
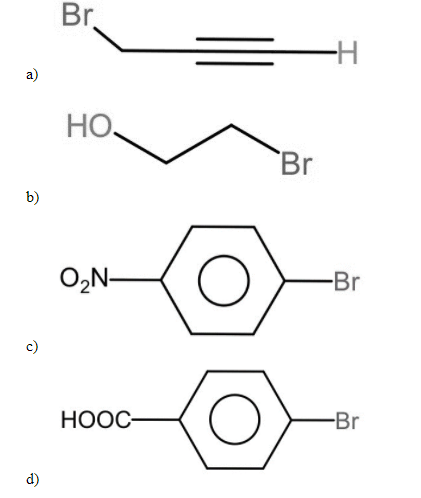
Which of the following halides does not form G.R when treated with magnesium in
the presence of ether?
This question has multiple correct options

Answer
596.1k+ views
Hint: The compounds formed by the reaction of an alkyl or aryl halide with magnesium are called Grignard reagents (G.R in short here). These are a part of an important named reaction called Grignard Reaction.
Complete answer:
Grignard reagents are named after Victor Grignard, who discovered them and gave their use as synthetic agents. In almost all the classes of organic compounds can be prepared from them. In the year 1912, Grignard finally earned one-half of a Nobel Prize for his revolutionary contribution to synthetic organic chemistry. The general reaction can be given as: \[\underset{Alkyl\,halide}{\mathop{R-X}}\,+Mg\xrightarrow{diethyl\,ether}\underset{Grignard\,\operatorname{Re}agent}{\mathop{R-Mg-X}}\,\]
where, R = alkyl or aryl group X = Cl, Br or I.
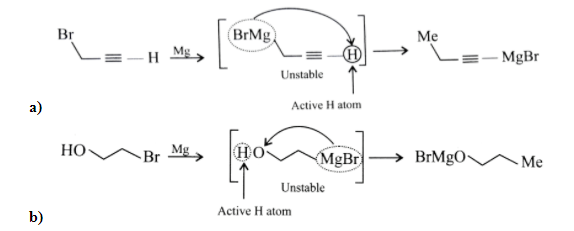
Since Grignard reagents are essentially the conjugate bases of alkanes, they’re also extremely strong bases, which means that sometimes in acid-base reactions they can compete with their nucleophilic addition reactions. As a result, it fails to give a Grignard reagent because the hydroxyl group protonates this reaction as soon as it is formed.
Now let’s see our options one by one:
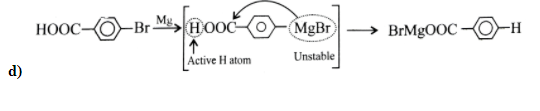
C) It is not possible to synthesize Grignard reagent from nitro chlorobenzene. A reaction occurs through the -nitro group, as it is an electron-withdrawing group and it oxidizes the Grignard reagent. Therefore, we can’t prepare Grignard reagent from nitro chlorobenzene.
Note: A suitable solvent must be used. Therefore, Grignard reagents are usually prepared in diethyl ether (\[C{{H}_{3}}C{{H}_{2}}OC{{H}_{2}}C{{H}_{3}}\]). The two ether oxygen atoms stabilize the reagent by making complex with reagent.
Complete answer:
Grignard reagents are named after Victor Grignard, who discovered them and gave their use as synthetic agents. In almost all the classes of organic compounds can be prepared from them. In the year 1912, Grignard finally earned one-half of a Nobel Prize for his revolutionary contribution to synthetic organic chemistry. The general reaction can be given as: \[\underset{Alkyl\,halide}{\mathop{R-X}}\,+Mg\xrightarrow{diethyl\,ether}\underset{Grignard\,\operatorname{Re}agent}{\mathop{R-Mg-X}}\,\]
where, R = alkyl or aryl group X = Cl, Br or I.
Since Grignard reagents are essentially the conjugate bases of alkanes, they’re also extremely strong bases, which means that sometimes in acid-base reactions they can compete with their nucleophilic addition reactions. As a result, it fails to give a Grignard reagent because the hydroxyl group protonates this reaction as soon as it is formed.
Now let’s see our options one by one:

C) It is not possible to synthesize Grignard reagent from nitro chlorobenzene. A reaction occurs through the -nitro group, as it is an electron-withdrawing group and it oxidizes the Grignard reagent. Therefore, we can’t prepare Grignard reagent from nitro chlorobenzene.

Note: A suitable solvent must be used. Therefore, Grignard reagents are usually prepared in diethyl ether (\[C{{H}_{3}}C{{H}_{2}}OC{{H}_{2}}C{{H}_{3}}\]). The two ether oxygen atoms stabilize the reagent by making complex with reagent.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




