
Unlike phenol, 2,4-dinitrophenol is soluble in sodium carbonate solution in water because?
A. presence of two - $N{{O}_{2}}$groups in the ring makes 2, 4 – dinitrophenol a stronger acid than phenol.
B. presence of two - $N{{O}_{2}}$groups in the ring makes 2, 4 – dinitrophenol a weaker acid than phenol.
C. presence of two - $N{{O}_{2}}$groups make the hydrogen bonding easier, making 2,4-dinitrophenol soluble.
D. nitro group reacts with $N{{a}_{2}}C{{O}_{3}}$while the -OH group does not.
Answer
591.9k+ views
Hint: To solve this question you should have a basic idea regarding the structures of phenol and 2,4-dinitrophenol and about electron withdrawing groups and its effect after adding to any compound. Phenol has a benzene ring with one hydroxyl group and 2,4-dinitrophenol has two nitrogen oxide molecules more than phenol.
Complete answer:
Structures of phenol and 2,4-dinitrophenol are drawn below.
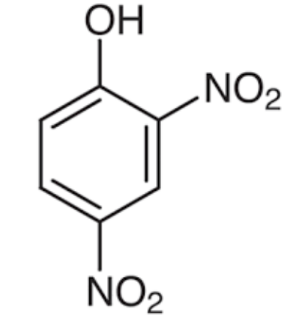
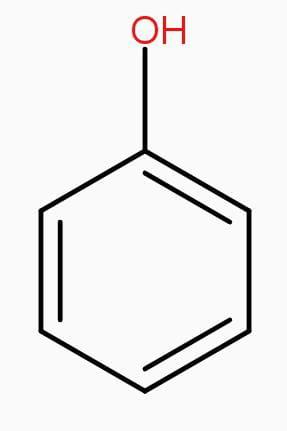
(2,4 – dinitrophenol) (phenol)
In 2,4 – dinitrophenol two - $N{{O}_{2}}$ groups are present. $N{{O}_{2}}$ has a strong electron withdrawing nature which increases acidity of the compound.
Electron withdrawing group (EWG): An atom or group that draws electron density from neighboring atoms towards itself, usually by resonance or inductive effects.
Therefore, presence of two electron withdrawing - $N{{O}_{2}}$groups in the ring makes 2,4- dinitrophenol a stronger acid than phenol.
Sodium Carbonate solution is basic in nature. Hence, Stronger the acid it is easily soluble in the basic solution.
Therefore, it reacts with $N{{a}_{2}}C{{O}_{3}}$solution to form sodium salt with the evolution of $C{{O}_{2}}$, thus making it soluble in$N{{a}_{2}}C{{O}_{3}}$.
Hence, unlike phenol, 2,4-dinitrophenol is soluble in sodium carbonate solution in water because presence of two - $N{{O}_{2}}$groups in the ring makes 2, 4 – dinitrophenol a stronger acid than phenol.
Hence, option A is the right answer.
Note:
Always remember, 2,4,6 – trinitrophenol > 2,4 – dinitrophenol > phenol, order of acidic nature of the compounds.
Orders of electron withdrawing group is -$N{{O}_{2}}$, -$CN$,-$CHO$,-$COR$,-$COOH$,-$COOR$, -$CON{{H}_{2}}$(strongest to weakest)
Complete answer:
Structures of phenol and 2,4-dinitrophenol are drawn below.


(2,4 – dinitrophenol) (phenol)
In 2,4 – dinitrophenol two - $N{{O}_{2}}$ groups are present. $N{{O}_{2}}$ has a strong electron withdrawing nature which increases acidity of the compound.
Electron withdrawing group (EWG): An atom or group that draws electron density from neighboring atoms towards itself, usually by resonance or inductive effects.
Therefore, presence of two electron withdrawing - $N{{O}_{2}}$groups in the ring makes 2,4- dinitrophenol a stronger acid than phenol.
Sodium Carbonate solution is basic in nature. Hence, Stronger the acid it is easily soluble in the basic solution.
Therefore, it reacts with $N{{a}_{2}}C{{O}_{3}}$solution to form sodium salt with the evolution of $C{{O}_{2}}$, thus making it soluble in$N{{a}_{2}}C{{O}_{3}}$.
Hence, unlike phenol, 2,4-dinitrophenol is soluble in sodium carbonate solution in water because presence of two - $N{{O}_{2}}$groups in the ring makes 2, 4 – dinitrophenol a stronger acid than phenol.
Hence, option A is the right answer.
Note:
Always remember, 2,4,6 – trinitrophenol > 2,4 – dinitrophenol > phenol, order of acidic nature of the compounds.
Orders of electron withdrawing group is -$N{{O}_{2}}$, -$CN$,-$CHO$,-$COR$,-$COOH$,-$COOR$, -$CON{{H}_{2}}$(strongest to weakest)
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




