Class 9 Science Chapter 2 Summary Notes PDF Download
In Chapter 2 of Class 9 Science, titled "Is Matter Around Us Pure?", you will explore the concept of purity in matter. This chapter delves into the different types of mixtures and pure substances, including solutions, suspensions, and colloids. It also covers the methods of separating mixtures and the importance of purity in various contexts. Our notes provide a comprehensive overview of these concepts with clear explanations, examples, and practice questions to help you understand and apply the principles of purity in matter. Download the FREE PDF to access all the essential information and tools you need to excel in this chapter and enhance your scientific knowledge. Visit the CBSE Class 9 Science Revision Notes and CBSE Class 9 Science Syllabus pages for more resources.
 Table of Content
Table of ContentCBSE Notes Class 9 Science Chapter 2 - Is Matter Around Us Pure - 2025-26
Access Revision Notes for Class 9 Science Chapter 2 Is Matter Around Us Pure?
Introduction:
The matter is made up of two or more components known as substances.
A substance is a sort of matter that cannot be divided into any other types of matter by physical means, according to science.
The term "pure substance" refers to a substance that has only one component and nothing else.
Substances are frequently mixed with one another, and the result is referred to as a mixture.
Pure and impure substances:
A pure material is one that has only one type of particle. Water, sulphur, hydrogen, carbon, and other pure substances (made up of only one type of particle) are known as pure substances since they can't be separated by any physical process. A pure substance has a constant composition, as well as a constant melting and boiling point.
Impure Substances: Impure substances are those that are made up of two or more types of particles (atoms or molecules) that may be separated using physical methods. All of the substances in the mixtures are impure. The salt solution, sugar solution, milk, seawater, air, sugarcane juice, soft beverages, sharbat, rocks, minerals, petroleum, LPG, biogas, tap water, tea, coffee, paint, wood, soil and bricks, are some examples of mixtures. The mixture can be homogeneous or heterogeneous. A mixture's composition, as well as its melting and boiling points, are not fixed.
Types of Pure Substances:
Pure substances are divided into two categories. These are elements and compounds respectively.
A pure substance's simplest or basic form, which cannot be broken down into anything simpler than it by physical or chemical techniques, is called an element.
Dalton's later research revealed that atoms are the simplest form of matter. It can now be defined as a pure substance made up of only one type of atom. Hydrogen, carbon, oxygen, and other elements are examples.
Solids, liquids, and gases are all examples of elements. At room temperature, sodium and carbon elements, for example, are solids, mercury and bromine elements are liquids, and hydrogen and oxygen elements are gases. In reality, solids make up the vast majority of the elements. Elements are further divided into three types:
Metals
Non-metals
Metalloids
Metals:
A metal is a malleable and ductile element that conducts electricity. Metals include Iron, Copper, Aluminium, Zinc, Silver, Gold, Platinum, Chromium, Sodium, Potassium, and Magnesium, to name a few.
Non-Metals:
Non-metals, as their name implies, are diametrically opposed to metals, implying that their properties are vastly different. They are relatively few in number, but they are vital to the survival of living organisms. Non-metals make up just approximately fourteen to fifteen percent of the elements in the periodic table. Carbon, Sulphur, Phosphorous, Hydrogen, and Oxygen are only a few examples.
Comparison among the Properties of Metals and Non-Metals:
Metals | Non-Metals |
|
|
|
|
|
|
|
|
|
|
|
|
Metalloids:
There are a few elements that have properties that are similar to both metals and non-metals. Metalloids are elements that exist on the edge of existence. Boron (B), Silicon (Si), Germanium (Ge), Arsenic (As), Antimony (Sb), Bismuth (Bi), Tellurium (Te), and Polonium are some examples of metalloids (Po).
Illustration – 1:
Give two reasons why you believe copper is metal and sulphur is not.
Solution: The following are the two qualities that indicate that copper is metal and sulphur is a non-metal:
Copper
Copper is ductile and malleable. It can be pulled into wires and pounded into thin sheets.
Copper is a good heat and electrical conductor.
Sulphur
Sulphur is neither ductile nor malleable. It's fragile. When hammered or strained, sulphur fractures into fragments.
Sulphur is a poor heat and electrical conductor.
Types of Mixture:
Compounds:
It's also a pure substance, similar to the elements. It does, however, indicate a chemically integrated mixture of two or more elements.
“A pure substance containing two or more elements mixed in a predetermined mass proportion”
Example:\[\text{H}{{\text{O}}_{\text{2}}}\](water), \[\text{C}{{\text{O}}_{\text{2}}}\](Carbondioxide), \[\text{N}{{\text{H}}_{\text{3}}}\](Ammonia) etc.
Compound Types: The compounds have been divided into two groups. These are the following:
Inorganic compounds: These compounds are usually made up of non-living materials like rocks and minerals. Common salt, marble, washing soda, baking soda, carbon dioxide, ammonia, sulphuric acid, and other inorganic compounds are examples.
Organic compounds: The term "organ" refers to several organs found in living organisms. As a result, organic chemicals are compounds derived from living organisms, such as plants and animals. It has been discovered that carbon is a fundamental component of all organic molecules. As a result, organic substances are frequently referred to as "carbon compounds."
Compound Characteristics: The following are key compound characteristics:
A pure compound is made up of the same constituents.
The properties of a pure compound are completely different from the properties of the element from which it is created.
Because a compound is generated by a chemical process, it has qualities that are distinct from the elements from which it is formed. Hydrogen gas, for example, is combustible, but oxygen is a supporter of combustion. The chemical reaction between the two gases results in the formation of water. It isn't combustible and doesn't enable burning.
It puts an end to or extinguishes combustion. We frequently use water to put out fires.
Chemical compounds' constituents cannot be separated mechanically. Compound formation necessitates energy exchange.
Because of the following factors, water has been termed a compound: Physical methods cannot separate water into its constituent’s hydrogen and oxygen.
Water's properties are vastly different from those of its constituents, hydrogen and oxygen. Hydrogen is flammable, but oxygen promotes combustion. Water differs from the other two in that it extinguishes fire.
When hydrogen and oxygen are burned to make water, heat and light are released. The chemical make-up of water is constant.
The components hydrogen and oxygen are present in a \[1:8\] mass ratio.
Under atmospheric pressure of 1 atmosphere, water has a stable boiling point of \[\text{100 }\!\!{}^\circ\!\!\text{ C}\] (or \[\text{373 K}\]) (or \[\text{760 mm}\]).
Mixture:
“A mixture is a combination of two or more substances (elements or compounds) that are not chemically combined but may also be present in any proportion.” There are two different kinds of mixtures:
Homogeneous mixture
Heterogeneous mixture
Compounds and elements are pure substances. In scientific terminology, mixtures are not pure substances.
Homogeneous mixture:
“When diverse ingredients or substances in a combination exist in one single phase with no obvious borders of separation, it is said to be homogenous. The composition of a homogenous mixture is consistent throughout.” Here are a few instances of homogenous mixtures:
Because the dissolved salt is evenly distributed all through the saltwater sample, it is a homogenous combination. All solutions are termed homogeneous since the dissolved component is present throughout the solution in the same quantity.
Air is also a mixture of gases such as nitrogen, oxygen, carbon dioxide, water vapours, inert gases, and others. All of the gases in the air combine to form a single-phase, the gaseous phase. Air can also be considered a solution.
The term "solution" refers to any homogenous mixture.
Heterogeneous Mixtures:
“If a combination does not have a homogeneous composition and visible borders of separation between parts, it is said to be heterogeneous.”
Here are some instances of heterogeneous mixtures:
A heterogeneous mixture is one that consists of sand and common salt. These are undoubtedly present in the same phase, namely the solid phase, yet they have distinct separation boundaries. The sand and ordinary salt particles are plainly visible in the combination.
Oil and water, too, produce a heterogeneous mixture. Both constituents are liquids, but their separation boundaries are different.
Oil and water can be found in different layers.
The distinction between compounds and a mixture:
Compounds | Mixtures |
A compound is made up of two or more components that have been chemically joined. | Two or more elements or compounds are merely blended in the mixture rather than chemically combined. |
The elements of a compound are present in a set mass ratio. This proportion will not change. | The ingredients of a mixture are present in a predetermined ratio. It can vary. |
Compounds are always homogeneous, meaning that their makeup is the same throughout. | In nature, mixtures can be either homogeneous or heterogeneous. |
The elements of a compound lose their identities, i.e., the constituting element's features are not visible in the compound. | The constituents of a mixture lose their identities, i.e., a mixture displays all of the constituents' qualities. In the development of a mixture, no energy change is observed. |
The physical separation of parts in a compound is impossible. | Physical means can easily separate the parts of a combination. |
Illustration – 2:
Explain why air is a mixture rather than a compound.
Solution: Because of the following factors, the air is considered a mixture:
By using a physical process or fractional distillation, air can be divided into its constituents such as oxygen, nitrogen, and other gases (or liquid air).
The properties of all the gases present in the air can be seen. For example, oxygen and air both enable burning; carbon dioxide turns lime-water milky, and the air turns lime-water milky as well, though at a far slower rate.
When air is generated by mixing the proper proportions of oxygen, nitrogen, carbon dioxide, argon, water vapour, and other gases, heat and light are neither given out nor absorbed.
Because different parts of the world have varying concentrations of various gases, the air has a variable makeup. There isn't a set formula for it.
There is no fixed boiling point for liquid air.
Sort the following items into element, compound, and mixture categories: Sodium, Soil, Sugar Solution, Silver, Calcium Carbonate, Tin Silicon, Coal, Air, Soap, Methane, Carbon Dioxide, Blood
Solution: The following is how we can categorise the provided materials into elements, compounds, and mixtures:
Sodium, Silver, Tin, and Silicon are some of the elements.
Calcium carbonate, soap, methane, and carbon dioxide are examples of compounds.
Soil, Sugar Solution, Coal, Air, and Blood are examples of mixtures.
List the elements included in the following compounds and their names:
Quicklime (a), hydrogen bromide (b), baking soda (c), and potassium sulphate (d)
Solution:
Calcium oxide (CaO) is quicklime. Calcium (Ca) and oxygen (\[{{\text{O}}_{\text{2}}}\]) are two of the elements found in it (O).
HBr stands for hydrogen bromide. Hydrogen (H) and Bromine (Br) are the elements present (Br).
Sodium hydrogencarbonate, or \[\text{NaHC}{{\text{O}}_{\text{3}}}\], is the chemical formula for baking soda. It contains the elements sodium (Na), hydrogen (H), carbon (C), and oxygen (O).
\[{{\text{K}}_{\text{2}}}\text{S}{{\text{O}}_{\text{4}}}\] is the chemical formula for potassium sulphate. It contains the elements potassium (K), sulphur (S), and oxygen (O).
Solutions, Suspensions and Colloids:
The instance of Solution Solute: A solute is a substance that is dissolved to form a solution, such as salt or sugar.
A solute's solubility is defined as its ability to dissolve in water. “A solute's solubility is defined as the greatest amount of solute that may be dissolved in 100 gm of solvent to form a saturated solution at a particular temperature.”
It is directly proportional to temperature.
$\text{Solubility = }\dfrac{\text{mass of solute}}{\text{mass of solvent}}\times 100$
On the basis of the solubility of the solute, solutions can be further split into two categories - Saturated and Unsaturated Solutions.
A solution is considered to be saturated if it contains the maximum amount of the solute dissolved in it at a given temperature and no more solute can be dissolved.
At a given temperature, a solution is said to be unsaturated if more solute can be dissolved in it.
Suspensions:
Are made up of compounds that are insoluble in water. “A heterogeneous mixture is one in which minute solid particles are dispersed throughout a liquid without dissolving in it.” Examples include chalk water, muddy water, milk of magnesia, and fluorine water.
Suspension Characteristics
A heterogeneous suspension is made up of two phases. One phase is made up of solid particles, while the other is made up of the liquid in which they are suspended or spread.
A suspension's particle size is greater than 100 microns (or \[{{10}^{-7}}\]m).
A suspension's particles can be observed with the naked eye as well as under a microscope.
Ordinary filter sheets may easily separate the solid particles present in the suspension. For this reason, no specific filter sheets are required.
Suspension particles are inherently unstable. When the suspension is not disrupted, they settle down after a while. This is referred to as precipitate.
It's worth noting that the terms suspension and precipitate are interchangeable. Suspension is represented by the solid particles in their suspended state. When they settle, they form a precipitate.
Colloids:
A colloid is a type of solution in which the size of the solute partials lies somewhere between real solutions and suspension. Colloidal solutions, like suspensions, are heterogeneous in nature, but the particles are smaller and more evenly dispersed. It is in the range of 1 nm to 100 nm, i.e., between real solution and suspension particle sizes. Because the particle sizes are so similar to what we see in solutions, most colloidal solutions appear to be homogeneous, just like genuine solutions. However, this is not the case.
In everyday life, we come across a wide range of colloidal solutions. Typical examples include smoke from factory chimneys, toothpaste, ink, blood, soap solutions, jellies, and starch solution in water.
Colloidal solutions are heterogeneous mixtures, as we already established. This signifies that the constituents aren't all present at the same time. In a colloidal solution, there are actually two phases. Dispersed phase and dispersion medium are the terms for these.
Characteristic of Colloidal Solutions:
Colloidal solutions appear to be homogeneous but are actually heterogeneous in nature.
This is due to the particle size (1 nm to 100 nm) in a colloidal solution, which is relatively near to the particle size in suspension. However, under a microscope, these can be seen.
Colloidal solutions are a two-phase system
We've already established that colloidal solutions are a two-phase system. Dispersed phase and dispersion medium are these terms. Because of this, colloidal solutions are diverse in character.
Colloidal particles pass through ordinary filter papers
Colloidal solutions flow through regular filter sheets like real solutions in the vast majority of circumstances. This is due to the dispersed phase's or colloidal particles' tiny size. To remove these particles from the dispersion media, special filter sheets called ultra-filter papers must be utilised.
Colloidal particles carry a charge
The dispersed phase particles in a colloidal solution remain scattered or suspended, as we have learned. They do not approach close to one other as they would if they were suspended. The presence of a charge (positive or negative) on these particles causes this. Please keep in mind that all of the particles in a colloidal solution have the same charge.
As a result, these particles with identical charges repel one other and remain dispersed or suspended. Haemoglobin, starch, gelatin, and metals such as copper, silver, gold, and metal sulphides, for example, contain negative charges on their particles.
Metal hydroxides, such as iron, aluminium, calcium, and others, have a positive charge on their particles.
Particles in a colloidal solution follow a zigzag path
Because of their small size, colloidal particles are generally invisible. Their route, though, can be observed under a microscope. These particles travel in a zigzag pattern. This motion can be observed while viewing a movie in a theatre. Dust particles are present in the beam of light that falls on the screen from behind. They walk in a zigzag pattern.
In 1828, Robert Brown, an English physicist, was the first to detect such colloidal particle movement. Brownian motion is the name for this type of motion.
Tyndall effect: Scattering of light by colloidal particles:
The particles in a colloidal solution are large enough to scatter light. This can be demonstrated as follows. When a light beam is focused on a colloidal solution (for example, soap solution) in a dark environment, the path of the light beam is lighted and visible when viewed from the side. Because colloidal particles are large enough to scatter light falling on them in all directions, the path of the light beam becomes visible. We can perceive the course of the light beam because of the scattered light that enters our eyes.
Property | Suspension | Colloidal solution | True solution |
| > 100 nm | 1 to 100 nm | < 1 nm |
| Possible | Not possible | Not possible |
| Settle of their own | Settle only on centrifugation | Do not settle |
| Opaque | Generally transparent | Transparent |
| Shows | Shows | Does not show |
| Do not diffuse | Diffuse slowly | Diffuse rapidly |
| May show | Show | May or may not shown |
| Heterogeneous | Homogeneous |
To tell the difference between a colloid and a solution. The Tyndall effect can be used to distinguish between colloids (or colloidal solutions) and real solutions. A soap solution, for example, scatters a ray of light travelling through it, making its path visible; thus, soap solution is a colloid (or colloidal solution). A beam of light travelling through the salt solution, on the other hand, is not scattered.
Classification of Colloids:
Colloids are classified according to the physical state of the dispersed phase (solute) and the dispersion medium (solvent). These are
Sol.
Solid sol.
Aerosol
Emulsion
Foam
Solid foam
Gel
Technical name of colloid | Dispersed phase | Dispersion medium | Examples |
| Solid | Liquid | Ink, soap solution, starch solution, most paints |
| Solid | Solid | Coloured gemstone (kike ruby glass) |
| (i) solid (ii) liquid | Gas Gas | Smoke, automobile exhausts hairspray, fog, mist, clouds |
| Liquid | Liquid | Milk, butter, face cream |
| Gas | Liquid | Fire-extinguisher foam, soap bubbles, shaving cream, beer foam |
| Gas | Solid | Insulating foam, foam rubber, sponge |
| Solid | Liquid | Jellies, gelatin |
Illustration – 3:
A solution contains 30 g of sugar dissolved in 370 g of water. Calculate the concentration of this solution.
Solution: We know that concentration of solution $=\dfrac{\text{Mass of solute}}{\text{Mass of solution}}\times 100$
Here, mass of solute (sugar) = 30 g and the mass of solvent (water) = 370 g
So, Mass of solution $=$ Mass of solute $+$ Mass of solvent \[=\text{ }30\text{ }+\text{ }370\text{ }=~400\]g
Now, putting the values of ‘mass of solute’ and ‘mass of solution’ in the above formula, we get:
Concentration of solution $=\dfrac{30}{400}\times 100=\dfrac{30}{4}=7.5%$
If 110 g of salt is present in 550 g of solution, calculate the concentration of solution.
Solution: Here, Mass of solute (salt) \[=\text{ }110\] g and, Mass of solution \[=\text{ }550\] g
Now, we know that; concentration of solution $=\dfrac{\text{Mass of solute}}{\text{Mass of solution}}\times 100$
$=\dfrac{110}{550}\times 100=20%$
If 2 mL of acetone is present in 45 mL of its aqueous solution calculate the concentration of this solution.
Solution: Here, volume of solute (acetone) \[=\text{ }2\] mL and, volume of solution \[=\text{ }45\] mL
Now, we know that: concentration of solution $=\dfrac{\text{Mass of solute}}{\text{Mass of solution}}\times 100$
$=\dfrac{2}{45}\times 100=4.4%$
12 grams of potassium sulphate dissolves in 75 grams of water 60°C. What is its solubility in water at that temperature?
Solution: Here we have been given that 75 grams of water dissolves 12 grams of potassium sulphate. We have to find how much potassium sulphate will dissolve in 100 grams of water. Now, 75 g of water dissolves \[=\text{ }12\] g of potassium sulphate.
So, 100 g of water will dissolve $=\dfrac{12}{75}\times 100=16$ g of potassium sulphate. Thus, the solubility of potassium sulphate in water is 16 g at \[\text{60 }\!\!{}^\circ\!\!\text{ C}\].
Separation of mixture:
To tell the difference between a colloid and a solution. The tyndall effect can be used to distinguish between colloids (or colloidal solutions) and real solutions. A soap solution, for example, scatters a ray of light travelling through it, making its path visible; thus, soap solution is a colloid (or colloidal solution). A beam of light travelling through salt solution, on the other hand, is not scattered.
A commonly used process which is used to separate the constituents of the mixture are:
Sublimation
Filtration
Centrifugation
Evaporation
Crystallization
Chromatography
Distillation
Fractional Distillation
Separating funnel
We'll look at the following three scenarios to discover how to separate mixtures:
A combination of two solids
A solid and a liquid mixture
A combination of two liquids.
Separation of a two-solid combination:
The following procedure is used to separate a mixture of two substances.
Using a suitable solvent (a mixture of sugar and sand)
Using the sublimation process (ammonium chloride and common salt)
Using a magnet (mixture of iron filling and sulphur power)
Separation of a mixture of solid and a liquid
By filtration
By centrifugation
By evaporation
By crystallization
By chromatography
By distillation
To Separate Cream From Milk
Centrifugation: It is a method for separating suspended particles of a substance from a liquid in which the mixture (or sperm) is rotated at a high speed in a centrifuge, forcing denser particles to the bottom and lighter particles to the top layer.
By the process of chromatography:
Water serves as the solvent in our ink, and the dye is soluble in it. The dye particles are carried away by the rising water on the filter paper. A dye is usually a blend of two or more colours. The colour component that is more soluble in the water rises faster, and the colours segregate as a result.
Chromatography is the technique of separating the components of a mixture. The Greek word chroma means "colour." Chromatography is a technique for separating those solutes that dissolve in the same solvent. It was first used to separate colours, hence the name.
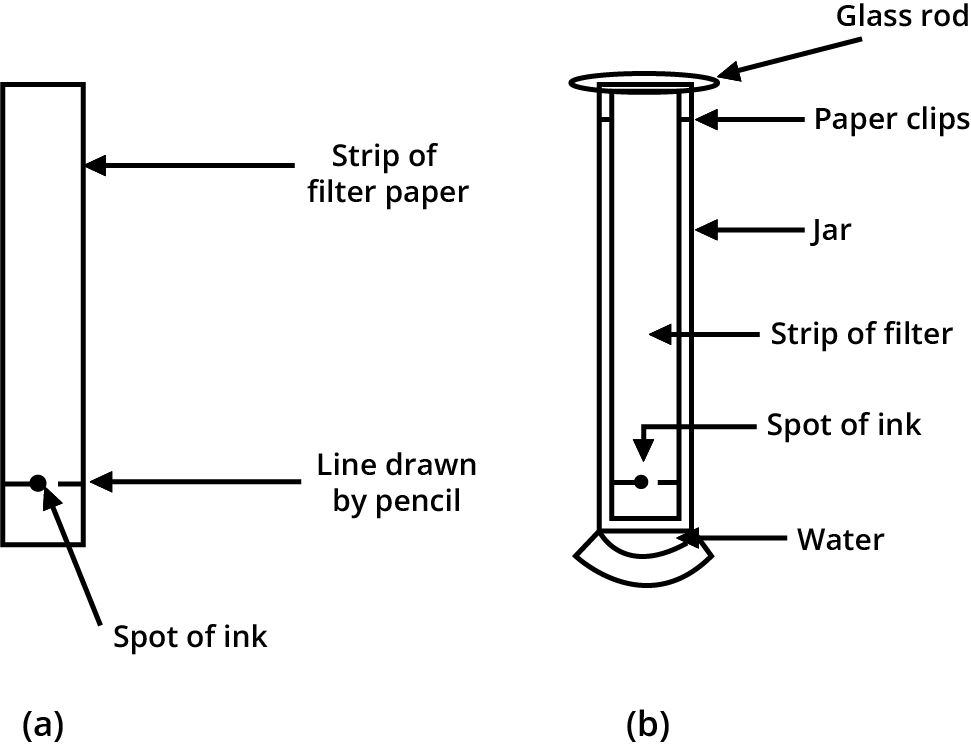
Separation of dyes in black ink using chromatography:
The two methods are used to separate a combination of two liquids: miscible liquid (which mixes together in all proportions and forms a single layer) and immiscible liquid (which does not mix with each other and forms distinct layers).
By the fractional distillation (for miscible liquid):
Fractional distillation is used to separate a combination of two or more miscible liquids with a difference in boiling points of less than 25 K, such as for the separation of different gases from air, distinct factions from petroleum products, and so on.
The apparatus is similar to that used for simple distillation, except that between the distillation flask and the condenser is a fractionating column.
A simple fractionating column is a glass bead-filled tube. The beads serve as a surface for the vapour to cool and condense on multiple occasions.
Separation of the Gases of the Air
Nitrogen, oxygen, argon, carbon dioxide, helium, neon, krypton, and xenon, among other gases, make up air. Fractional distillation of liquid air separates the various gases of air from one another. This distinction is made due to the fact that the boiling points of the various gases in the atmosphere differ (when in liquid form).
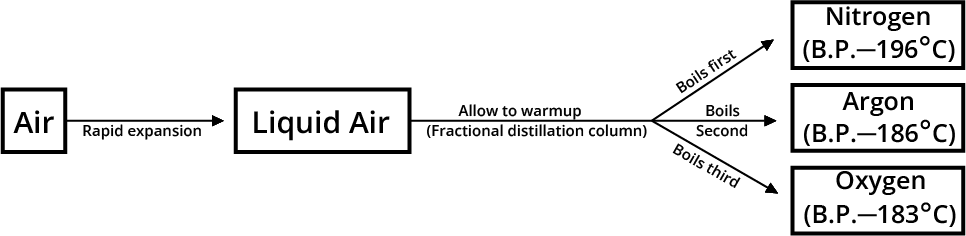
Illustration – 4: A mixture of sand, water, and mustard oil is administered to you. What method will you use to separate the various components of this mixture?
Solution: There are three ingredients in this mixture: sand, water, and mustard oil. Sand is now a solid that is insoluble in both water and mustard oil. Mustard oil and water are incompatible liquids.
The sand, water, and mustard oil combination is filtered. As a residue, sand is left on the filter paper. The filtrate is made up of water and mustard oil.
A separating funnel is used to collect the filtrate, which contains both water and mustard oil. In a separating funnel, the lower layer is water, and the upper layer is mustard oil. The bottom layer of water is removed first by opening the separating funnel's stop-cock. Mustard oil stays in the separating funnel and can be extracted individually.
Key Points of Is Matter Around Us Pure Class 9 Notes CBSE Science Chapter 2 Notes
The mixture consists of two or more pure substances that can be isolated by any physical method.
There are Two Types of Mixtures
1. Homogenous Mixtures: When all the components present in a mixture is mixed well and there are no boundaries of separation between any of them, then it is called a homogenous mixture. For example, sugar in water.
2. Heterogeneous Mixture: A mixture is heterogeneous when all the components in the mixture are not mixed in a fixed ratio and there appears visible boundaries of separation in them. For example, sand in water.
Class 9 Science Ch 2 Notes: Solution And Its Properties
Chapter 2 Class 9 Science notes also tell us that a solution is a homogeneous mixture of more than one substance. For example, soda water, lemonade, etc.
Any solution has two components namely solvent and solute.
1. Solvent - The component of any solution in which the solute particles are dissolved is called a solvent.
2. Solute - The component of any solution that is dissolved in the solvent is called a solute.
Science Class 9 Chapter 2 Notes: Points to Remember
A solution is always said to be a homogeneous mixture.
The particle of any solution is smaller than 1nm in its diameter and it is not visible through the naked eye.
It is not capable of scattering a beam of light that passes through it. Hence the path is not visible in a solution.
Solute particles are impossible to separate from the mixture through filtration.
A solution is very stable and the solute particles in a solution can not settle when they are left undisturbed.
Class 9 is Matter Around Us Pure Notes: Types of Solution & Concentration
The amount of solute present in the given solution is called the concentration of the solution. The amount of solute that can dissolve in a given volume or mass of solvent is known as the concentration of the solution.
a) Suspension and Its Properties
The suspension is any heterogeneous mixture in which any solute particle cannot dissolve but they remain suspended in a bulk of the specific medium. Example: Chalk mixed in water, smoke released in the air.
Properties:
It is a heterogeneous mixture.
Particles are visible to the naked eye.
Size is greater than 100nm.
Solvent and solute can be separated if any solution is passed through it.
b) Colloidal Solution and Its Properties
A colloidal solution is a heterogeneous mixture where the particle’s size lies in between the suspensions and the true solutions. They can scatter a beam of visible light very easily. This is called the Tyndall effect.
Properties:
Particles are not visible to naked eyes.
Solvent and solutions cannot be separated by filtration.
The size of the particles is smaller than 100Nm.
A stable mixture.
Particles don’t settle down when they are left undisturbed.
Class 9th Science Chapter 2 Notes: Separations of the components of mixtures
Several methods are used for the separation to get a specific component from any mixture. Heterogeneous mixtures are capable of separating by using physical methods such as sieving, handpicking, filtration, etc.
Physical and Chemical Changes
Any process that brings in a change in the physical property but doesn’t produce any new substance is known as a physical change. Common physical changes are changes in rigidity, colour, density, fluidity, boiling point.
The process by which a new chemical substance is formed is called chemical changes. Some of the chemical properties are inflammability and odour.
Class 9 Ch 2 Science notes cover explanations of physical an chemical changes. It states that a pure substance can be an element or a compound. Element is a matter that cannot be broken into simpler substances. The compound is any substance that is composed of more than two elements by combining them.
Is Matter Around Us Pure Class 9 Notes CBSE Science Chapter 2 - Extra Questions and Answers for Practice
What is a homogenous mixture?
Ans. A homogenous mixture is a composition that remains uniform throughout the mixture.
Name the three alloys which are commonly used.
Ans. Three alloys that are commonly used are as follows:
Stainless steel
Brass
Bronze
What is the meaning of ‘Dispersed phase medium’?
Ans. The phase which is dispersed or is present in colloidal particle form is called ‘Dispersed phase medium’
Important Topics of Class 9 Science Chapter 2 Is Matter Around Us Pure? you shouldn’t Miss!
These topics provide a thorough understanding of the purity of matter and how mixtures can be analysed and separated.
Pure Substances: Understanding what constitutes a pure substance, including elements and compounds, and their characteristics.
Mixtures: Learning about different types of mixtures—homogeneous and heterogeneous—and their properties. Examples include solutions, suspensions, and colloids.
Separation Techniques: Familiarizing yourself with methods to separate mixtures, such as filtration, distillation, and chromatography, and their practical applications.
Solutions: Studying the components of solutions (solvent and solute), concentration, and the process of dissolution.
Colloids and Suspensions: Differentiating between colloids and suspensions, understanding their properties, and recognizing examples in everyday life.
Importance of Science Chapter 2 Is Matter Around Us Pure? Class 9 Notes
Class 9 Chemistry Chapter 2 Notes help you with essential knowledge about the nature of matter, enhancing your understanding of chemistry and its applications.
Foundation for Chemistry: This chapter introduces fundamental concepts about the purity of substances, which are essential for understanding more advanced topics in chemistry.
Understanding Mixtures and Pure Substances: It helps you differentiate between pure substances and mixtures, understanding their properties and behaviours in various contexts.
Practical Applications: Knowledge of separation techniques is crucial for real-life applications such as purifying drinking water, separating components in laboratories, and various industrial processes.
Conceptual Clarity: The chapter provides a clear understanding of how solutions, colloids, and suspensions differ, which is key for solving problems and conducting experiments.
Scientific Inquiry: Learning about purity and separation methods encourages critical thinking and practical problem-solving skills, which are valuable for scientific experiments and everyday life.
Tips for Learning the Class 9 Science Chapter 2 Is Matter Around Us Pure?
Understand Key Definitions: Make sure you clearly understand the definitions of pure substances, mixtures, solutions, suspensions, and colloids. Knowing these terms will help you grasp the concepts more effectively.
Use Visual Aids: Create diagrams or charts to visualise different types of mixtures and their properties. Visual aids can help you better understand and remember the distinctions between them.
Practice Separation Techniques: Familiarize yourself with practical methods like filtration, distillation, and chromatography. Try simple experiments at home or in the lab to see these techniques in action.
Relate to Real-Life Examples: Connect the concepts to everyday examples, such as how tea is brewed (solution), how sand can be separated from water (suspension), or how milk is an example of a colloid.
Solve Practice Problems: Work on problems related to mixtures and separation techniques to reinforce your understanding and application of the concepts.
Conclusion
Chapter 2 of Class 9 Science, "Is Matter Around Us Pure?", provides essential insights into the nature of matter and the methods used to separate mixtures. By understanding the distinctions between pure substances and various types of mixtures, and mastering separation techniques, you gain a solid foundation in chemistry. Applying real-life examples and practising key concepts will enhance your comprehension and problem-solving skills. Use these notes to reinforce your learning and prepare effectively for exams, ensuring a thorough grasp of the purity of matter and its practical implications.
Related Study Materials for Class 9 Science Chapter 2 Is Matter Around Us Pure?
Students can also download additional study materials provided by Vedantu for Class 9 Science Chapter 2 Is Matter Around Us Pure?
S. No | Is Matter Around Us Pure? Related Study Materials |
1 | Class 9 Science Is Matter Around Us Pure? Important Questions |
2 |
Chapter-wise Revision Notes Links for Class 9 Science
S. No | Links for Chapter-wise Class 9th Science Notes FREE PDF Download |
1 | |
2 | |
3 | |
4 | |
5 | |
6 | |
7 | |
8 | |
9 | |
10 | |
11 |
Important Study Materials for Class 9 Science
S. No | Related Study Materials Links for Class 9 Science |
1. | |
2. | |
3. | |
4. |
FAQs on CBSE Notes Class 9 Science Chapter 2 - Is Matter Around Us Pure - 2025-26
1. What is the best way to use these revision notes for Chapter 2, "Is Matter Around Us Pure"?
Start by quickly scanning the key topics like pure substances, mixtures, and separation techniques. Use these notes to recap the main definitions and then focus on the diagrams and examples provided to strengthen your understanding before an exam.
2. What key concepts are summarised in the Class 9 Science Chapter 2 notes?
These notes summarise core concepts, including the differences between elements, compounds, and mixtures. They also provide a quick overview of solutions, suspensions, and colloids, along with essential separation methods like evaporation and chromatography.
3. How do these notes help in remembering the difference between homogeneous and heterogeneous mixtures?
The notes provide a clear, side-by-side summary. They highlight key differences in composition and appearance, using simple examples like salt in water (homogeneous) and sand in water (heterogeneous) to make the concepts easy to recall during revision.
4. Do these revision notes cover the main separation techniques from Chapter 2?
Yes, the notes offer a quick review of all major separation techniques from the NCERT syllabus. This includes:
- Evaporation
- Centrifugation
- Sublimation
- Chromatography
- Distillation and Fractional Distillation
5. While revising, what is the most common point of confusion between compounds and mixtures?
A common point of confusion is that both involve combining two or more substances. During revision, focus on this key difference: a compound is a new substance with a fixed chemical composition, while a mixture contains components that are not chemically bonded and have no fixed ratio.
6. Why is it important to revise the properties of solutions, suspensions, and colloids together?
Revising them together helps you effectively compare and contrast their key properties, such as particle size, stability, and visibility. This comparative approach is very useful for answering application-based questions, which are common in exams.
7. How can I use these notes to quickly revise the applications of different separation techniques?
For a quick revision, mentally connect each technique to a real-world application mentioned in the notes. For example, associate centrifugation with separating cream from milk, and chromatography with separating colours in ink. This makes the concepts easier to remember.
8. Besides definitions, what should I focus on in these notes for a last-minute revision of "Is Matter Around Us Pure"?
For a last-minute revision, focus on the summary tables and diagrams. Pay close attention to the table comparing the properties of solutions, suspensions, and colloids. This visual, example-based approach helps in quickly recalling the chapter's most important ideas.




















 Watch Video
Watch Video















