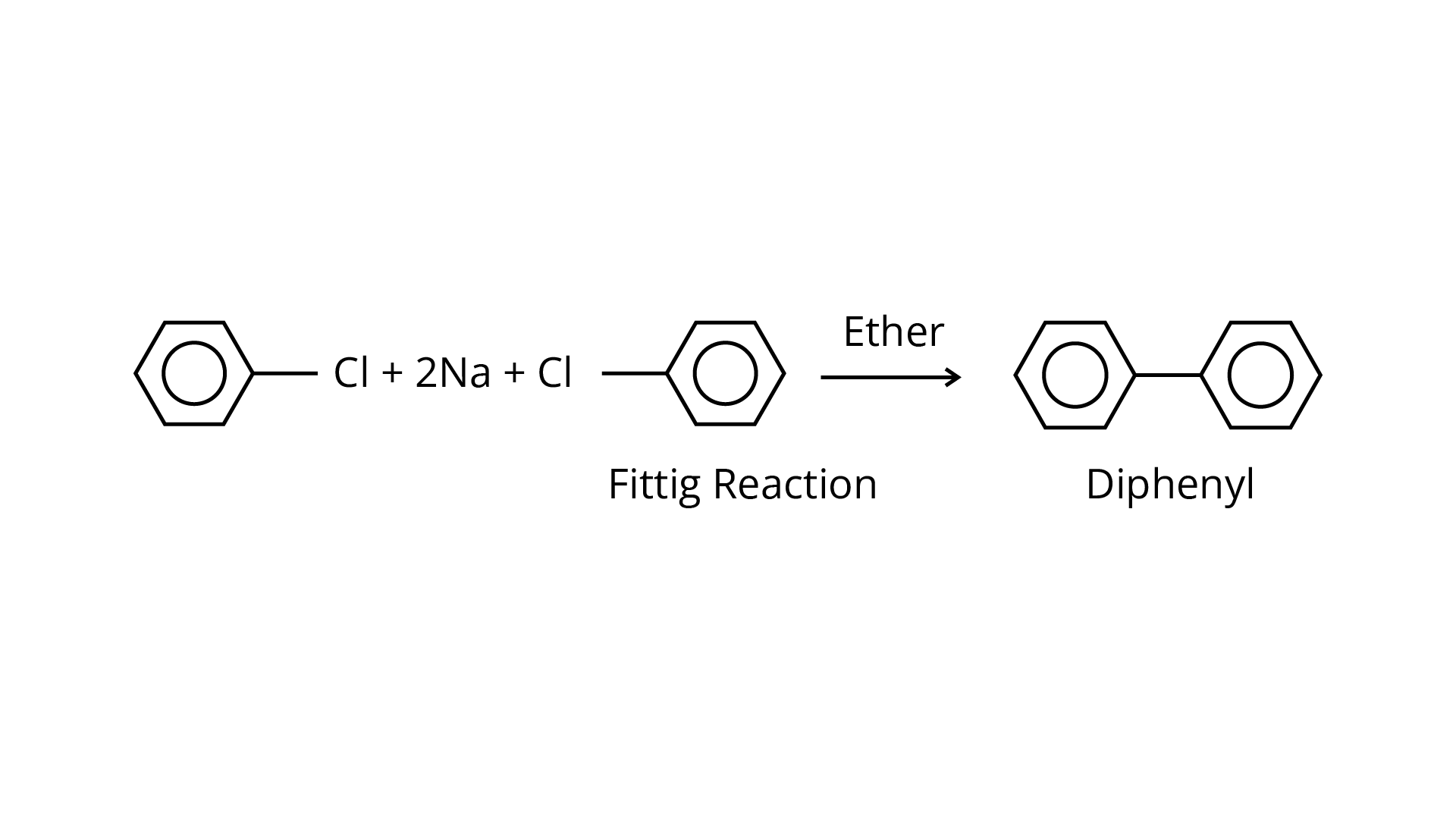
69. Diphenyls are potential threat to the environment. How are these produced from arylhalides?
Ans: Diphenyls, such as p,p-dichlorodiphenyltrichloroethane (DDT), which have been employed as organic pesticides, are a possible hazard to the environment since it was shown to have significant toxicity towards fish during its early years of widespread use, and many insects acquired resistance to it. The issue was exacerbated by DDT's chemical persistence and fat solubility. In terms of chemistry, DDT is not metabolised or solubilized well by mammals. The fatty tissues are where it is deposited and kept. DDT builds up within the animal over time if it is consumed at a constant pace, harming the ecosystem.
The following two techniques can be used to make di-phenyls from aryl halides:
(i) The aryl halides are reacted with sodium metal in the presence of dry ether to generate substituted aryl compounds, such as biphenyl compounds, in the Fittig reaction.
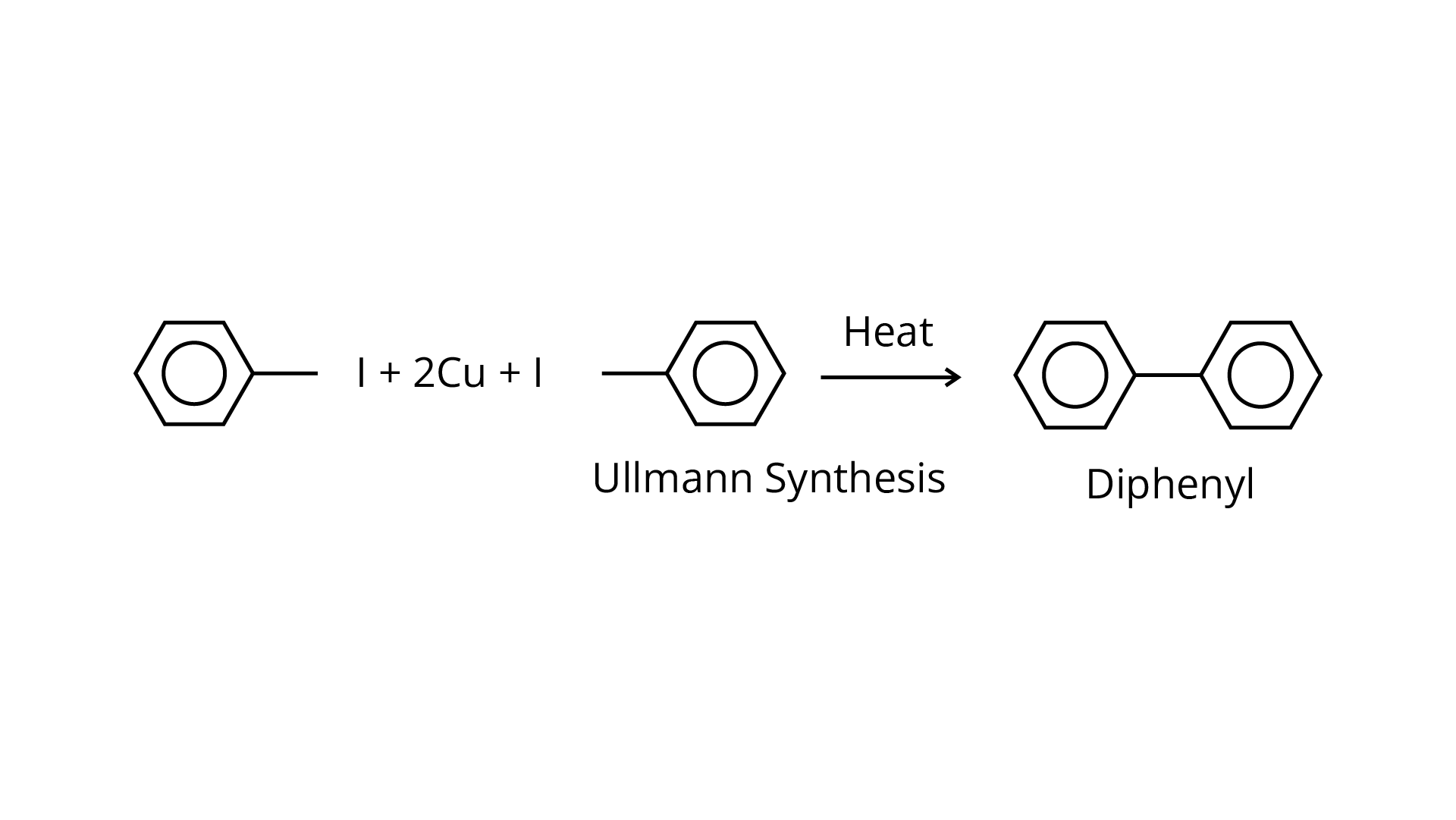
(ii) Aryl halides are heated in the presence of \[{\text{Cu}}\] to create biphenyl/diphenyl compounds in the Ullmann synthesis, which is a coupling process.
70. What are the IUPAC names of the insecticide DDT and benzene hexachloride? Why is their use banned in India and other countries?
Ans: The IUPAC names for DDT and benzene hexachloride are 2, 2-bis (4-chlorophenyl)-1, 1-trichloroethane and 1, 2, 3, 4, 5, 6-hexachlorocyclohexane, respectively.
They are poisonous and non-bio digestible at the same time. They are fat soluble, and their concentration in the food chain continues to rise. As a result, they are prohibited in India and other nations.
71. Elimination reactions (especially \[{\text{\beta }}\]-elimination) are as common as the nucleophilic substitution reaction in case of alkyl halides. Specify the reagents used in both cases.
Ans: Nucleophilic substitution and elimination (β-elimination) reactions are both possible with alkyl halides.
Although, with the appropriate reaction conditions and reagent selection, a specific product can be produced. In most cases, the elimination reaction is best suited to strong and bigger bases, as well as high temperatures. The substitution reaction, on the other hand, is best for weaker and smaller bases at lower temperatures.
$\text{C}{{\text{H}}_{\text{3}}}\text{C}{{\text{H}}_{\text{2}}}\text{Br+alc}\text{.KOH}\xrightarrow{473-523\text{K}}\text{C}{{\text{H}}_{\text{2}}}\text{=C}{{\text{H}}_{\text{2}}}\text{ + HBr}$
$C{{H}_{3}}C{{H}_{2}}Br+aqKOH\to C{{H}_{3}}C{{H}_{2}}OH+KBr$
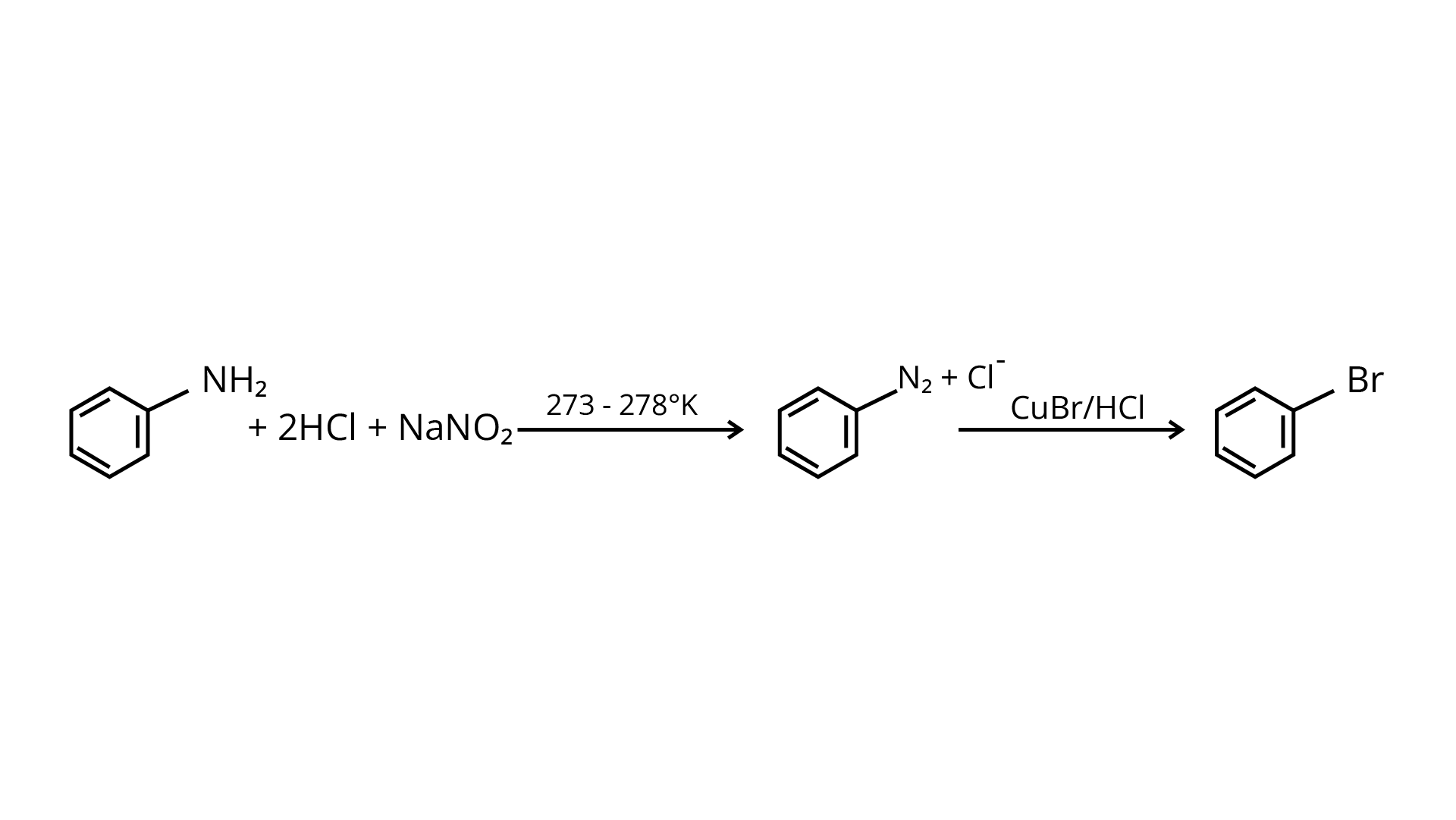
72. How will you obtain mono-bromobenzene from aniline?
Ans: Sandmeyer's reaction may be used to make mono-bromobenzene from aniline (\[{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}{\text{N}}{{\text{H}}_{\text{2}}}\]). A diazonium salt is produced when aniline suspended in cold mineral acid is treated with sodium nitrite. When the diazonium salt is combined with cuprous bromide, the diazonium ion is replaced by \[{\text{--Br}}\], resulting in mono-bromobenzene.
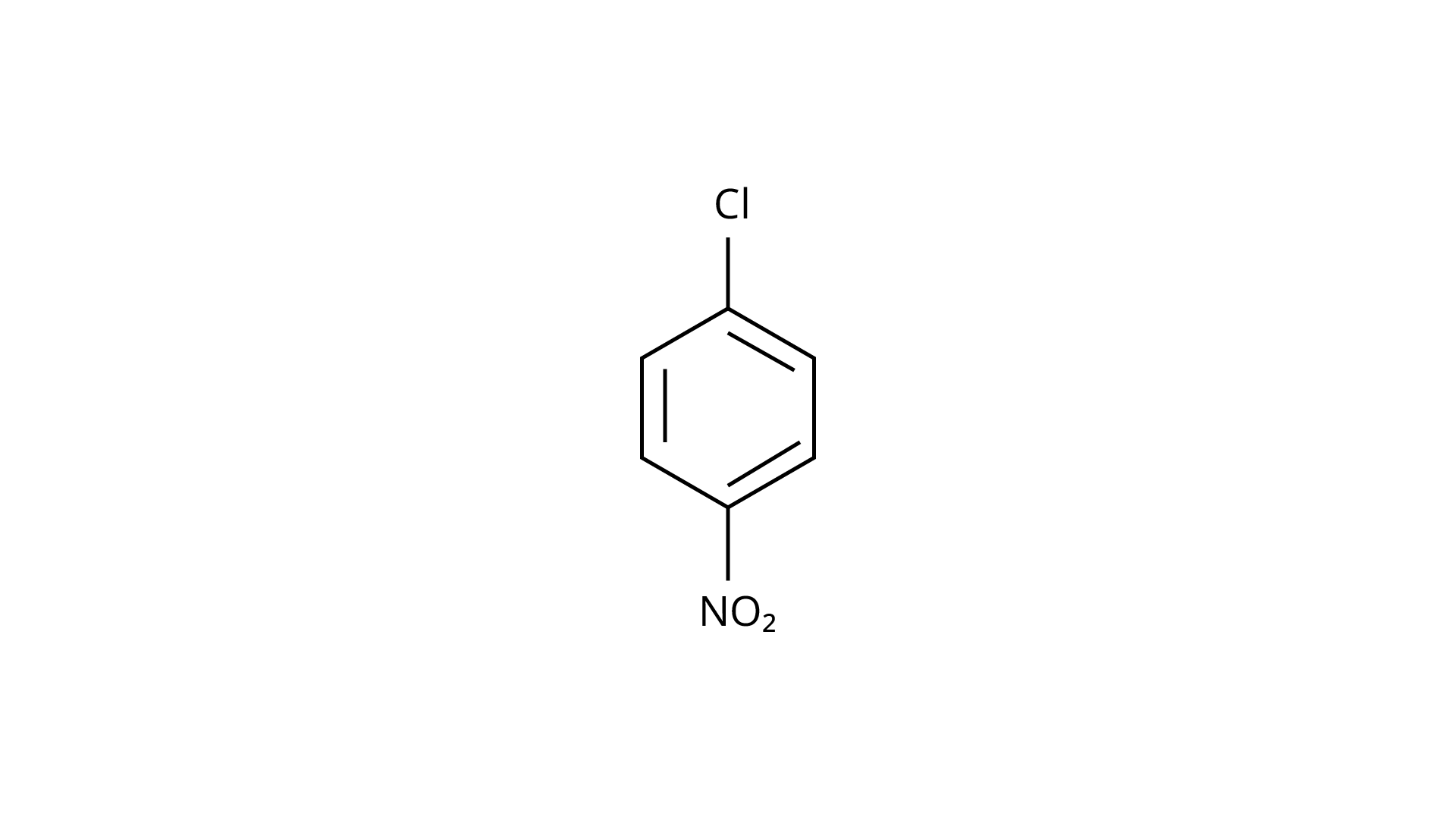
73. Aryl halides are extremely less reactive towards nucleophilic substitution. Predict and explain the order of reactivity of the following compounds towards nucleophilic substitution:
(I)
(II)
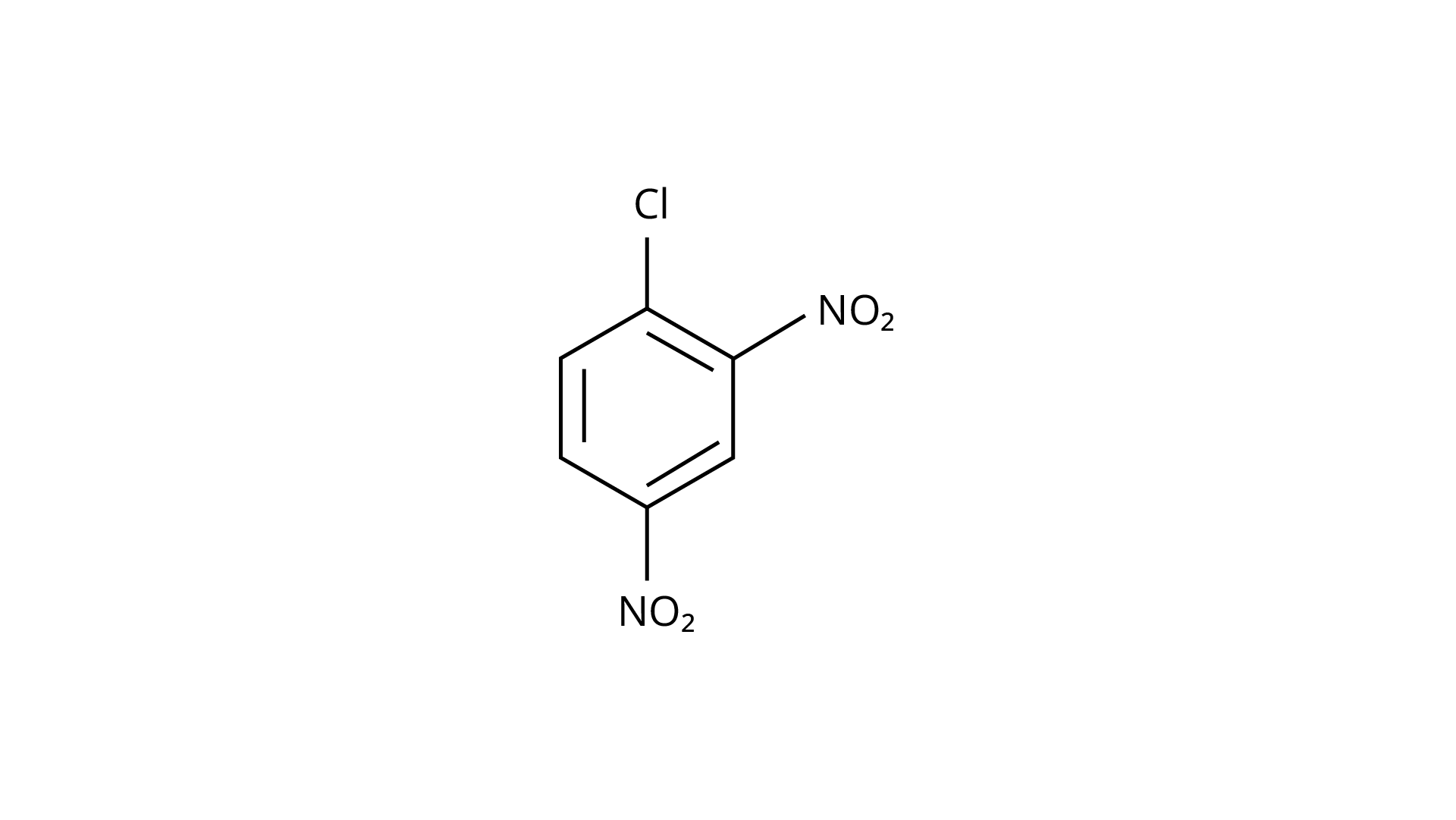
(III)
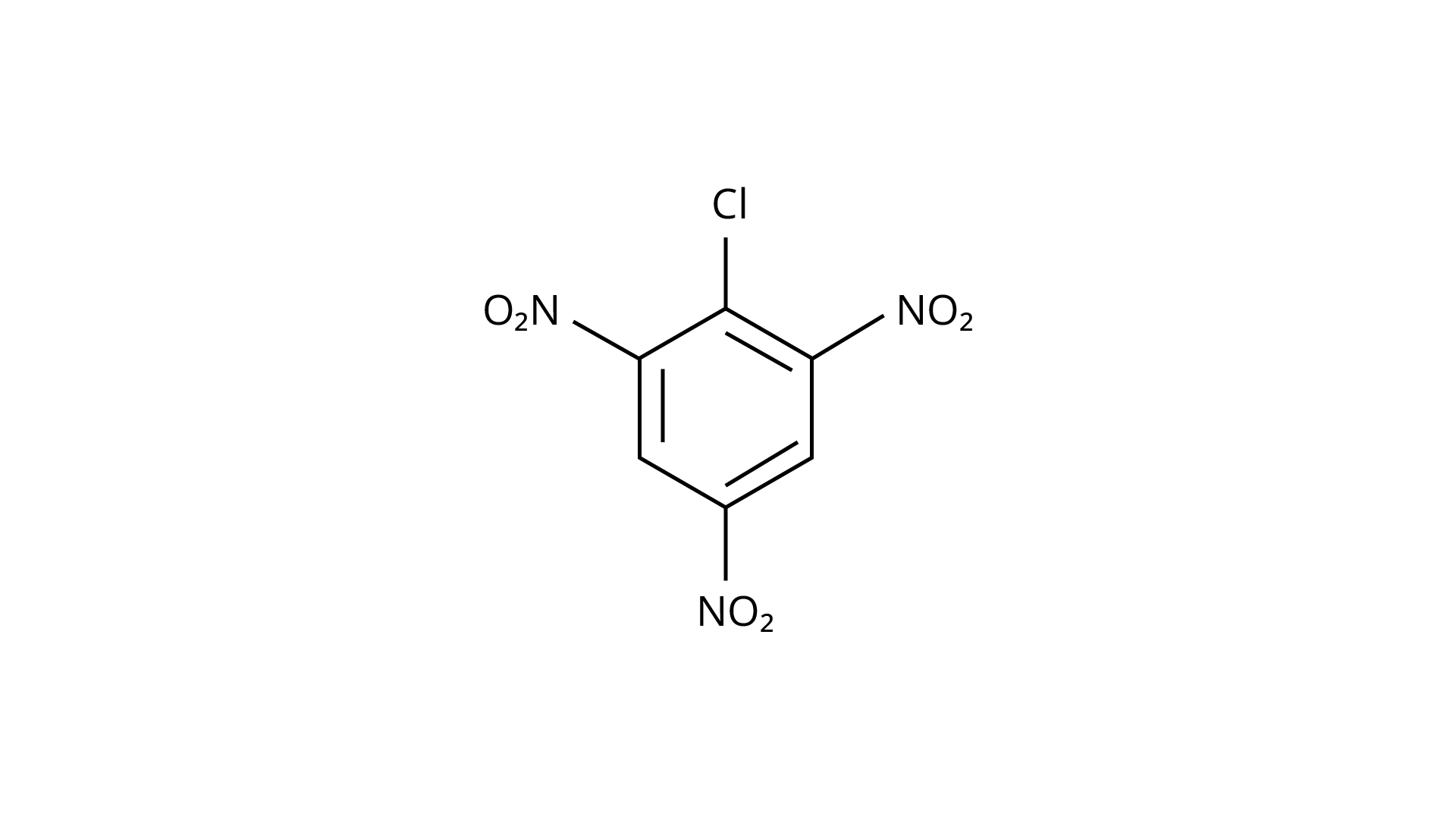
Ans:Because of resonance stabilisation, aryl halides are less reactive to nucleophilic substitution. The inclusion of a \[{\text{--N}}{{\text{O}}_{\text{2}}}\], an electron-withdrawing group in the ortho or para position, enhances the aryl halide's sensitivity to substitution. The more electron withdrawing groups there are in the aryl halide, the more reactive it is. The decreasing sequence of reactivity, according to this theory, is III > II > I.
74. tert-Butyl Bromide reacts with aq. \[NaOH\] by \[{S_N}1\] mechanism while n-butyl bromide reacts by \[{S_N}2\] mechanism. Why?
Ans: Tert-butyl bromide is substituted via the \[{{\text{S}}_{\text{N}}}1\] process because it may produce a stable carbocation in the first step after the halide group is cleaved. The nucleophile \[{\text{OH - }}\] interacts with the carbocation next. The primary halide n-butylbromide, on the other hand, is unable to create a stable carbocation, thus it undergoes the \[{{\text{S}}_{\text{N}}}2\] process, which is a one-step substitution involving \[{\text{OH - }}\] attack and concomitant \[{\text{X - }}\] leaving to generate n-butyl alcohol.
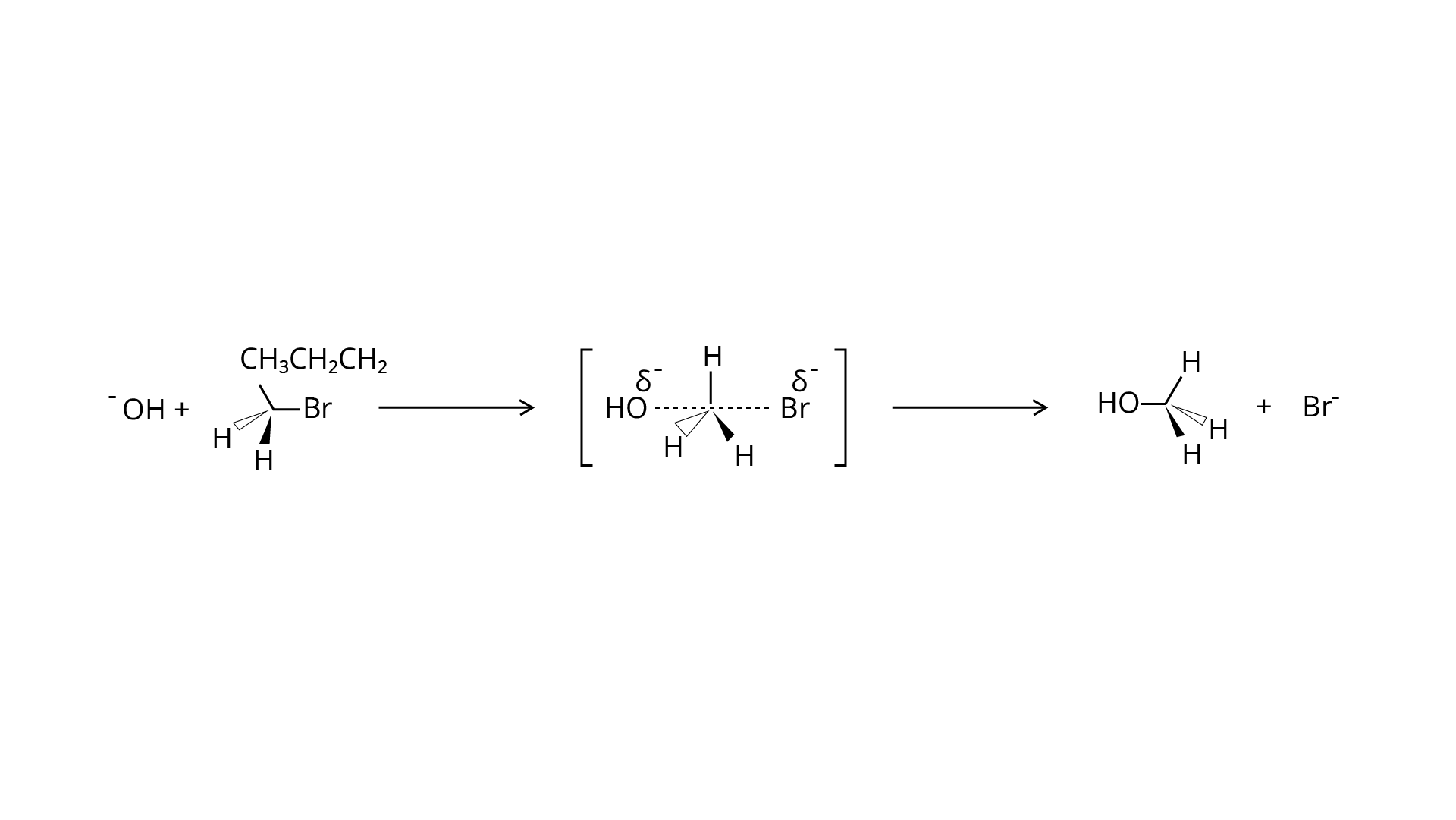
\[C{H_3} - \mathop C\limits_{\mathop |\limits_{C{H_3}} }^{\mathop |\limits^{C{H_3}} } - Br\xrightarrow{{Ionization/B{r^ - }slow}}C{H_3} - \mathop {{C^ + }}\limits_{\mathop |\limits_{C{H_3}} }^{\mathop |\limits^{C{H_3}} } \]
(tert-butyl carbocation)
stable
\[C{H_3} - \mathop {{C^ + }}\limits_{\mathop |\limits_{C{H_3}} }^{\mathop |\limits^{C{H_3}} } - Br\xrightarrow{{OH/fast}}C{H_3} - \mathop C\limits_{\mathop |\limits_{C{H_3}} }^{\mathop |\limits^{C{H_3}} } - OH\]
(tert-butyl alcohol
75. Predict the major product formed when \[{\text{HCl}}\]is added to isobutylene. Explain the mechanism involved.
Ans:The reaction of \[{\text{HCl}}\] and isobutylene is given as:
\[C{H_3} - \mathop C\limits_{\mathop |\limits_{C{H_3}} } = C{H_2} + HCl \to C{H_3} - \mathop C\limits_{\mathop |\limits_{C{H_3}} }^{\mathop |\limits^{Cl} } - C{H_3}\]
Isobutylene 2-methyl-2-chloropropane
Steps in reaction involves are:
Step 1
\[\text{3}{}^\circ \text{ Carbocation}\] is more stable then \[1{}^\circ \text{ Carbocation}\]
Step 2
\[C{H_3} - \mathop C\limits_{\mathop |\limits_{C{H_3}} }^ + - C{H_3}\xrightarrow{{C{l^ - }}}C{H_3} - \mathop C\limits_{\mathop |\limits_{C{H_3}} }^{\mathop |\limits^{|Cl} } - C{H_3}\]
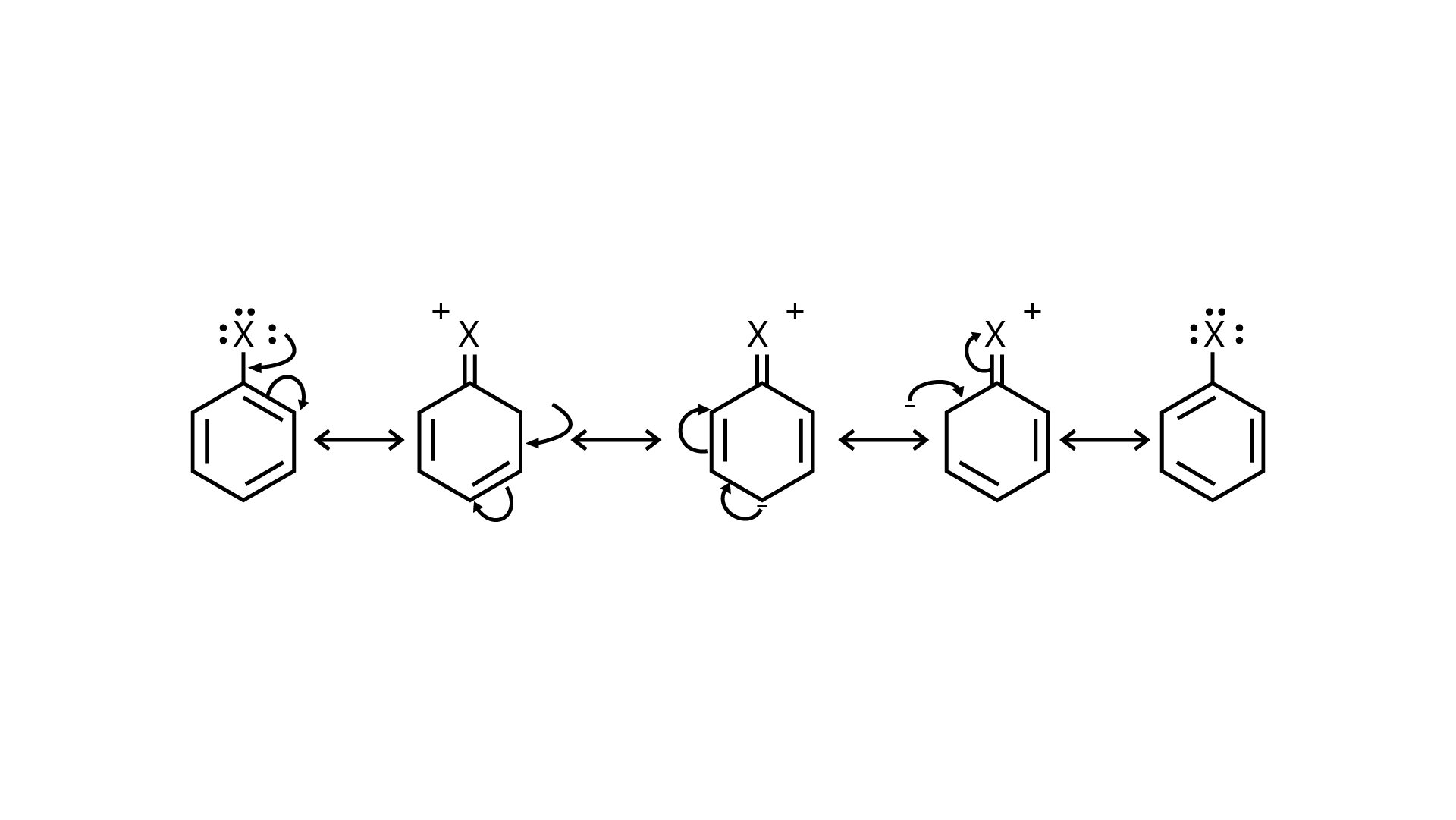
76. Discuss the nature of \[C -- X\]bond in the haloarenes.
Ans:: Resonance effect: Because the \[{\text{C -- X}}\]bond has the characteristics of a partial double bond, it is extremely difficult to break.
(i) The \[{\text{C -- X}}\]bond in haloarenes is extremely less reactive to nucleophilic groups
(ii) The C atom linked to the halogen is \[{\text{s}}{{\text{p}}^2}\] hybridised in the \[{\text{C -- X}}\]bond. Carbon that has been \[{\text{s}}{{\text{p}}^2}\] hybridised with a higher \[{\text{s}}\]-character is more electronegative in nature and can retain the electron pair of the \[{\text{C -- X}}\] bond more tightly than carbon that has been \[{\text{s}}{{\text{p}}^{\text{3}}}\] hybridised with a lower \[{\text{s}}\] -character in haloalkanes.
77. How can you obtain iodoethane from ethanol when no other iodine containing reagent except \[{\text{NaI}}\] is available in the laboratory?
Ans: In the presence of \[{\text{ZnC}}{{\text{l}}_{\text{2}}}\], ethanol can be treated with \[{\text{HCl}}\] to produce chloroethane. Iodoethane is formed when chloroethane reacts with \[{\text{NaI}}\].
\[{{\text{C}}_2}{{\text{H}}_5}{\text{OH}} + {\text{HCl}}\mathop {\xrightarrow{{}}}\limits^{{\text{ZnC}}{{\text{l}}_2}} {{\text{C}}_2}{{\text{H}}_5}{\text{Cl}}\mathop {\xrightarrow{{}}}\limits^{{\text{ NaI }}} {{\text{C}}_2}{{\text{H}}_5}{\text{I}}\]
78. Cyanide ion acts as an ambident nucleophile. From which end it acts as a stronger nucleophile in aqueous medium? Give reason for your answer.
Ans: Ambident nucleophiles are groups that include two nucleophilic centres, such as cyanide and nitrile. In the cyanide group, linkage can occur from both the carbon and nitrogen ends. The carbon end functions as a greater nucleophile in an aqueous media because it leads to the creation of a \[{\text{C---C}}\] sbond, which is stronger than a \[{\text{N---C}}\] bond in the identical molecule.
IV.Matching Type:
79. Match the compounds given in Column I with the effects given in Column II.
Column I | Column II |
(i) Chloramphenicol | (a) Malaria |
(ii) Thyroxine | (b) Anaesthetic |
(iii) Chloroquine | (c) Typhoid fever |
(iv) Chloroform | (d) Goiter |
(e) Blood substituent |
Ans: The medicinal uses are linked to the halogen derivatives. Chloramphenicol is an antibiotic used to treat typhoid fever, thyroxine is a hormone whose dysregulation causes goitre, chloroquine is a malaria medication, and chloroform is often used as an anaesthetic.
Correct Answer: (i) → (c) (ii) → (d) (iii) → (a) (iv) → (b)
80. Match the items of Column I and Column II.
Column I | Column II |
(i) \[{SN}^1\] reaction | (a) vic-dibromides |
(ii) Chemicals in fire extinguisher | (b) gem-dihalides |
(iii) Bromination of alkenes | (c) Racemisation |
(iv) Alkylidene halides | (d) Saytzeff rule |
(v) Elimination of \[HX\] from alkylhalide | (e) Chlorobromocarbons |
Ans: Racemization is the process of converting enantiomers into a racemic mixture, which is a mixture comprising two optically active enantiomers in equal quantities, from the results of the \[{{\text{S}}_{\text{N}}}{\text{1}}\] reaction. Chemical fire extinguishers are chlorobromocarbons. Bromination of alkenes produces vic-dibromides, which are formed when halogen atoms of the same kind are linked to nearby carbon atoms of the molecule. Alkylidene dihalides, sometimes called gem-dihalides, are dihalides that have two halogen atoms bonded to the same carbon atom. The Saytzeff rule governs the removal of \[{\text{HX}}\] from alkyl halides: “In dehydrohalogenation processes, the desired product is that alkene with the greatest number of alkyl groups linked to the doubly bound carbon atoms.”
Correct Answer: (i) → (c) (ii) → (e) (iii) → (a) (iv) → (b) (v) → (d)
81. Match the structures of compounds given in Column I with the classes of compounds given in Column II.
Column I | Column II |
(i) \[C{H_2} = \mathop {CH}\limits_{\mathop |\limits_X } - C{H_3}\] | (a) Aryl halide |
(ii) \[C{H_2} = CH - C{H_2} - X\] | (b) Alkyl halide |
(iii) 
| (c) Vinyl halide |
(iv) \[C{H_2} = CH - X\] | (d) Allyl halide |
Ans:Because it includes a halogen atom bound to an \[{\text{s}}{{\text{p}}^3}\] hybridised carbon atom, which is subsequently linked to additional alkyl groups, the first molecule is an alkyl halide.
An allyl halide is a compound in which the halogen atom is linked to a carbon atom that is bound to an \[{\text{s}}{{\text{p}}^3}\] hybridised carbon atom that is attached to a carbon-carbon double bond.
The third molecule is an aryl halide, which is a chemical in which the halogen atom is \[{\text{s}}{{\text{p}}^{\text{2}}}\] hybridised with an aromatic ring carbon atom.
In a carbon-carbon double bond, the halogen atom is linked to a \[{\text{s}}{{\text{p}}^{\text{2}}}\] hybridised carbon atom in the fourth molecule, a vinyl halide.
Correct Answer:(i) → (b) (ii) → (d) (iii) → (a) (iv) → (c)
82. Match the reactions given in Column I with the types of reactions given in Column II.
Column I | Column II |
(i) 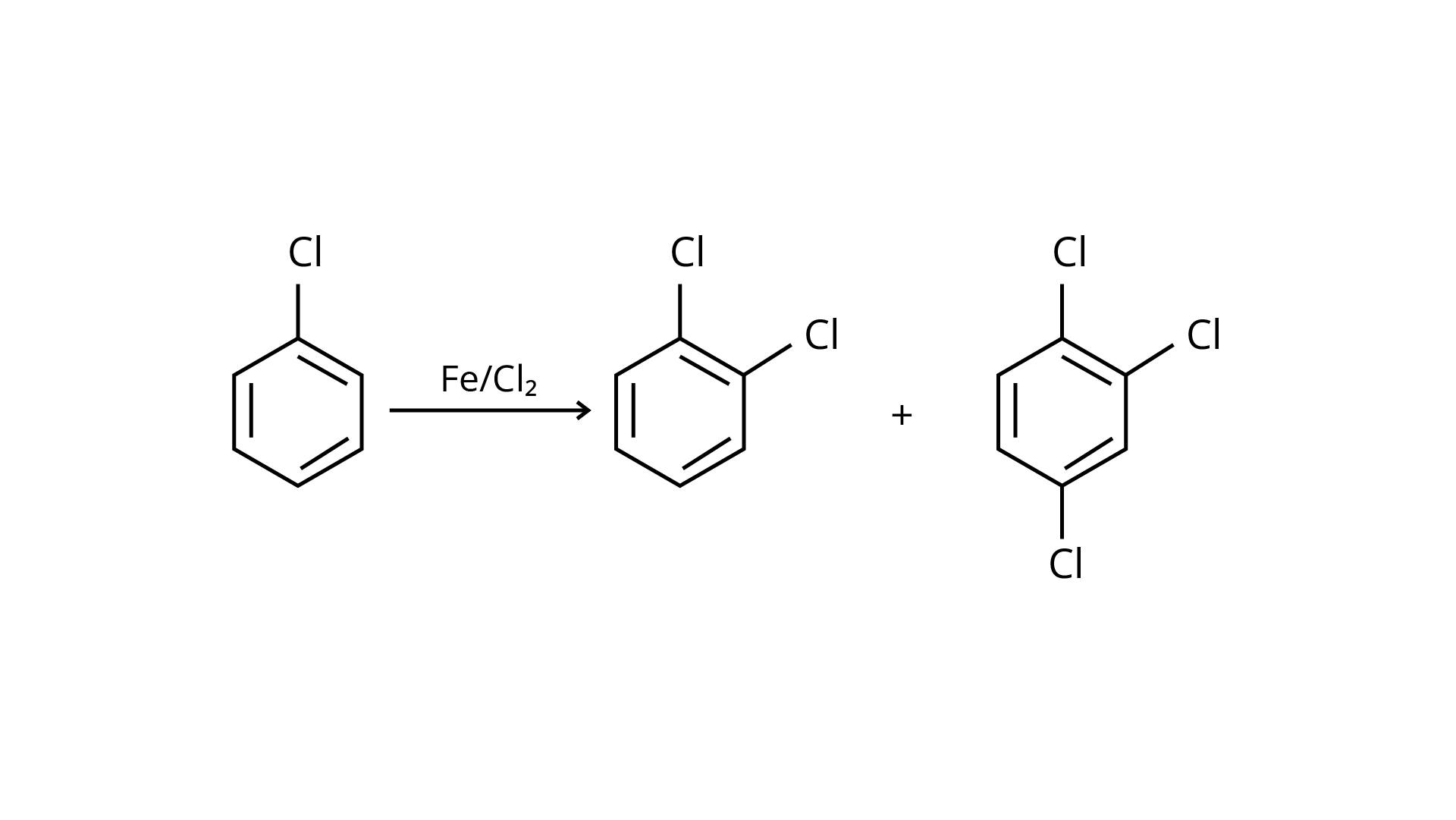
| (a) Nucleophilic aromatic |
(ii) | (b) Electrophilic aromatic substitution |
(iii) 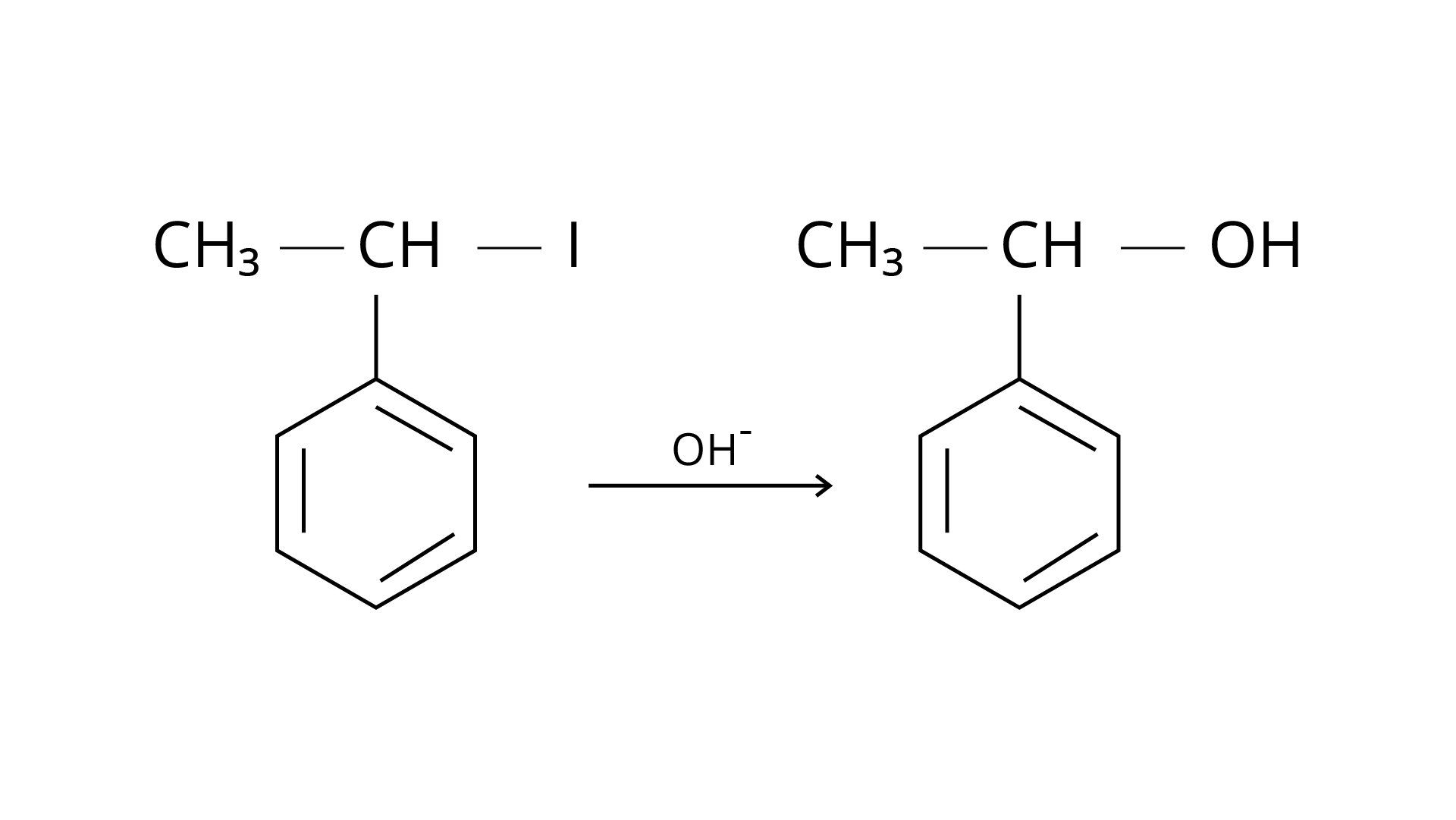
| (c) Saytzeff elimination |
(iV) 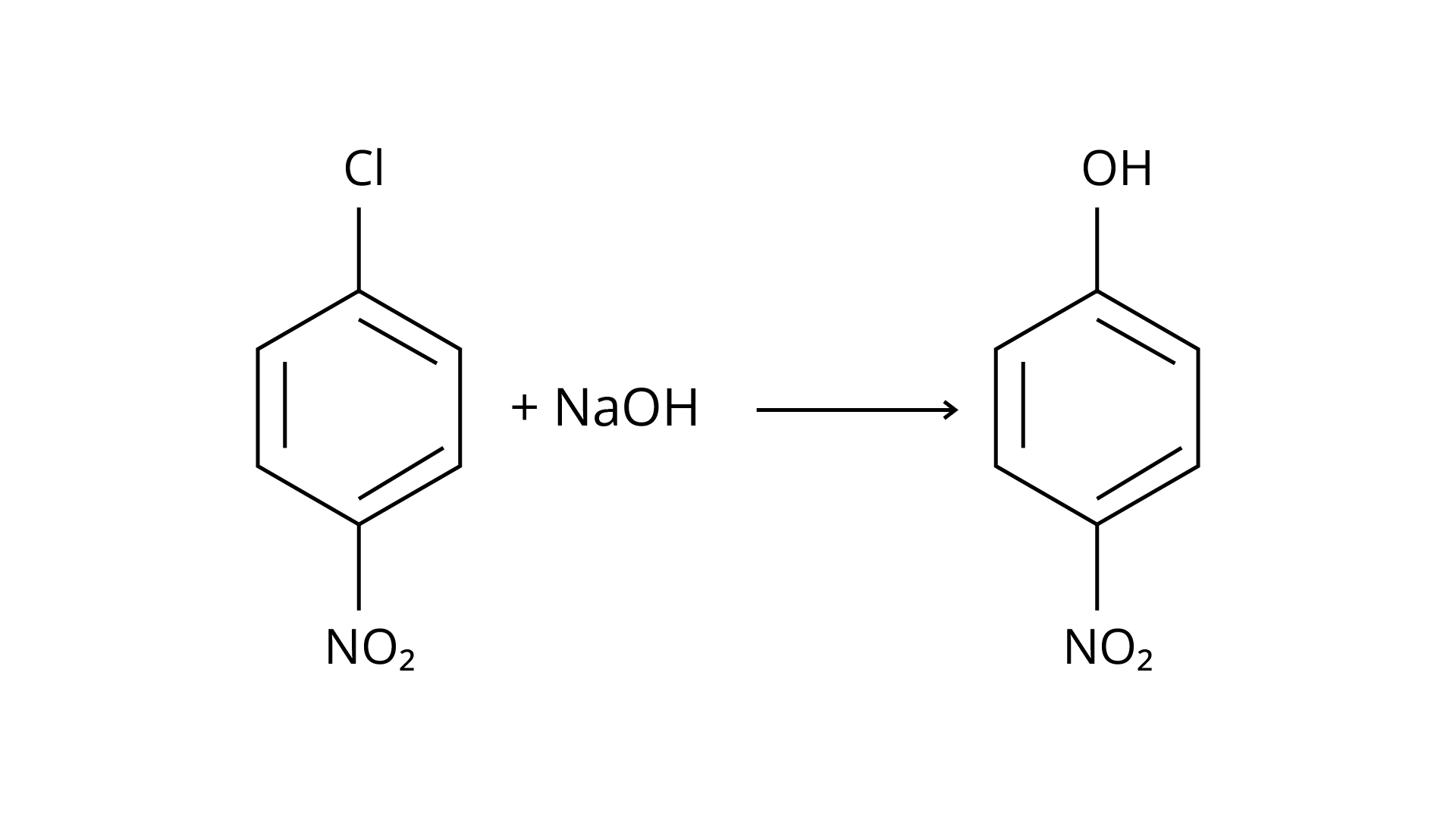
| (d) Electrophilic addition |
(v)\[\mathop {C{H_3}C{H_2}CHC{H_3}}\limits_{\mathop |\limits_{Br} } \xrightarrow{{alkaline/KOH}}C{H_3}CH = CHC{H_3}\] | (e) Nucleophilic substitution |
Ans: The first reaction is an electrophilic substitution process in which \[{\text{C}}{{\text{l}}^{\text{ + }}}\] from the \[{\text{C}}{{\text{l}}_{\text{2}}}\] and \[{\operatorname{FeCl} _3}\] combination attacks and substitutes the benzene ring.
Following Markownikoff's rule, the second reaction is an electrophilic addition reaction in which \[{\text{HBr}}\] is added across the double bond.
The third reaction is a nucleophilic substitution process that uses the \[{{\text{S}}_{\text{N}}}{\text{1}}\] mechanism to replace the \[{\text{--I}}\] group with \[{\text{--OH}}\].
The nucleophilic aromatic substitution process, in which the \[{\text{--Cl}}\] group is replaced by \[{\text{--OH}}\], is the fourth reaction.The fifth reaction is a dehydrohalogenation reaction, in which the alkyl halide is eliminated by Saytzeff.
Correct Answer: (i) → (b) (ii) → (d) (iii) → (e) (iv) → (a) (v) → (c)
83. Match the structures given in Column I with the names in Column II.
Column I | Column II |
(i) 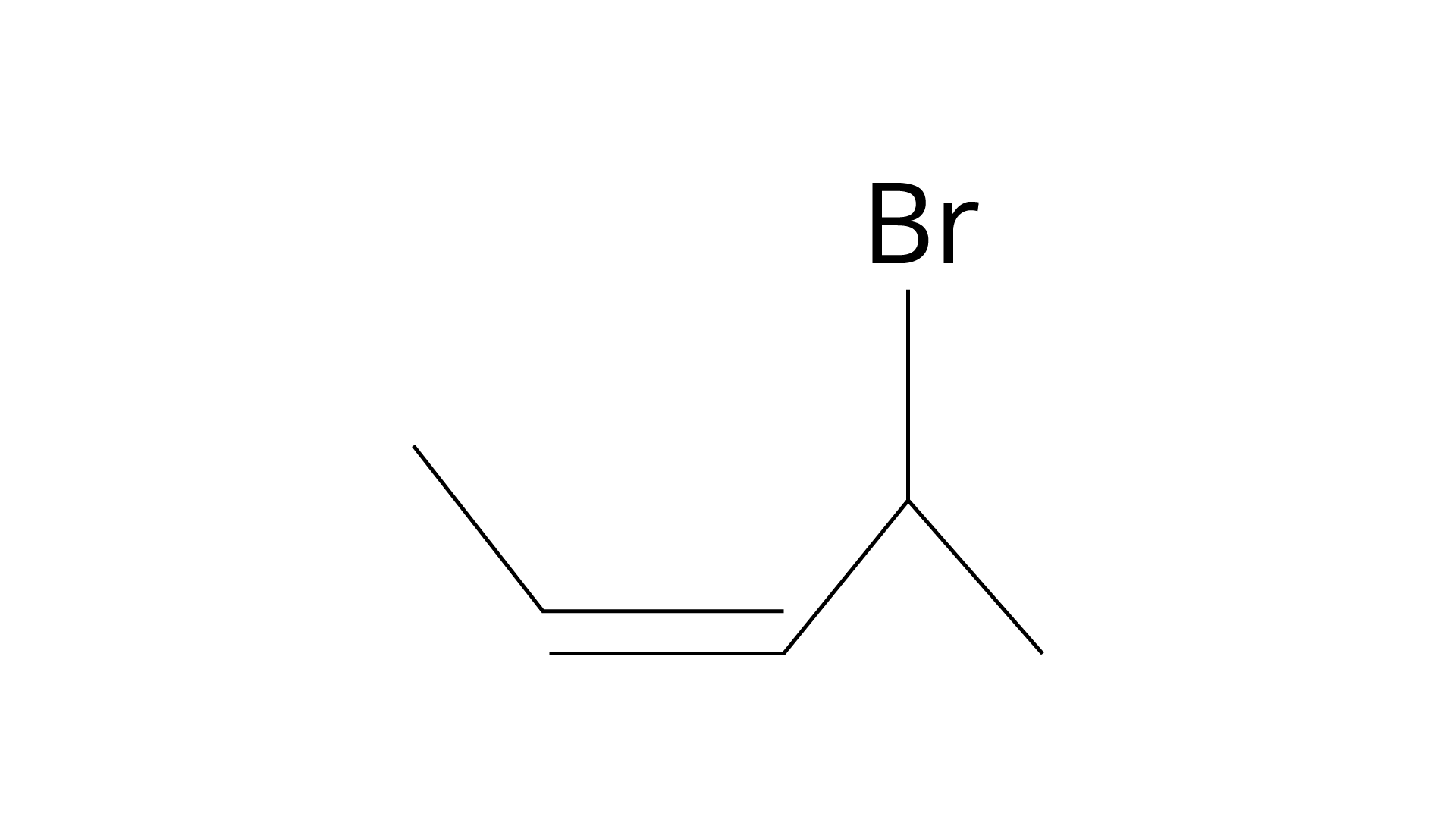
| (a) 4 -Bromopent-2-ene |
(ii) 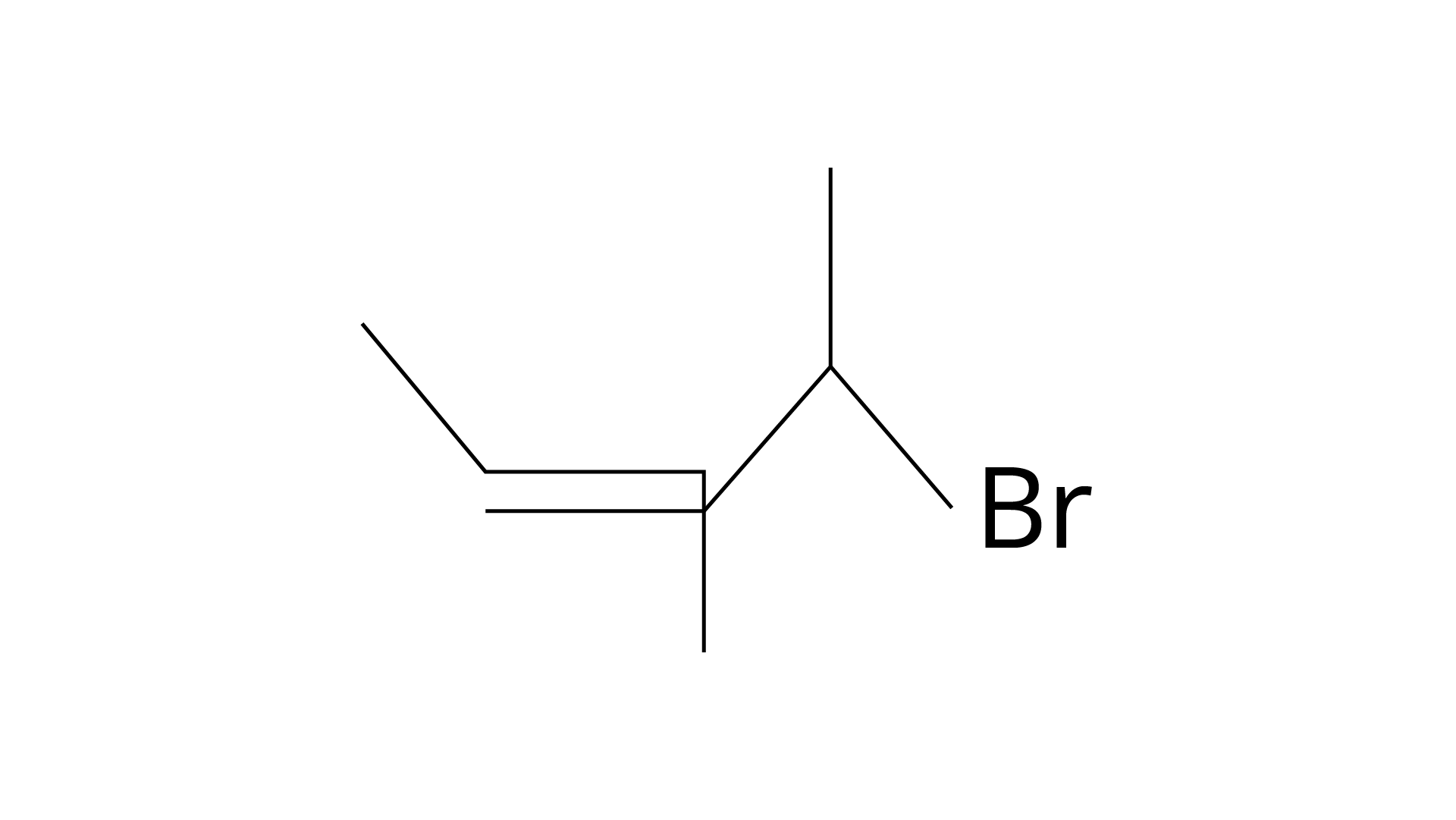
| (b) 4 -Bromo-3-methylpent-2-ene |
(iii) 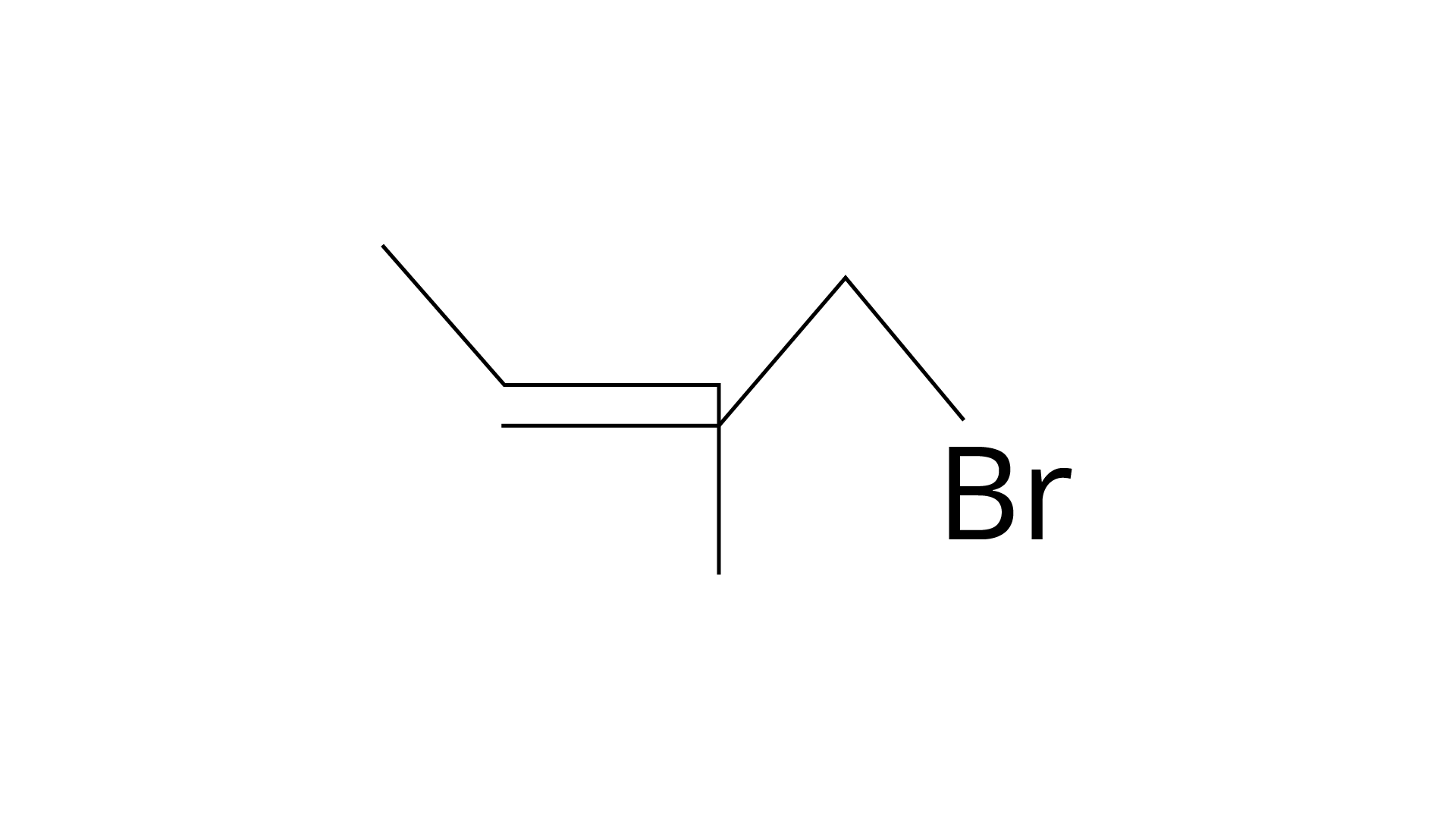
| (c) 1 -Bromo-2-methylbut-2-ene |
(iV) 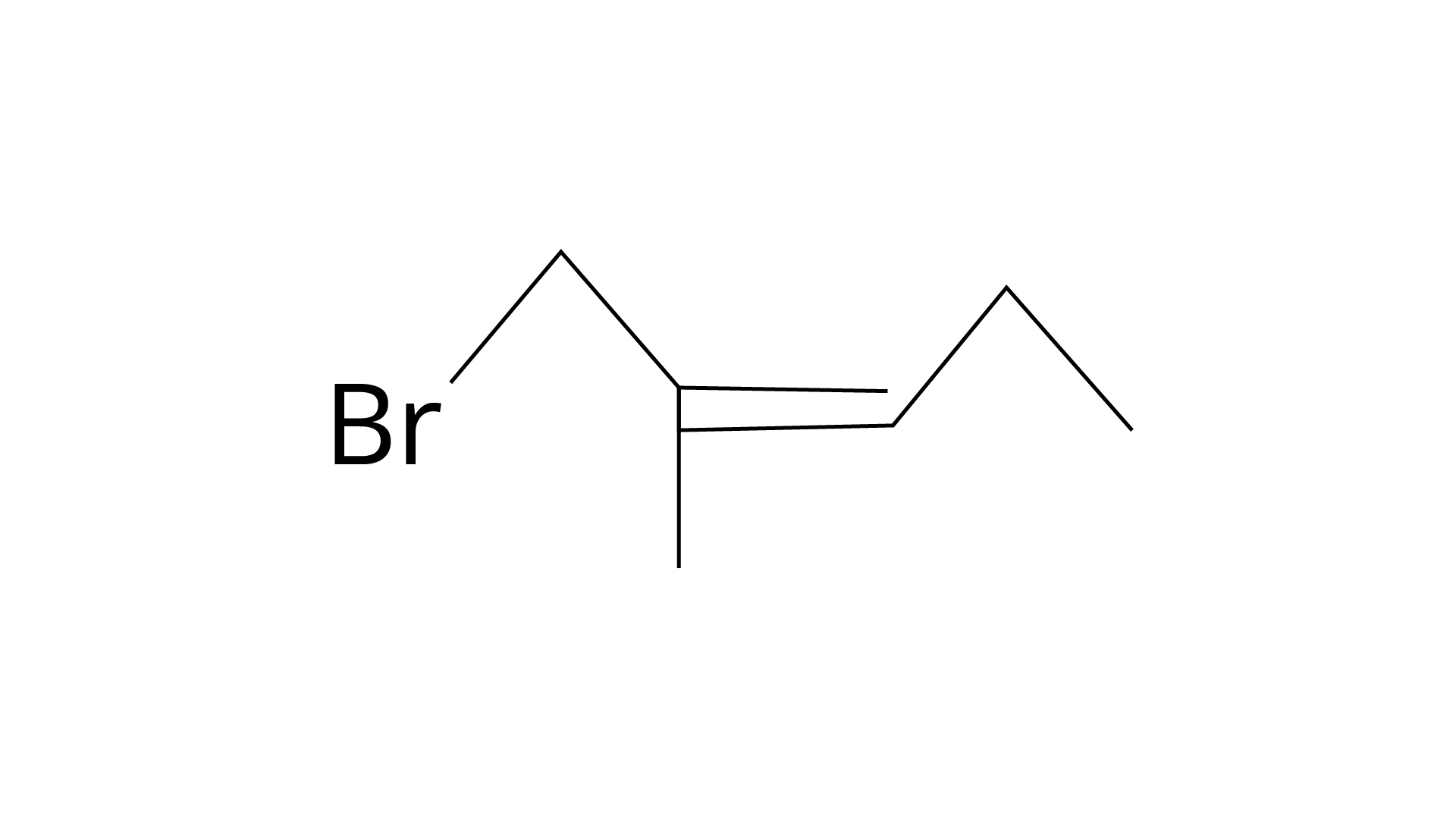
| (d) 1 -Bromo-2-methylpent-2-ene |
Ans: (i) A: 4-bromopent-2-ene.
(ii) B: 4-bromo-3-methylpent-2-ene.
(iii) C: 1-bromo-2-methylbut-2-ene.
(iv) D: 1 -bromo-2-methylpent-2-ene.
Correct Answer: (i) → (a) (ii) → (c) (iii) → (b) (iv) → (d)
84. Match the reactions given in Column I with the names given in Column II.
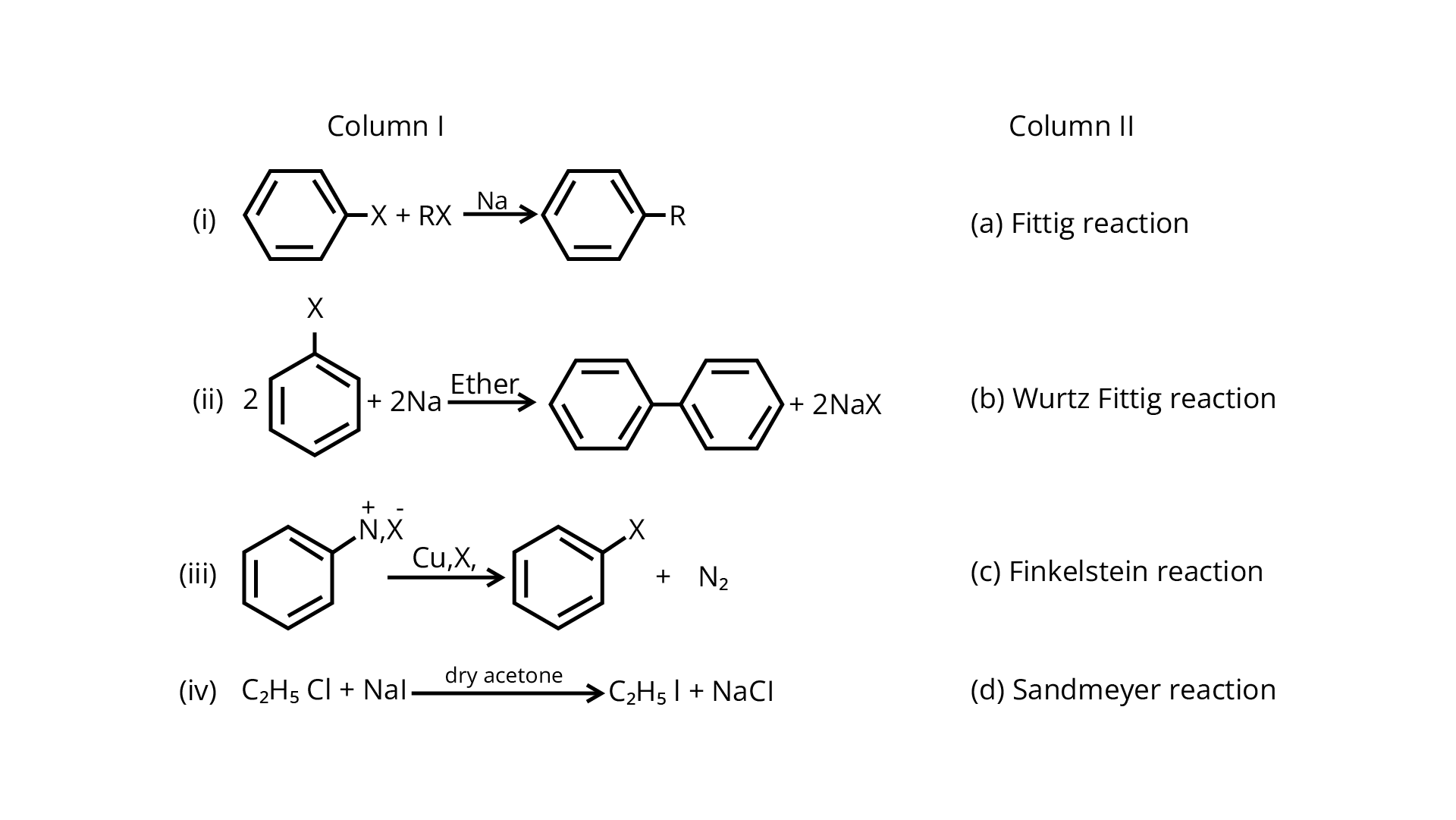
Ans: In the presence of \[{\text{Na}}\] metal in dry ether, an alkyl halide and an aryl halide combine to create alkyl arene in the Wurtz-Fittig reaction.
The Fittig reaction, which involves a coupling reaction between two aryl halides in dry ether in the presence of \[{\text{Na}}\] metal, yielding diaryl compounds, is the second reaction.
The Sandmeyer reaction produces aryl halides from primary amines by converting them to diazonium salts and treating them with cuprous bromide.
The Finkelstein reaction, which produces alkyl iodides by reacting alkyl chlorides or bromides with \[{\text{NaI}}\] in dry acetone, is the fourth process.
Correct Answer: (i) → (b) (ii) → (a) (iii) → (d) (iv) → (c)
V. Assertion and Reason Type
In the following questions a statement of Assertion (A) followed by a statement of Reason (R) is given. Choose the correct option out of the choices given below each question.
(i) Assertion and reason both are correct and reason is correct explanation of assertion.
(ii) Assertion and reason both are wrong statements.
(iii) Assertion is correct but reason is wrong statement.
(iv) Assertion is wrong but reason is correct statement.
(v) Assertion and reason both are correct statements but reason is not correct explanation of assertion.
85.Assertion : Phosphorus chlorides (tri and penta) are preferred over thionyl chloride for the preparation of alkyl chlorides from alcohols.
Reason : Phosphorus chlorides give pure alkyl halides.
(i) Assertion and reason both are correct and reason is correct explanation of assertion.
(ii) Assertion and reason both are wrong statements.
(iii) Assertion is correct but reason is wrong statement.
(iv) Assertion is wrong but reason is correct statement.
(v) Assertion and reason both are correct statements but reason is not correct explanation of assertion.
Ans: Thionyl chloride is favoured over phosphorus chlorides because the by-products produced, in addition to the alkyl halides, include \[{\text{S}}{{\text{O}}_{\text{2}}}\] and \[{\text{HCl}}\], which are gaseous and so may escape the reaction, leaving just pure halides.
Correct Answer: Option (ii)
86. Assertion: The boiling points of alkyl halides decrease in the order:
\[RI > RBr > RCl > RF\]
Reason : The boiling points of alkyl chlorides, bromides and iodides are considerably higher than that of the hydrocarbon of comparable molecular mass.
(i) Assertion and reason both are correct and reason is correct explanation of assertion.
(ii) Assertion and reason both are wrong statements.
(iii) Assertion is correct but reason is wrong statement.
(iv) Assertion is wrong but reason is correct statement.
(v) Assertion and reason both are correct statements but reason is not correct explanation of assertion.
Ans: Because of the size of the halogen atom, the boiling temperatures of alkyl halides drop in the specified sequence. With the greatest atomic number, iodide has the most electrons, resulting in increased Van Der Waals forces and a higher boiling point.
Correct Answer: Option (v)
87.Assertion: \[KCN\] reacts with methyl chloride to give methyl isocyanide
Reason: \[C{N^ - }\]is an ambident nucleophile.
(i) Assertion and reason both are correct and reason is correct explanation of assertion.
(ii) Assertion and reason both are wrong statements.
(iii) Assertion is correct but reason is wrong statement.
(iv) Assertion is wrong but reason is correct statement.
(v) Assertion and reason both are correct statements but reason is not correct explanation of assertion.
Ans: Haloalkanes react with \[{\text{AgCN}}\] to produce alkyl isocyanides as the primary product, whereas \[{\text{KCN}}\] produces alkyl cyanides.
Correct Answer: Option (iv)
88.Assertion: tert-Butyl bromide undergoes Wurtz reaction to give \[2,2,3,3\]-tetramethylbutane.
Reason : In the Wurtz reaction, alkyl halides react with sodium in dry ether to give hydrocarbons containing double the number of carbon atoms present in the halide.
(i) Assertion and reason both are correct and reason is correct explanation of assertion.
(ii) Assertion and reason both are wrong statements.
(iii) Assertion is correct but reason is wrong statement.
(iv) Assertion is wrong but reason is correct statement.
(v) Assertion and reason both are correct statements but reason is not correct explanation of assertion.
Ans: Because tertiary butyl bromide interacts with \[{\text{NaI}}\] in dry ether to create 2, 2, 3, 3-tetramethylbutane, it undergoes the Wurtz reaction to yield 2, 2, 3, 3-tetramethylbutane.
Correct Answer: Option (i)
89.Assertion: Presence of a nitro group at ortho or para position increases the reactivity of haloarenes towards nucleophilic substitution.
Reason : Nitro group, being an electron withdrawing group decreases the electron density over the benzene ring.
(i) Assertion and reason both are correct and reason is correct explanation of assertion.
(ii) Assertion and reason both are wrong statements.
(iii) Assertion is correct but reason is wrong statement.
(iv) Assertion is wrong but reason is correct statement.
(v) Assertion and reason both are correct statements but reason is not correct explanation of assertion.
Ans:The nitro group on the ortho and para positions of haloarenes acts as an electron-withdrawing group, making it more reactive to nucleophilic substitution reactions due to the electron deficit.
Correct Answer: Option (i)
90.Assertion : In monohaloarenes, further electrophilic substitution occurs at ortho and para positions.
Reason : Halogen atom is a ring deactivator.
(i) Assertion and reason both are correct and reason is correct explanation of assertion.
(ii) Assertion and reason both are wrong statements.
(iii) Assertion is correct but reason is wrong statement.
(iv) Assertion is wrong but reason is correct statement.
(v) Assertion and reason both are correct statements but reason is not correct explanation of assertion.
Ans:Because halogen atoms are ortho and para directing, rather than ring deactivators, further electrophilic substitution occurs at ortho and para locations.
Correct Answer: Option (v)
91. Assertion: Aryl iodides can be prepared by reaction of arenes with iodine in the presence of an oxidising agent.
Reason : Oxidising agent oxidises \[{I_2}\] into \[HI\].
(i) Assertion and reason both are correct and reason is correct explanation of assertion.
(ii) Assertion and reason both are wrong statements.
(iii) Assertion is correct but reason is wrong statement.
(iv) Assertion is wrong but reason is correct statement.
(v) Assertion and reason both are correct statements but reason is not correct explanation of assertion.
Ans: Oxidising chemicals such as \[{\text{HI}}{{\text{O}}_{\text{3}}}\] oxidise \[{\text{HI}}\] to \[{{\text{I}}_{\text{2}}}\] because the presence of HI causes the aryl iodides to revert to arenes in their absence.
Correct Answer: Option (iii)
92. Assertion- It is difficult to replace chlorine by \[ - OH\] in chlorobenzene in comparison to that in chloroethane.
Reason: Chlorine-carbon (\[C - Cl\]) bond in chlorobenzene has a partial double bond character due to resonance.
(i) Assertion and reason both are correct and reason is correct explanation of assertion.
(ii) Assertion and reason both are wrong statements.
(iii) Assertion is correct but reason is wrong statement.
(iv) Assertion is wrong but reason is correct statement.
(v) Assertion and reason both are correct statements but reason is not correct explanation of assertion.
Ans: Due to resonance, a partial double bond character occurs in the bond between the \[{\text{C}}\] and \[{\text{Cl}}\] atoms in chlorobenzene, and we all know that changing a partial double bond character is more difficult than replacing a single bond as in the \[{\text{C - Cl}}\] bond in chloroethane.
Correct Answer: Option (i)
93. Assertion : Hydrolysis of \[( - ) - 2\]-bromooctane proceeds with inversion of configuration.
Reason : This reaction proceeds through the formation of a carbocation.
(i) Assertion and reason both are correct and reason is correct explanation of assertion.
(ii) Assertion and reason both are wrong statements.
(iii) Assertion is correct but reason is wrong statement.
(iv) Assertion is wrong but reason is correct statement.
(v) Assertion and reason both are correct statements but reason is not correct explanation of assertion.
Ans: The \[{{\text{S}}_{\text{N}}}2\] mechanism is demonstrated by the hydrolysis of alkyl halides with inversion of configuration. This is a one-step method that does not need the production of carbocation.
Correct Answer: Option (iii)
94. Assertion- Nitration of chlorobenzene leads to the formation of \[{\text{m}}\]-nitrochlorobenzene
Reason :\[ - N{O_2}\] group is a m -directing group.
(i) Assertion and reason both are correct and reason is correct explanation of assertion.
(ii) Assertion and reason both are wrong statements.
(iii) Assertion is correct but reason is wrong statement.
(iv) Assertion is wrong but reason is correct statement.
(v) Assertion and reason both are correct statements but reason is not correct explanation of assertion.
Ans: Because m-nitrochlorobenzene is not a stable molecule, the \[{\text{---N}}{{\text{O}}_{\text{2}}}\] group is a meta-directing group, and the reactions' products include nitro groups at the o- and p- positions.
Correct Answer: Option (iv)
VI. Long Answer Type:
95. Some alkylhalides undergo substitution whereas some undergo elimination reaction on treatment with bases. Discuss the structural features of alkyl halides with the help of examples which are responsible for this difference.
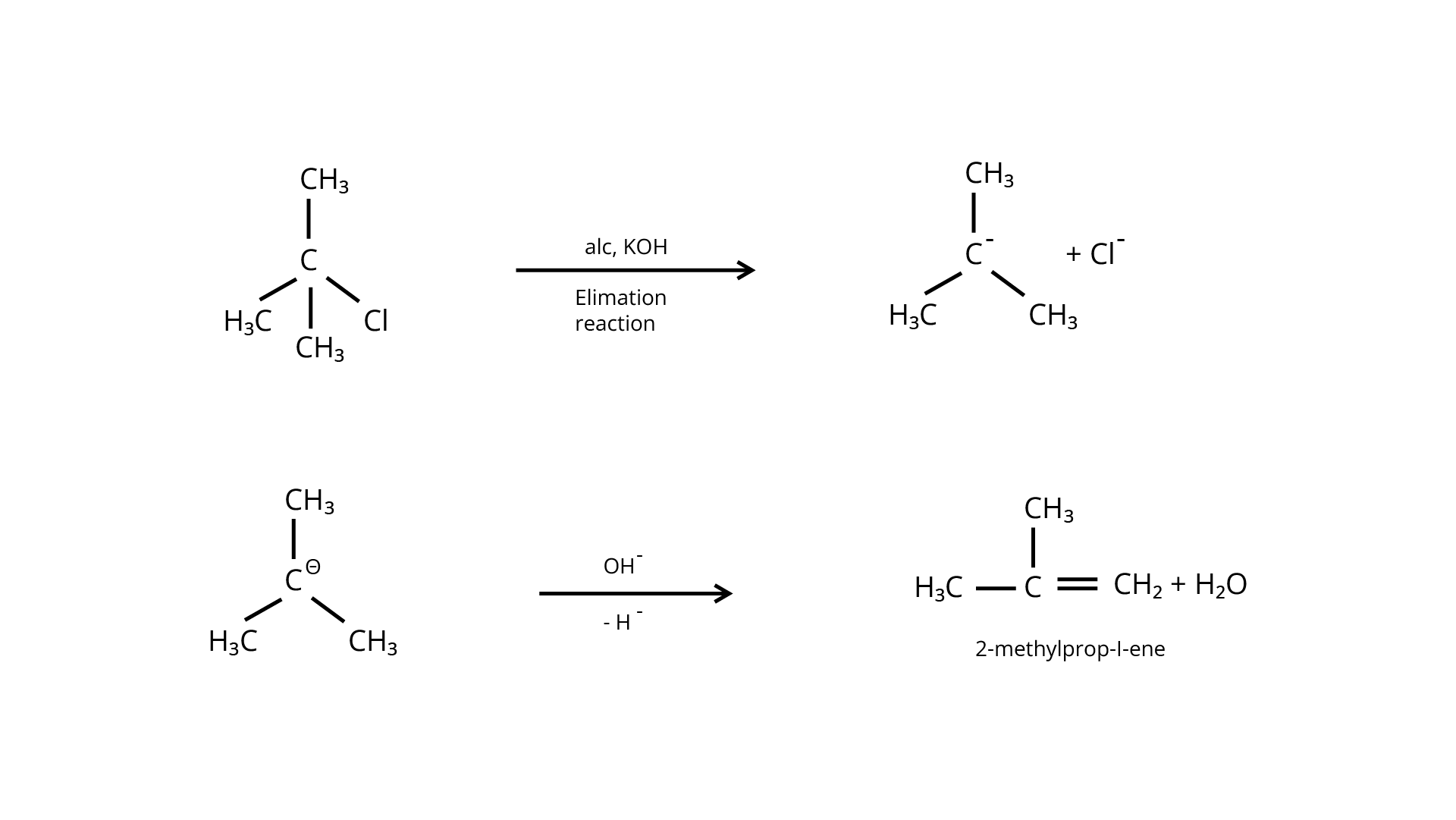
Ans: The structure of the alkyl halide as well as the chemicals used in the reaction dictate whether it undergoes substitution or elimination. The reactivity of alkyl halides to substitution processes can be examined to better understand their structural characteristics. The \[{{\text{S}}_{\text{N}}}{\text{2}}\] mechanism, which involves cleavage of the halide from the carbon atom and simultaneous attachment of the attacking nucleophile, is preferred by primary alkyl halides for substitution reactions.
Tertiary halides, on the other hand, go through an elimination process due to the production of stable carbocation. Tertiary halides also prefer to go through the \[{{\text{S}}_{\text{N}}}1\] reaction, which is a two-step reaction including the creation of a stable carbocation once the halide atom is cleaved. Depending on the solvent and temperature circumstances, secondary halides exhibit intermediate reactivity in both elimination and substitution processes.
The given reaction is for a primary alkyl halide undergoing \[{{\text{S}}_{\text{N}}}{\text{2}}\] substitution.
The provided reaction is for an \[{{\text{S}}_{\text{N}}}1\] substitution of a tertiary alkyl
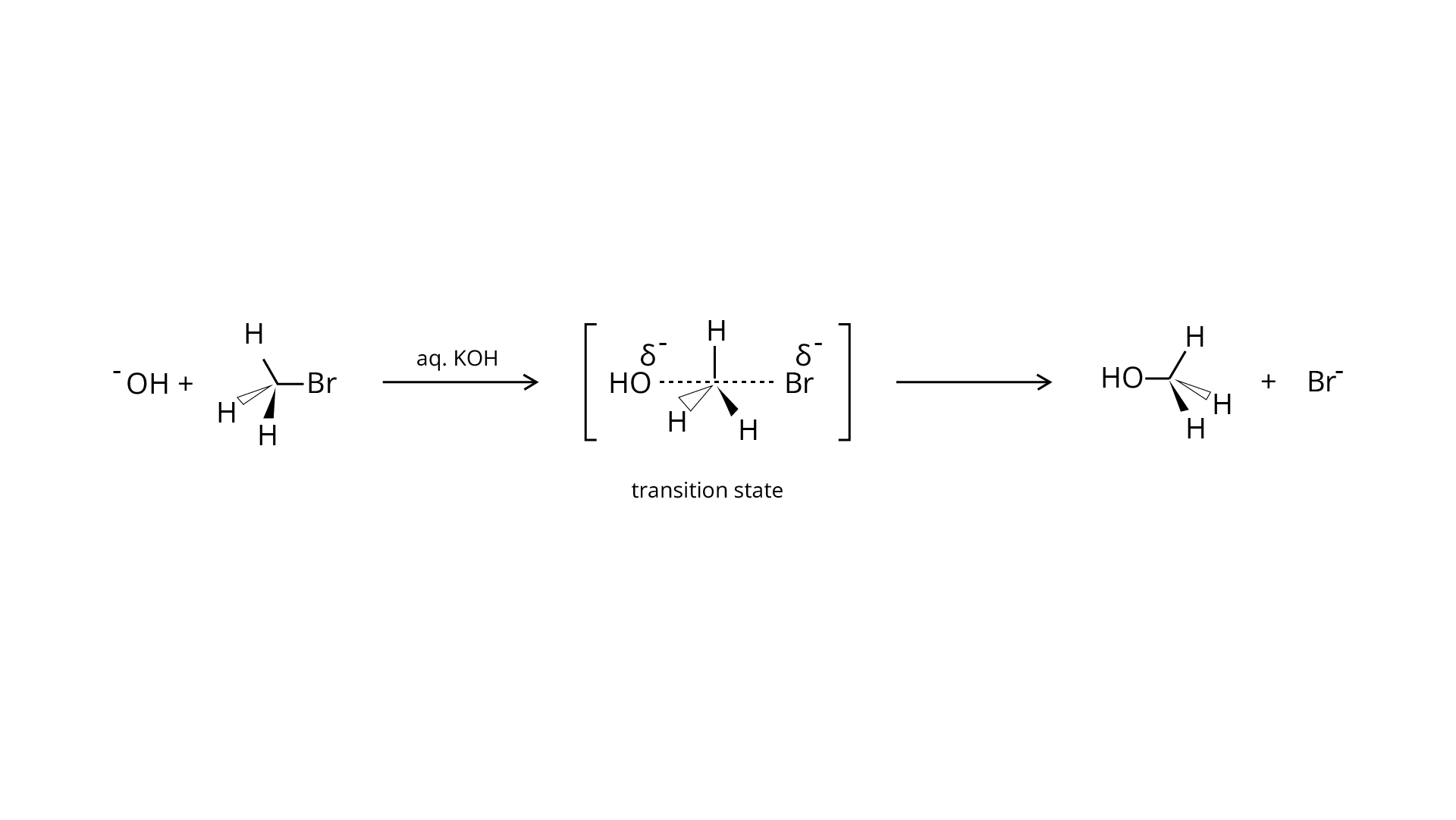
halide. The strength of the base utilised determines the mechanism. When using a weak base, such as aqueous \[{\text{KOH}}\], the reaction is substitution, but when using a strong base, such as an alcoholic base, the reaction is elimination.
\[C{H_3} - \mathop C\limits_{\mathop |\limits_{C{H_3}} }^{\mathop |\limits^{C{H_3}} } \xrightarrow{{Ionization/B{r^ - }slow}}C{H_3} - \mathop {{C^ + }}\limits_{\mathop |\limits_{C{H_3}} }^{\mathop |\limits^{C{H_3}} } \]
Tert-butyl carbocation
(stable)
\[C{H_3} - \mathop {{C^ + }}\limits_{\mathop |\limits_{C{H_3}} }^{\mathop |\limits^{C{H_3}} } \xrightarrow{{OH/fast}}C{H_3} - \mathop C\limits_{\mathop |\limits_{C{H_3}} }^{\mathop |\limits^{C{H_3}} } - OH\]
(tert-butyl alcohol)
96. Some halogen containing compounds are useful in daily life. Some compounds of this class are responsible for exposure of flora and fauna to more and more of UV light which causes destruction to a great extent. Name the class of these halocompounds. In your opinion, what should be done to minimise harmful effects of these compounds.
Ans: Multiple halogen groups are present in these carbon compounds, which have a wide range of applications in industry. Polyhalogen compounds are what they're called. The following are the different chemicals and their impact on life:
1. Dichloromethane, commonly known as methylene chloride, is a solvent that is used as a paint remover, an aerosol propellant, a process solvent in drug manufacturing, and a metal cleaning and finishing solvent. The central nervous system has been documented to be harmed by dichloromethane. Low amounts of this chemical might cause mild hearing and visual impairment, while larger levels in the air can induce dizziness, nausea, tingling, and numbness in the fingers and toes. Methylene chloride produces severe burning and moderate skin redness in humans when it comes into close contact with the skin. The cornea can be burned by direct contact with the eyes.
2. Trichloromethane or Chloroform: This is mostly utilised as a solvent for lipids, alkaloids, iodides, and other compounds, and it is also used to make Freon R-22. It used to be used as an anaesthetic in medicine, but now it's replaced with less harmful alternatives like ether. Chronic exposure to chloroform, which is transformed to phosgene and causes liver and kidney damage as well as skin ulcers, is deadly.
3. Tetrachloromethane, also known as carbon tetrachloride, was commonly utilised to make cleaning solutions, chlorofluorocarbons, and aerosol propellants. The use of \[{\text{CC}}{{\text{l}}_{\text{4}}}\] has resulted in the loss of the ozone layer, which shields the planet from UV radiation, leading to an increase in skin cancer and other diseases.
4. DDT, or p,p'-dichlorodiphenyltrichloroethane, was a commonly used pesticide. They're chlorinated organic pesticides that became popular after WWII due to their excellent effectiveness against malaria-causing mosquitoes and typhus-carrying lice. The major consequences began with insects developing resistance to DDT and a high toxicity to fish. The issue was exacerbated by DDT's chemical persistence and fat solubility. In terms of chemistry, DDT is not metabolised or solubilized well by mammals. The fatty tissues are where it is deposited and kept. DDT builds up within the animal over time if it is consumed at a constant pace, harming the ecosystem.
97. Why are aryl halides less reactive towards nucleophilic substitution reactions than alkyl halides? How can we enhance the reactivity of aryl halides?
Ans: Because of the following reasons, aryl halides are less reactive towards nucleophilic substitution processes than alkyl halides:
(i) The halogen atom's electrons are conjugated to the aryl ring's -electrons, giving the \[{\text{C---X}}\] bond a partial double bond character. The whole molecule is then resonance stabilised, allowing for the formation of the following configurations. \[{\text{C---X}}\] bond cleavage in haloarenes is more difficult than in haloalkanes due to this bond creation.
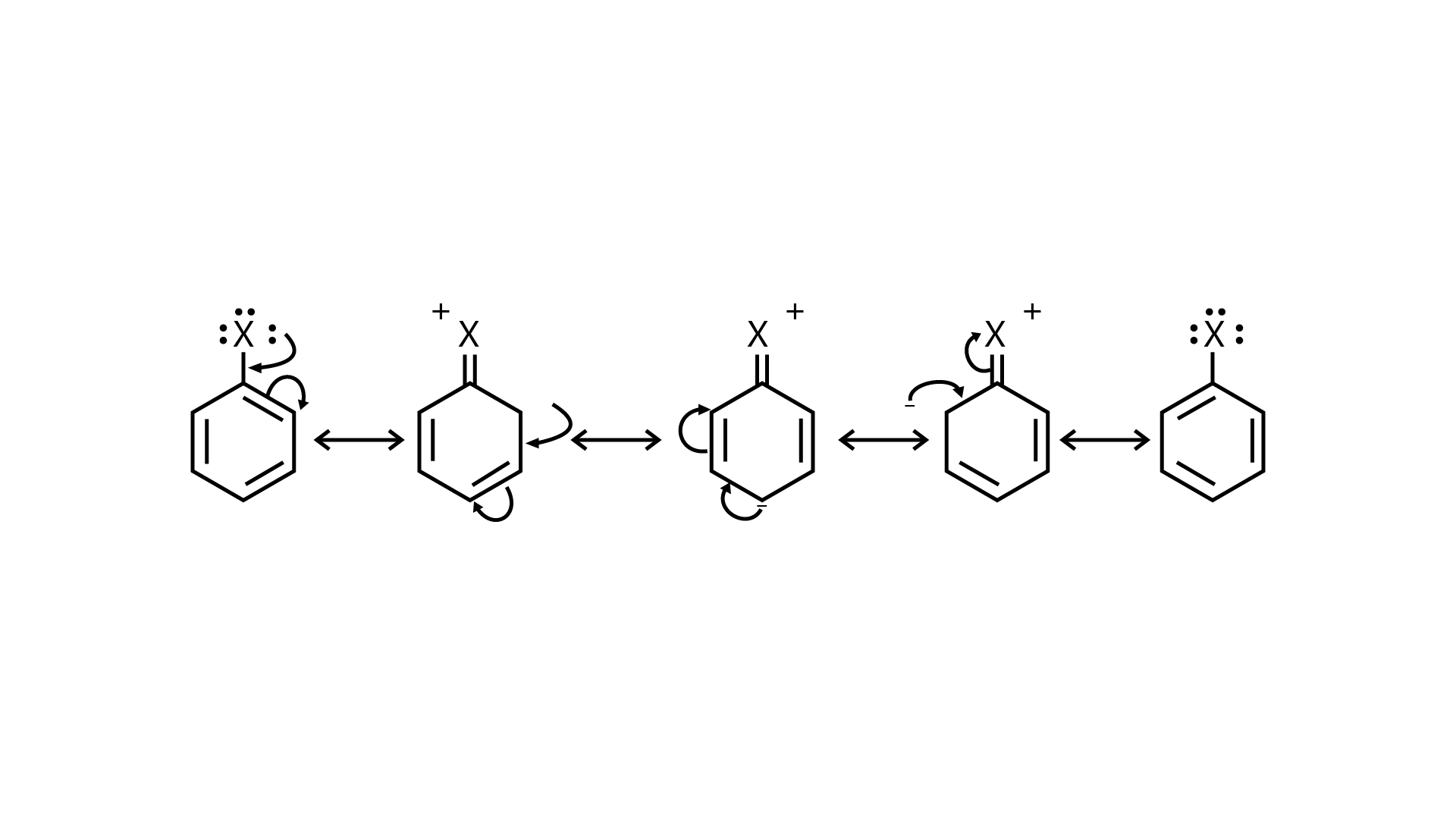
(ii) The carbon linked to the halogen in haloalkanes is \[{\text{s}}{{\text{p}}^{\text{3}}}\] hybridised, whereas the carbon coupled to the halogen in haloarene is \[{\text{s}}{{\text{p}}^2}\] hybridised. In haloalkanes, the \[{\text{s}}{{\text{p}}^2}\] hybridised carbon atom binds the halogen atom more firmly than a connection between \[{\text{s}}{{\text{p}}^{\text{3}}}\] carbon atom and halogen atom. Haloarenes have a shorter \[{\text{C---X}}\]bond than haloalkanes, making them more difficult to break.
(iii) Because the phenyl cation is not resonance stabilised, the \[{S_N}1\] mechanism of substitution cannot be used.
(iv) Because of the potential for repulsion between electron-rich arenes and approaching electron-rich nucleophiles, aryl halides are less reactive than alkyl halides.
The inclusion of an electron withdrawing group at the ortho- and para-positions can enhance the reactivity of aryl halides. At the meta-position, there is no impact. The presence of an electron withdrawing group, such as the nitro group, at ortho- and para- locations pulls electrons away from the benzene ring, making it easier for the nucleophile to attack haloarene. The carbanion is stabilised as a result of resonance. The \[{\text{--N}}{{\text{O}}_{\text{2}}}\] group stabilises the negative charge at ortho- and para-positions with regard to the halogen substituent, but none of the resonant structures in meta-nitrobenzene contain the negative charge on the carbon atom bearing the \[{\text{--N}}{{\text{O}}_{\text{2}}}\] group. As a result, the inclusion of a meta-position electron withdrawing group has no influence on the aryl halide's reactivity.
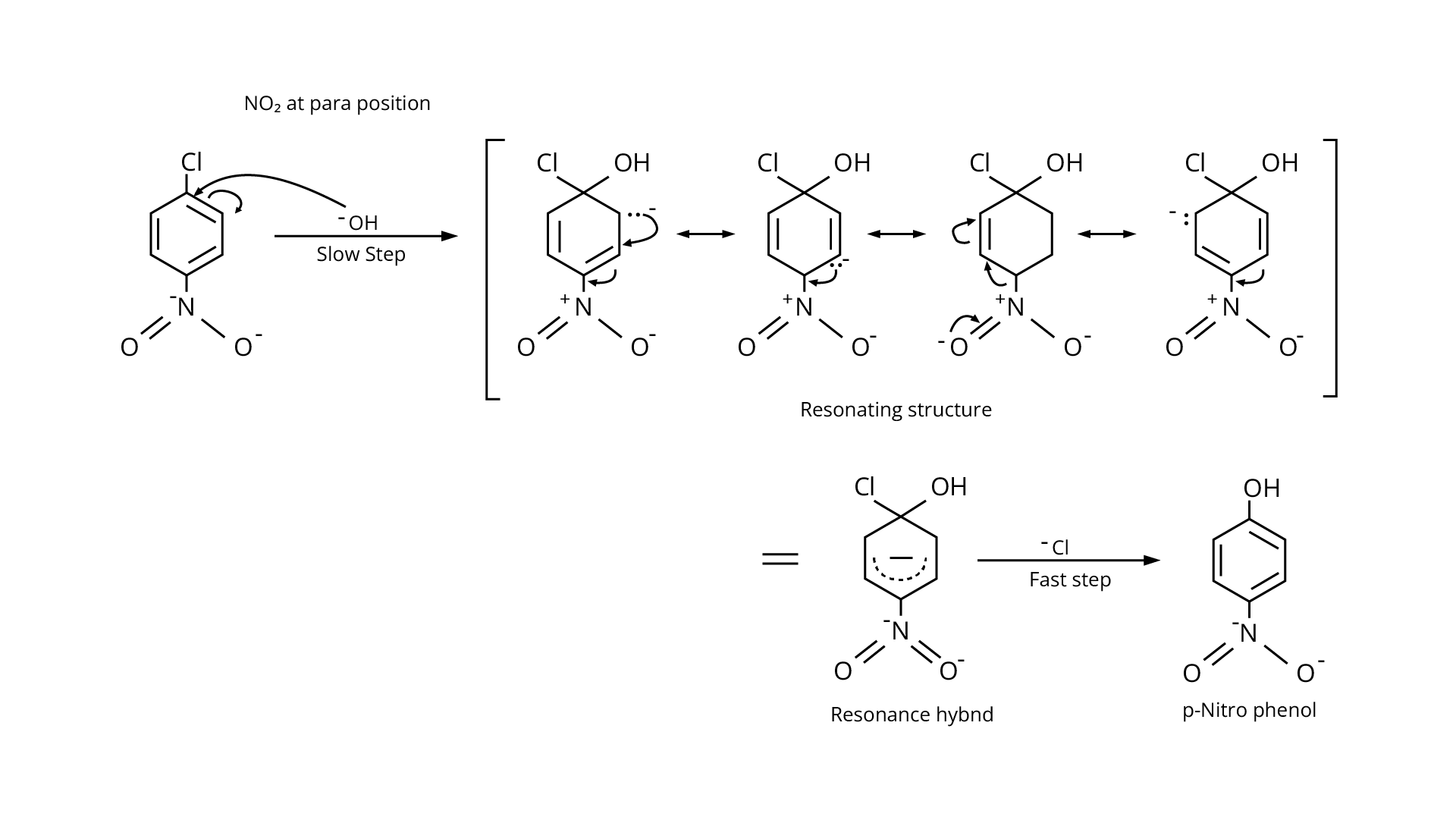
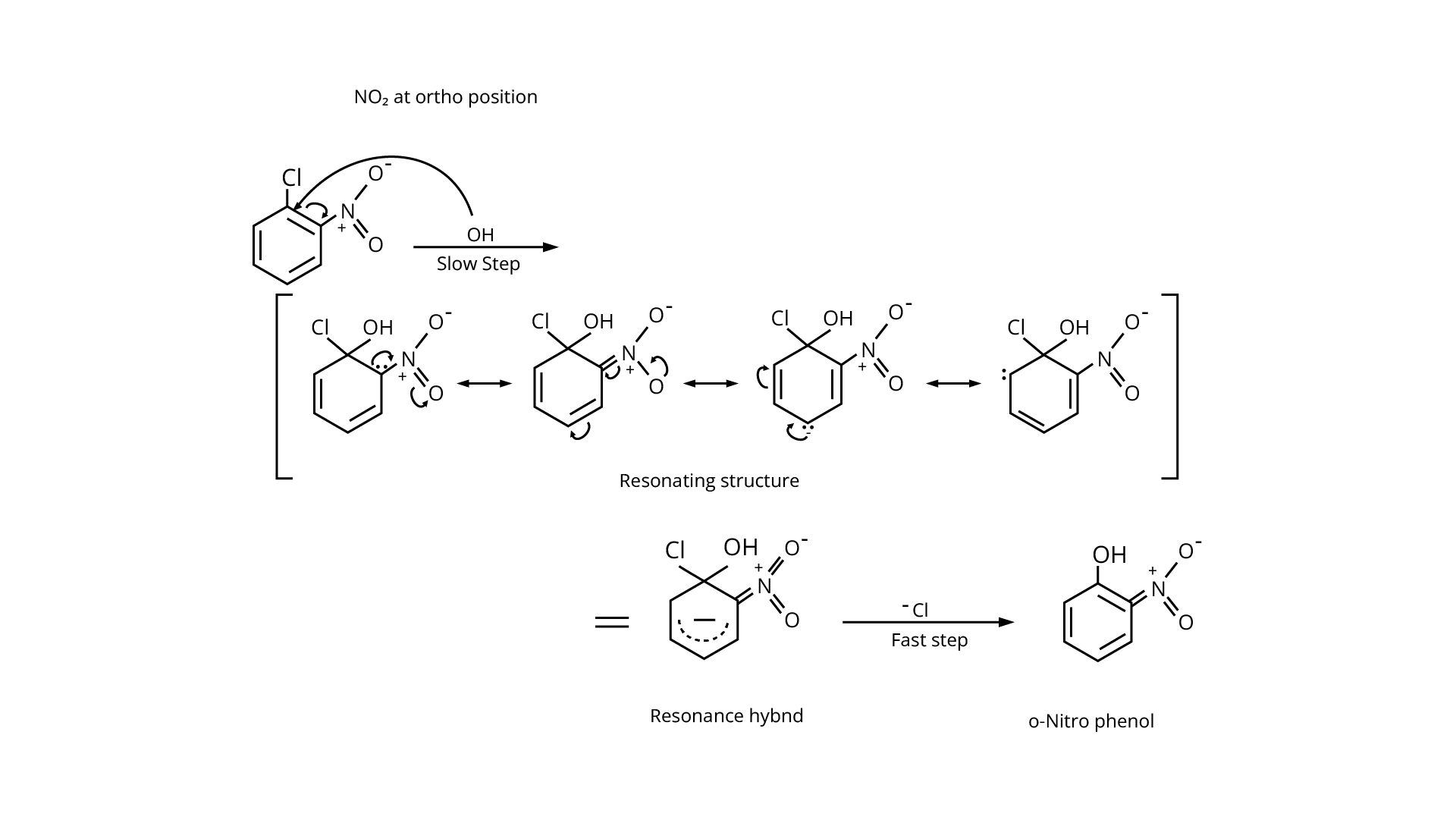
Key Things To Keep in Mind While Studying Organic Chemistry
Make sure that you are not skipping any chapter
Everything is interrelated, and that is why it is important to make sure that you are revising properly and regularly
Keep your notes and study material handy
Practice a lot
Use the free resources from Vedantu to boost your scores!
Benefits of Using Solutions for Class 12 NCERT Exemplar for Haloalkanes and Haloarenes
NCERT Exemplar is an essential textbook. Most students do not take the right advantage of this brilliant resource, but we highly recommend students to start studying from this book. By studying from the free PDF that covers the solutions from Class 12 NCERT Exemplar for the chapter Haloalkanes and Haloarenes, students can:-
Score well in JEE, NEET, and other competitive examinations
Score well in their school level examinations
Practice a mix of easy and high-level questions at once
Work on weaker concepts easily
Clear their doubts immediately by studying from this well-detailed solution PDF
Understand the chapter easily in a very short period.
Haloalkanes and Haloarenes is a very important topic from Organic chemistry and thus needs the right quality of resources to study from. Luckily, with Vedantu, students don’t have to worry. Download the free PDF now and let the learning begin!
FAQs on NCERT Exemplar for Class 12 Chemistry Chapter-10 (Book Solutions)
1. What are Haloalkanes and Haloarenes?
This is a very important question concerning school-level-based exams. A lot of times there have been questions in the test that ask for the differences between Haloalkanes and Haloarenes or simply the definition of the two terms. Hence dedicating an entirely different page that covers everything related to these two terms is what Vedantu did. Check out this page from Vedantu to understand what are Haloalkanes and Haloarenes, their differences, and their uses.
2. Why is Organic Chemistry so tough?
The simple answer is, that it is not. Organic chemistry surely requires a lot of attention and good focus, but it does not mean that it is difficult. Many students refrain from studying topics from Organic Chemistry in the first place because they believe that it is too difficult, which is a huge mistake. We can guarantee that once students patiently sit down and study organic chemistry, they will realize that it is not that difficult and is simple to understand. You just need to practice.
3. What are some of the most important chapters from Class 12 Organic Chemistry?
No chapter can be called “less important” when it comes to both organic and inorganic chemistry. All of these chapters are interconnected and are strongly related to each other. You cannot miss out on any of them if your goal is to have a strong level of understanding by the end of your study session and to score good marks. These chapters need a lot of focus and require a good amount of time. Study every chapter if you wish to score the best (sorry).
4. Organic Chemistry or Inorganic Chemistry. Which is more important for my class 12 Examinations?
Another question is where the ability to choose fails to make a decision. Both Organic and Inorganic Chemistry are very different from each other. They are two different ideas. Inorganic can be considered as a simpler alternative out of the two, but at the end of the day, both of these topics carry a good chunk of marks. So both of them are very important for your class 12 board examinations.
Do not skip them at any cost and make sure to go through the study material thoroughly.
5. Is NCERT exemplar enough for Class 12 School-Level Exams?
NCERT Exemplar is a brilliant resource to study from. When it comes to school-level examinations, students are advised to study their NCERT textbook first. Thoroughly and in detail, make sure that students are covering the content from NCERT first and only then go to other resources like NCERT Exemplar. Combine NCERT and NCERT exemplar, and you have a brilliant study strategy to score well.

























