
The total number of structural isomers possible for an amine with a molecular formula of ${{C}_{4}}{{H}_{11}}N$ is:
A. 5
B. 6
C. 7
D. 8
Answer
595.2k+ views
Hint: Think about the types of amines that are formed and what defines them as primary secondary or tertiary amines. Isolate that group and visualize structural isomers with the remaining carbon and hydrogen atoms.
Complete step by step answer:
We will consider all the isomers of primary, secondary, and tertiary amines individually
- First, let us consider all the structures that can be formed if the given amine is a primary amine. A primary amine is an amine where the nitrogen atom is attached to only one carbon atom and the other two bonds are formed with hydrogen. So according to this, the general molecular formula of a primary amine (in this case) will be ${{C}_{4}}{{H}_{9}}N{{H}_{2}}$. So, for a primary amine, we can arrange the four atoms of carbon in four different ways. They are as follows:
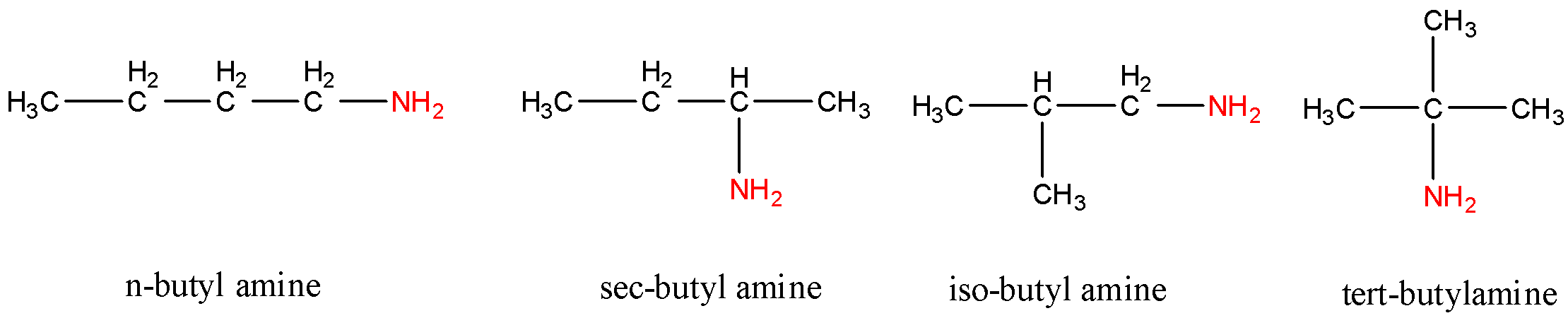
Count the number of all the hydrogen atoms and carbon atoms to verify that the number of atoms in the structure and the molecular formula are the same.
- Now, let us look at the isomeric structures that involve a secondary amine group. Secondary amines are amines where the nitrogen atom is bonded to two carbon atoms and one hydrogen atom. The general formula for secondary amines (in this case) will be ${{C}_{4}}{{H}_{10}}NH$. So, for secondary amines, we can arrange the four atoms of carbon in three different ways. They are as follows:
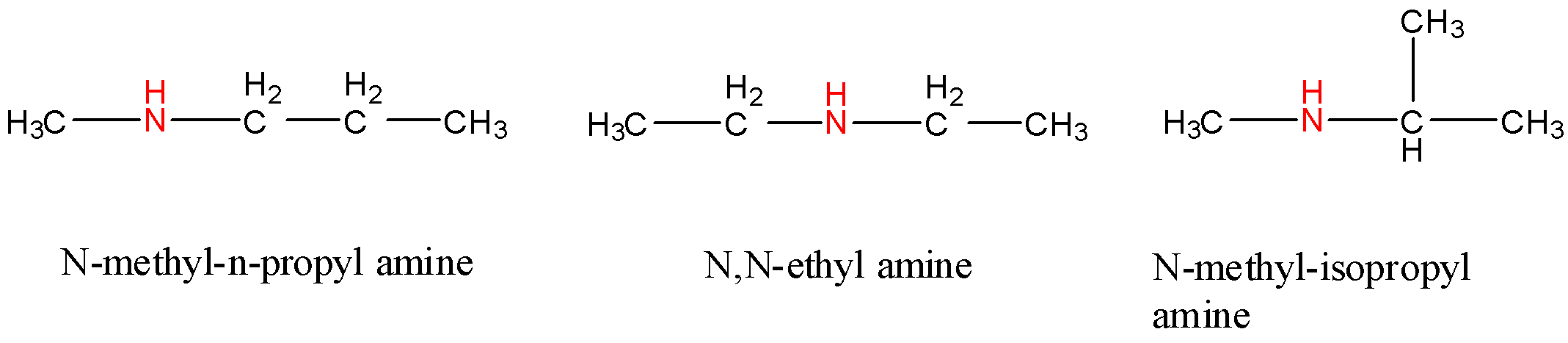
- Lastly, let us consider the isomeric structures that can be formed with a tertiary amino group and four carbon atoms. In tertiary amines, all the bonds that are formed by the nitrogen atom are with carbon atoms. The nitrogen atom is not directly attached to any hydrogen atoms in tertiary amines. The general molecular formula for tertiary amines (in this case) will be ${{C}_{4}}{{H}_{11}}N$. For a tertiary amine, we can arrange the four molecules of carbon in only one way. It is as follows:
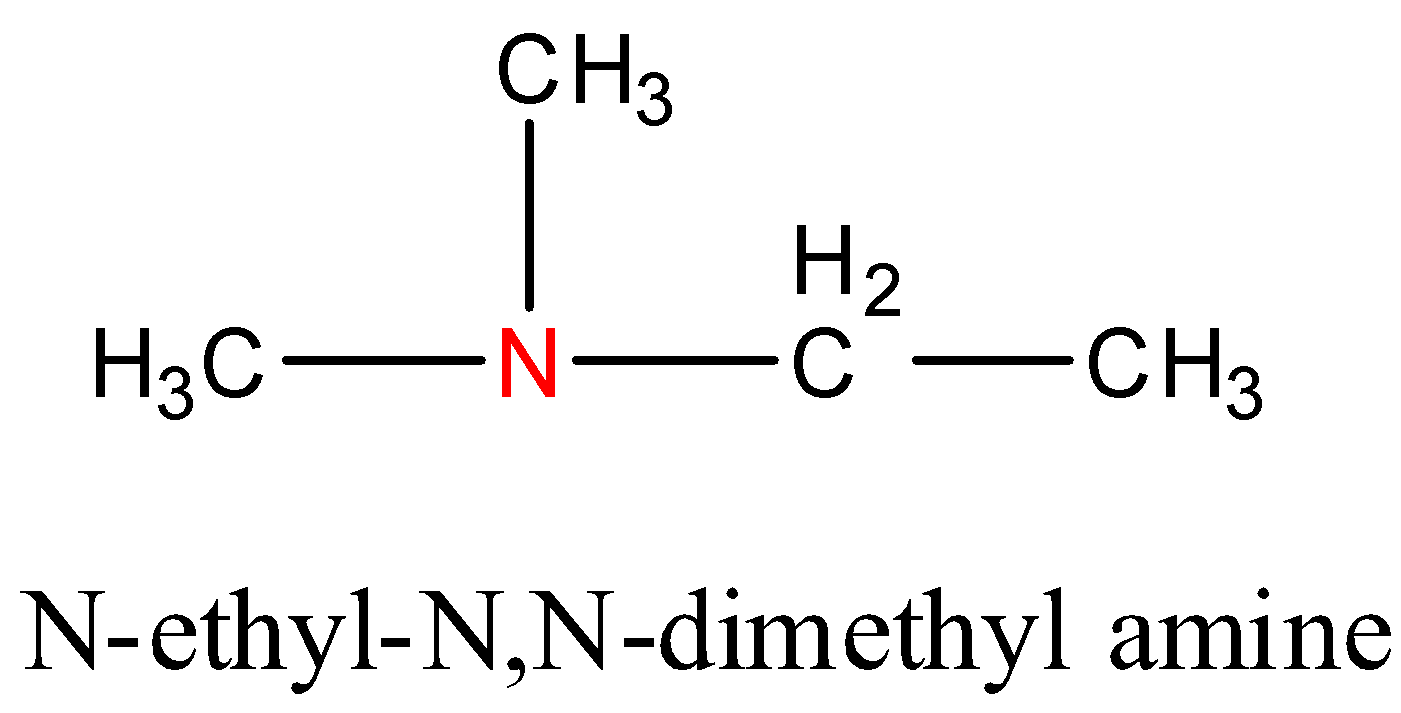
Hence, from this we can deduce that we can form 8 isomers that have the molecular formula ${{C}_{4}}{{H}_{11}}N$.
So, the correct answer is “Option D”.
Note: Remember that the names primary, secondary, and tertiary do not refer to the carbon that the amine group is attached to but the amine group itself. Usually when we say tert-butyl chloride, it means that the chlorine atom is attached to the tertiary carbon that is present. But when we talk about a ‘tertiary amine’ it indicates that the nitrogen atom is attached to three carbon atoms and not that the nitrogen atom is attached to a tertiary carbon.
Complete step by step answer:
We will consider all the isomers of primary, secondary, and tertiary amines individually
- First, let us consider all the structures that can be formed if the given amine is a primary amine. A primary amine is an amine where the nitrogen atom is attached to only one carbon atom and the other two bonds are formed with hydrogen. So according to this, the general molecular formula of a primary amine (in this case) will be ${{C}_{4}}{{H}_{9}}N{{H}_{2}}$. So, for a primary amine, we can arrange the four atoms of carbon in four different ways. They are as follows:

Count the number of all the hydrogen atoms and carbon atoms to verify that the number of atoms in the structure and the molecular formula are the same.
- Now, let us look at the isomeric structures that involve a secondary amine group. Secondary amines are amines where the nitrogen atom is bonded to two carbon atoms and one hydrogen atom. The general formula for secondary amines (in this case) will be ${{C}_{4}}{{H}_{10}}NH$. So, for secondary amines, we can arrange the four atoms of carbon in three different ways. They are as follows:

- Lastly, let us consider the isomeric structures that can be formed with a tertiary amino group and four carbon atoms. In tertiary amines, all the bonds that are formed by the nitrogen atom are with carbon atoms. The nitrogen atom is not directly attached to any hydrogen atoms in tertiary amines. The general molecular formula for tertiary amines (in this case) will be ${{C}_{4}}{{H}_{11}}N$. For a tertiary amine, we can arrange the four molecules of carbon in only one way. It is as follows:

Hence, from this we can deduce that we can form 8 isomers that have the molecular formula ${{C}_{4}}{{H}_{11}}N$.
So, the correct answer is “Option D”.
Note: Remember that the names primary, secondary, and tertiary do not refer to the carbon that the amine group is attached to but the amine group itself. Usually when we say tert-butyl chloride, it means that the chlorine atom is attached to the tertiary carbon that is present. But when we talk about a ‘tertiary amine’ it indicates that the nitrogen atom is attached to three carbon atoms and not that the nitrogen atom is attached to a tertiary carbon.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE

Explain sex determination in humans with line diag class 12 biology CBSE

Organisms of a higher trophic level which feed on several class 12 biology CBSE




