
Which among the following solids is a nonpolar solid ?
A. Hydrogen chloride
B. Sulphur dioxide
C. Water
D. Carbon dioxide
Answer
601.5k+ views
Hint: Let's first understand what non polar solids are .Non polar solids or Non polar molecular solids are made up of weak dispersion forces and they are soft in nature. Their molecules are held by weak London forces or dispersion forces. In room temperature and pressure non polar solids mostly exist in gaseous or liquid state.
Complete step by step solution:
Non polar solids have evenly distribution of electric charge across the molecule .Some example of non polar solids are carbon dioxide ,benzene ,methane, diatomic gases \[({N_2},{O_2},C{l_2})\] .Now will understand about non polar nature of carbon dioxide ,as we know non polar compound have symmetry that means all of the sides around the centre are identical .Carbon dioxide is covalently bonded .We will understand this by Lewis structure of $C{O_2}$ as it has two oxygen atom and one carbon atom ,both oxygen have two lone pair of electrons but these electrons cancels effect of charges .We can see symmetry in the structure ,here carbon is the central atom and both sides around carbon atom is identical so it is a non polar molecule.
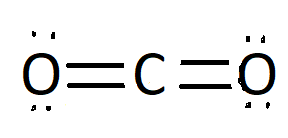
Lewis Structure of Carbon dioxide
Note: We should know the reason behind polarity of molecules. In the given option hydrogen chloride, sulphur dioxide, water are polar molecule as they don’t have symmetry around the central atom while carbon dioxide has a linear molecular geometry .The bond angle between carbon and and two oxygen atom is ${180^\circ }$. We have also considered carbon dioxide’s Lewis structure where we see equal distribution of charges.
Complete step by step solution:
Non polar solids have evenly distribution of electric charge across the molecule .Some example of non polar solids are carbon dioxide ,benzene ,methane, diatomic gases \[({N_2},{O_2},C{l_2})\] .Now will understand about non polar nature of carbon dioxide ,as we know non polar compound have symmetry that means all of the sides around the centre are identical .Carbon dioxide is covalently bonded .We will understand this by Lewis structure of $C{O_2}$ as it has two oxygen atom and one carbon atom ,both oxygen have two lone pair of electrons but these electrons cancels effect of charges .We can see symmetry in the structure ,here carbon is the central atom and both sides around carbon atom is identical so it is a non polar molecule.
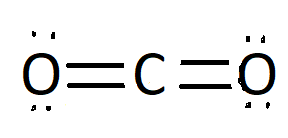
Lewis Structure of Carbon dioxide
Note: We should know the reason behind polarity of molecules. In the given option hydrogen chloride, sulphur dioxide, water are polar molecule as they don’t have symmetry around the central atom while carbon dioxide has a linear molecular geometry .The bond angle between carbon and and two oxygen atom is ${180^\circ }$. We have also considered carbon dioxide’s Lewis structure where we see equal distribution of charges.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




