
Write the IUPAC name of the following compounds.
a) Iso-butane
b) Ethyl methyl ketone
Answer
591.6k+ views
Hint: Start by labelling the parent alkane so that carbons having substituents will get lower numbers. Remember that iso-butane is a structural isomer of butane. As far as ketones are concerned, the suffix of functional group ketones is -one.
Complete step by step answer:
Let us go through each of these cases one at a time.
- Butane, or \[{{C}_{4}}{{H}_{10}}\], has two structural isomers called normal butane, and isobutane. According to IUPAC nomenclature, these isomers are called simply butane and 2-methylpropane. As you know, isomers are molecules that have the same molecular formula but different chemical structures.
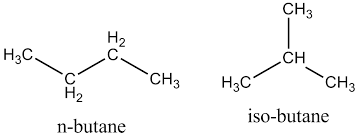
- Here, while naming iso-butane, notice that the parent chain can only be 3 carbons long. Thus, we can say that our IUPAC name will end with propane. Notice that no matter how we number the chain, the methyl substituent will be on carbon number 2. Now, to name the compound, mention the position of the substituent, then the name of the substituent, and finally the name of the parent alkane.
Hence, the IUPAC name of iso-butane will be 2-methylpropane
Now, let us come to the case of ethyl methyl ketone.
- Ethyl methyl ketone is also known as butan-2-one. It is colourless transparent liquid, with a low boiling point. It is famous for its outstanding dissolving capacity and dry characteristic. Ethyl methyl ketone can dissolve in water and ethyl alcohol, ether, benzene, toluene and other organic solvents.
- Let us now analyse the structure of Ethyl methyl ketone.
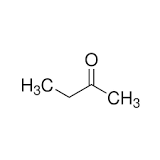
Following rules of IUPAC nomenclature:
Prefix – Number of carbons on the longest carbon chain is 4. Therefore, the prefix for this compound is butan-.
Suffix- The functional group in this compound is the carbonyl unit sandwiched between two alkyl groups, making this a ketone group. Therefore, the suffix of this compound is -one.
Numbering of functional groups – The functional group’s lowest position on the carbon chain is 2. Therefore, the numbering of functional groups results in -2-.
Now, to formulate the IUPAC name, write the prefix for the parent alkane, then mention the position at which the functional group is placed and then the suffix for the functional group.
With all these components in mind, we conclude that the IUPAC name of this compound is butan-2-one.
Note: While labelling the parent alkane make sure that all the functional groups and substituents involved have the lowest possible number and the chain is the longest that can be found on the molecule without interruptions by non-carbon atoms. Write the position of the functional group or substituent just before their respective suffixes or prefixes. As we can see in this problem, the place where we write the position of the substituent differs.
Complete step by step answer:
Let us go through each of these cases one at a time.
- Butane, or \[{{C}_{4}}{{H}_{10}}\], has two structural isomers called normal butane, and isobutane. According to IUPAC nomenclature, these isomers are called simply butane and 2-methylpropane. As you know, isomers are molecules that have the same molecular formula but different chemical structures.

- Here, while naming iso-butane, notice that the parent chain can only be 3 carbons long. Thus, we can say that our IUPAC name will end with propane. Notice that no matter how we number the chain, the methyl substituent will be on carbon number 2. Now, to name the compound, mention the position of the substituent, then the name of the substituent, and finally the name of the parent alkane.
Hence, the IUPAC name of iso-butane will be 2-methylpropane
Now, let us come to the case of ethyl methyl ketone.
- Ethyl methyl ketone is also known as butan-2-one. It is colourless transparent liquid, with a low boiling point. It is famous for its outstanding dissolving capacity and dry characteristic. Ethyl methyl ketone can dissolve in water and ethyl alcohol, ether, benzene, toluene and other organic solvents.
- Let us now analyse the structure of Ethyl methyl ketone.

Following rules of IUPAC nomenclature:
Prefix – Number of carbons on the longest carbon chain is 4. Therefore, the prefix for this compound is butan-.
Suffix- The functional group in this compound is the carbonyl unit sandwiched between two alkyl groups, making this a ketone group. Therefore, the suffix of this compound is -one.
Numbering of functional groups – The functional group’s lowest position on the carbon chain is 2. Therefore, the numbering of functional groups results in -2-.
Now, to formulate the IUPAC name, write the prefix for the parent alkane, then mention the position at which the functional group is placed and then the suffix for the functional group.
With all these components in mind, we conclude that the IUPAC name of this compound is butan-2-one.
Note: While labelling the parent alkane make sure that all the functional groups and substituents involved have the lowest possible number and the chain is the longest that can be found on the molecule without interruptions by non-carbon atoms. Write the position of the functional group or substituent just before their respective suffixes or prefixes. As we can see in this problem, the place where we write the position of the substituent differs.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




