
Write names and chemical formulae of monomers used in preparing Buna-S
Answer
591.6k+ views
Hint: Buna-S is a synthetic rubber. It is made from two monomers. Here, one of the monomers contains four carbon chains with two conjugated double bonds and the other consists of a benzene ring which is bonded to a vinyl group.
Complete answer:
Styrene-butadiene rubber is a type of synthetic rubber and we commonly know it by the name Buna-S. It is abbreviated to Buna-S where ‘Bu’- is for butadiene, ‘na’- is for sodium or natrium and ‘S’ stands for styrene.
Buna-S is a synthetic replacement for natural rubber. As we can understand from the name itself that it is made from two monomers. A monomer is a unit which is repeated to form the whole polymer. Here, we use the butadiene and styrene as monomers to prepare Buna-S.
We mix these two monomers in a ratio of 1 parts butadiene and 3 parts styrene by the process of emulsion polymerisation in presence of a peroxide catalyst at 5 degree Celsius. We can write the reaction as-
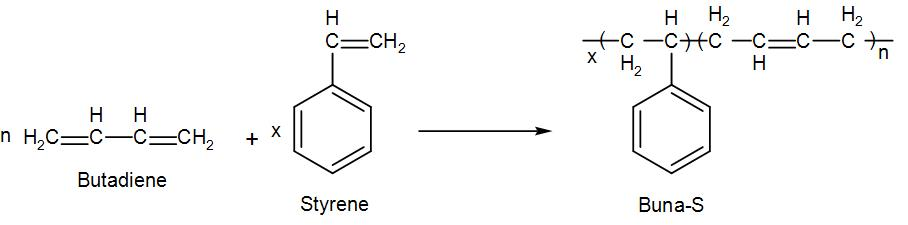
As we can see from the above reaction, the name of the monomers used for the preparation is butadiene and styrene. Chemical formula of butadiene is ${{C}_{4}}{{H}_{6}}$ and chemical formula of styrene is ${{C}_{8}}{{H}_{8}}$.
The prepared Buna-S from the above reaction is then vulcanized with sulphur which makes it different from the natural rubber. Vulcanizing rubber has better elasticity, low water absorption tendency and is resistant to attack by organic solvents and oxidizing agents unlike natural rubber.
Note:
Buna-S is also known as cold rubber because it is prepared at a low temperature of 5degree Celsius. We use Buns-S for the manufacture of motorcycle and scooter tyres and also cycle tyres and tubes. It is also used for the manufacture of conveyor belts, shoe soles, hoses and electrical insulation.
Complete answer:
Styrene-butadiene rubber is a type of synthetic rubber and we commonly know it by the name Buna-S. It is abbreviated to Buna-S where ‘Bu’- is for butadiene, ‘na’- is for sodium or natrium and ‘S’ stands for styrene.
Buna-S is a synthetic replacement for natural rubber. As we can understand from the name itself that it is made from two monomers. A monomer is a unit which is repeated to form the whole polymer. Here, we use the butadiene and styrene as monomers to prepare Buna-S.
We mix these two monomers in a ratio of 1 parts butadiene and 3 parts styrene by the process of emulsion polymerisation in presence of a peroxide catalyst at 5 degree Celsius. We can write the reaction as-

As we can see from the above reaction, the name of the monomers used for the preparation is butadiene and styrene. Chemical formula of butadiene is ${{C}_{4}}{{H}_{6}}$ and chemical formula of styrene is ${{C}_{8}}{{H}_{8}}$.
The prepared Buna-S from the above reaction is then vulcanized with sulphur which makes it different from the natural rubber. Vulcanizing rubber has better elasticity, low water absorption tendency and is resistant to attack by organic solvents and oxidizing agents unlike natural rubber.
Note:
Buna-S is also known as cold rubber because it is prepared at a low temperature of 5degree Celsius. We use Buns-S for the manufacture of motorcycle and scooter tyres and also cycle tyres and tubes. It is also used for the manufacture of conveyor belts, shoe soles, hoses and electrical insulation.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




