
Which of the following is true about \[KCl\] ?
A.It has fcc unit cell
B.It has bcc unit cell
C.It has sc unit cell
D.It has two allotropes one with fcc unit cell and other with bcc unit cell
Answer
591.9k+ views
Hint:
$KCl$ is the chemical formula for Potassium chloride which is commonly known as potassium salt. It is a salt, composed of potassium ($K$ ) metal and chlorine ($Cl$ ) halide and thus known as metal halide salt. It is white or colorless and odorless crystal. The crystals can dissolve readily in water, and its solutions have a salt-like taste.
Complete step by step answer:
-Crystallography is the branch of chemistry dealing with the science of determining the arrangement of atoms within crystalline solids. It was first discovered by Max von Laue for which he had been awarded the Nobel Prize in 1914.
-Depending on the shape of the crystal, crystals can be of seven major types. The seven types are cubic, hexagonal, tetragonal, orthorhombic, trigonal, monoclinic, and triclinic. For example, the cubic crystal system further consists of three different types of unit cells:
(1) Simple cubic or sc, (2) face-centered cubic or fcc, and (3) body-centered cubic or bcc.
-A unit cell is considered to be the smallest repeating unit in a crystal lattice. Unit cells occur in many different varieties. Crystal lattice can be defined as the arrangement of atoms, molecules, or ions of a crystal in the form of a space lattice. The unit cell completely reflects the symmetry and structure of the entire crystal, a crystalline solid. The crystalline solid can be built up by repetitive translation of the unit cell.
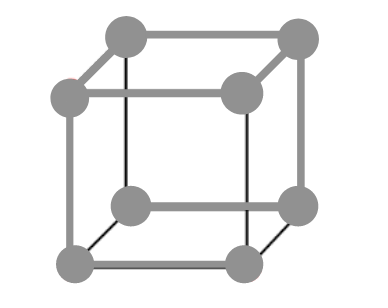
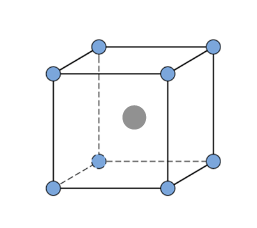
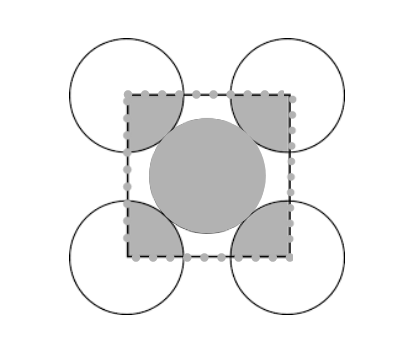
-Sodium chloride $NaCl$ has a cubic unit cell. It is considered as a face centered cubic, in which anions are arranged with an interpenetrating FCC cation lattice. The cell looks similar, no matter from where we start, with anions or cations on the corners. Each ion has a 6 coordinate and has local octane hedral geometry. The crystal structure of $KCl$ is the same as that of $NaCl$. It also adopts a face-centered cubic structure.
So, the correct option is A.
Note:
Potassium chloride $KCl$ is also known as potassium salt. It is used to treat hypokalemia. It is also used in cardiac surgery to stop the heartbeat, because cardiac surgery cannot be performed on beating heart.
$KCl$ is the chemical formula for Potassium chloride which is commonly known as potassium salt. It is a salt, composed of potassium ($K$ ) metal and chlorine ($Cl$ ) halide and thus known as metal halide salt. It is white or colorless and odorless crystal. The crystals can dissolve readily in water, and its solutions have a salt-like taste.
Complete step by step answer:
-Crystallography is the branch of chemistry dealing with the science of determining the arrangement of atoms within crystalline solids. It was first discovered by Max von Laue for which he had been awarded the Nobel Prize in 1914.
-Depending on the shape of the crystal, crystals can be of seven major types. The seven types are cubic, hexagonal, tetragonal, orthorhombic, trigonal, monoclinic, and triclinic. For example, the cubic crystal system further consists of three different types of unit cells:
(1) Simple cubic or sc, (2) face-centered cubic or fcc, and (3) body-centered cubic or bcc.
-A unit cell is considered to be the smallest repeating unit in a crystal lattice. Unit cells occur in many different varieties. Crystal lattice can be defined as the arrangement of atoms, molecules, or ions of a crystal in the form of a space lattice. The unit cell completely reflects the symmetry and structure of the entire crystal, a crystalline solid. The crystalline solid can be built up by repetitive translation of the unit cell.



-Sodium chloride $NaCl$ has a cubic unit cell. It is considered as a face centered cubic, in which anions are arranged with an interpenetrating FCC cation lattice. The cell looks similar, no matter from where we start, with anions or cations on the corners. Each ion has a 6 coordinate and has local octane hedral geometry. The crystal structure of $KCl$ is the same as that of $NaCl$. It also adopts a face-centered cubic structure.
So, the correct option is A.
Note:
Potassium chloride $KCl$ is also known as potassium salt. It is used to treat hypokalemia. It is also used in cardiac surgery to stop the heartbeat, because cardiac surgery cannot be performed on beating heart.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




